Advanced Ga2O3/Lignin and ZrO2/Lignin Hybrid Microplatforms for Glucose Oxidase Immobilization: Evaluation of Biosensing Properties by Catalytic Glucose Oxidation
Abstract
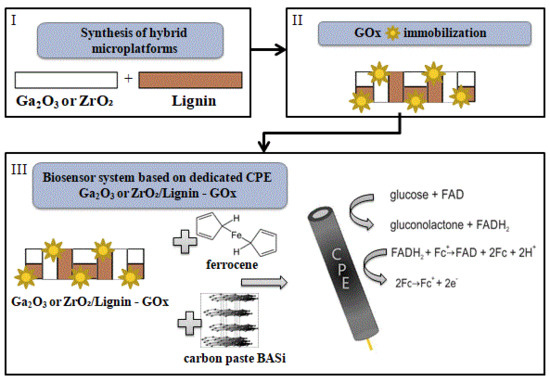
:1. Introduction
2. Results and Discussion
2.1. Surface Morphology
2.2. Zeta Potential, Mean Hydrodynamic Diameter, PdI, and Elemental Analysis of Microplatforms
2.3. Fourier Transform Infrared Spectroscopy (FT-IR) Analysis
2.4. Thermal Stability
2.5. Efficiency of Glucose Oxidase (GOx) Immobilization
2.6. Electrochemical Properties of Electrocatalytic Microplatforms with Immobilized Glucose Oxidase
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Modification of Metal Oxide Materials
3.3. Activation of Lignin and Synthesis of Ga2O3/Lignin and ZrO2/Lignin Supports
3.4. Immobilization of Glucose Oxidase on Ga2O3/Lignin and ZrO2/Lignin Supports
3.5. Construction of an Enzymatic Biosensor
3.6. Physicochemical Analysis
3.7. Electrochemical Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nanko, M. Definitions and categories of hybrid materials. AZojomo 2009, 6, 1–8. [Google Scholar]
- Chung, D.D.L. Composite Materials, Kirk-Othmer Encyclopedia of Chemical Technology; Wiley-VCH: New York, NY, USA, 2011. [Google Scholar]
- Jesionowski, T.; Klapiszewski, Ł.; Milczarek, G. Structural and electrochemical properties of multifunctional silica/lignin materials. Mat. Chem. Phys. 2014, 147, 1049–1057. [Google Scholar] [CrossRef]
- Klapiszewski, Ł.; Nowacka, M.; Milczarek, G.; Jesionowski, T. Physicochemical and electrokinetic properties of silica/lignin biocomposites. Carbohydr. Polym. 2013, 94, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Jędrzak, A.; Rębiś, T.; Klapiszewski, Ł.; Zdarta, J.; Milczarek, G.; Jesionowski, T. Carbon paste electrode based on functional GOx/silica-lignin system to prepare an amperometric glucose biosensor. Sens. Actuators B 2018, 256, 176–185. [Google Scholar] [CrossRef]
- Kickelbick, G. Hybrid Materials: Synthesis, Characterization and Applications; John Wiley & Sons: Wien, Austria, 2007. [Google Scholar]
- Gomez-Romero, P.; Sanchez, C. Functional Hybrid Materials; John Wiley & Sons: Darmstadt, Germany, 2006. [Google Scholar]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Drisko, D.L.; Sanchez, C. Hybridization in Materials Science—Evolution, Current State, and Future Aspirations. Eur. J. Inorg. Chem. 2012, 32, 5097–5105. [Google Scholar] [CrossRef]
- Yoon, K.; Takahashi, S.; Nge, T.T.; Nakagawa-izumi, A.; Ohi, H.; Yamada, T. Characterization of lignin derivatives in alkaline polyethylene glycol-treated soda cooking black liquor powder. BioResources 2016, 3, 6426–6437. [Google Scholar] [CrossRef] [Green Version]
- Bajwa, D.S.; Wang, X.; Sitz, E.; Loll, T.; Bhattacharje, S. Application of bioethanol derived lignin for improving physico-mechanical properties of thermoset biocomposites. Int. J. Biol. Macromol. 2016, 89, 265–272. [Google Scholar] [CrossRef]
- Espinoza-Acosta, J.L.; Torres-Chávez, P.I.; Ramírez-Wong, B.; López-Saiz, C.M.; Montaño-Leyva, B. Antioxidant, antimicrobial, and antimutagenic properties of technical lignins and their applications. BioResources 2016, 11, 5452–5481. [Google Scholar] [CrossRef]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme immobilization by adsorption: A review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef] [Green Version]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. Developments in support materials for immobilization of oxidoreductases: A comprehensive review. Adv. Colloids Interf. 2018, 258, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.; Marzuki, N.; Buang, N.; Huyop, F.; Wahab, R. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Zdarta, J.; Klapiszewski, Ł.; Jędrzak, A.; Nowicki, M.; Moszynski, D.; Jesionowski, T. Lipase B from Candida antarctica Immobilized on a Silica-Lignin Matrix as a Stable and Reusable Biocatalytic System. Catalysts 2017, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Zdarta, J.; Jędrzak, A.; Klapiszewski, Ł.; Jesionowski, T. Immobilization of Cellulase on a Functional Inorganic–Organic Hybrid Support: Stability and Kinetic Study. Catalysts 2017, 7, 374. [Google Scholar] [CrossRef] [Green Version]
- Bankar, S.B.; Bule, M.V.; Singhal, R.S.; Ananthanarayan, L. Glucose oxidase—An overview. Biotechnol. Adv. 2009, 27, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Batule, B.S.; Park, K.S.; Gautam, S.; Cheon, H.J.; Kim, M.I.; Park, H.G. Intrinsic peroxidase-like activity of sonochemically synthesized protein copper nanoflowers and its application for the sensitive detection of glucose. Sens. Actuator B-Chem. 2018, 283, 749–754. [Google Scholar] [CrossRef]
- Baghayeri, M.; Veisi, H.; Ghanei-Motlagh, M. Amperometric glucose biosensor based on immobilization of glucose oxidase on a magnetic glassy carbon electrode modified with a novel magnetic nanocomposite. Sens. Actuator B-Chem. 2017, 249, 321–330. [Google Scholar] [CrossRef]
- Wong, C.M.; Wong, K.H.; Chen, X.D. Glucose oxidase: Natural occurrence, function, properties and industrial applications. Appl. Microbiol. Biotechnol. 2008, 78, 927–938. [Google Scholar] [CrossRef]
- Wang, J. Nanomaterial-based electrochemical biosensors. Analyst 2005, 130, 421–426. [Google Scholar] [CrossRef]
- Valentini, F.; Palleschi, G. Nanomaterials and analytical chemistry. Anal. Lett. 2008, 41, 479–520. [Google Scholar] [CrossRef]
- Wang, D.; Lou, Y.; Wang, R.; Wang, P.; Zheng, X.; Zhang, Y.; Jiang, N. Humidity sensor based on Ga2O3 nanorods doped with Na+ and K+ from GaN powder. Ceramics Int. 2015, 41, 14790–14797. [Google Scholar] [CrossRef]
- Nikolaev, V.I.; Maslov, V.; Stepanov, S.I.; Pechnikov, A.I.; Krymov, V.; Nikitinac, I.P.; Guzilova, L.I.; Bougrov, V.E.; Romanov, A.E. Growth and characterization of beta-Ga2O3 crystals. J. Crystal Growth 2016, 457, 132–136. [Google Scholar] [CrossRef]
- Ramana, C.V.; Rubio, E.J.; Barraza, C.D.; Gallardo, A.M.; Mcpeak, S. Chemical bonding, optical constants, and electrical resistivity of sputter-deposited gallium oxide thin films. J. Appl. Phys. 2014, 115, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ai, M.; Guo, D.; Qu, Y.; Cui, W.; Wu, Z.; Li, P.; Li, L.; Tang, W. Fast-response solar-blind ultraviolet photodetector with a graphene/β-Ga2O3/graphene hybrid structure. J. Alloy. Compd. 2016, 692, 634–638. [Google Scholar] [CrossRef]
- Jung, J.; Cho, W.; Kim, J.; Hwang, K.; Kang, E.; Han, K. Morphological and crystal structural characterization of Ga2O3 particles synthesized by a controlled precipitation and polymerized complex method. Ceram. Int. 2016, 42, 2582–2588. [Google Scholar] [CrossRef]
- Jena, K.; Narayan, R.; Raju, K. Surface functionalized zinc oxide (ZnO) nanoparticle filled organic–inorganic hybrid materials with enhanced thermo-mechanical properties. Prog. Org. Coat. 2015, 89, 82–90. [Google Scholar] [CrossRef]
- Siddiqui, M.R.H.; Al-Wassil, A.I.; Al-Otaibi, A.M.; Mahfouz, R.M. Effects of Precursor on the Morphology and Size of ZrO2 Nanoparticles, Synthesized by Sol-gel Method in Non-aqueous Medium. Mater. Res. 2012, 15, 986–989. [Google Scholar] [CrossRef] [Green Version]
- Deshmane, V.G.; Adewuyi, Y.G. Synthesis of thermally stable, high surface area, nanocrystalline mesoporous tetragonal zirconium dioxide (ZrO2): Effects of different process parameters. Micropor. Mesopor. Mat. 2012, 148, 88–100. [Google Scholar] [CrossRef]
- Jayakumar, S.; Ananthapadmanabhan, P.V.; Perumal, K. Characterization of nano-crystalline ZrO2 synthesized via reactive plasma processing. Mater. Sci. Eng. B 2011, 176, 894–899. [Google Scholar] [CrossRef]
- Mallakpour, S.; Dinari, M.; Neamani, S. Surface Treatment of ZrO2 Nanoparticles with Biosafe Citric Acid and Its Utilization for the Synthesis of L-Leucine Based Poly(amide-imide) Nanocomposites. Polym. Plast. Technol. Eng. 2015, 54, 1634–1643. [Google Scholar] [CrossRef]
- Sidhu, G.K.; Kaushik, A.K.; Rana, S.; Bhansali, S. Photoluminescence quenching of Zirconia nanoparticle by surface modification. Appl. Surf. Sci. 2015, 334, 216–221. [Google Scholar] [CrossRef]
- Wang, J.; Sun, X.; Wei, A. Zinc oxide nanocomb biosensor for glucose detection. Appl. Phys. Lett. 2006, 88, 233106. [Google Scholar] [CrossRef]
- Marie, M.; Mandal, S.; Manasreh, O. An electrochemical glucose sensor based on zinc oxide nanorods. Sensors 2015, 15, 18714–18723. [Google Scholar] [CrossRef] [PubMed]
- Patil, D.; Jung, H.; Dung, N. Enzymatic glucose biosensor based on CeO2 nanorods synthesized by non-isothermal precipitation. Biosens. Bioelectron. 2012, 31, 176–181. [Google Scholar] [CrossRef]
- Golikova, E.; Lakina, N.; Grebennikova, O.; Matveeva, V.; Sulman, E. A study of biocatalysts based on glucose oxidase. Faraday Discuss. 2017, 202, 303–314. [Google Scholar] [CrossRef]
- Wang, Y.; Li, N.; Duan, P.; Sun, X.; Chu, B.; He, Q.; He, Q. Properties and Photocatalytic Activity of β -Ga2O3 Nanorods under Simulated Solar Irradiation. J. Nanomater. 2015, 16, 1–5. [Google Scholar]
- Sathyaseelan, B.; Elayaperumal, M.; Baskaran, I.; Senthilnathan, K.; Sivakumar, K.; Moodley, M.; Ladchumananandasivam, R.; Maaza, M. Studies on structural and optical properties of ZrO2 nanopowder for opto-electronic applications. J. Alloy. Compd. 2017, 694, 556–559. [Google Scholar] [CrossRef]
- Goudarzi, A.; Lin, L.T.; Ko, F.K. X-ray diffraction analysis of kraft lignins and lignin-derived carbon nanofibers. J. Nanotechnol. Eng. Med. 2014, 5, 021006. [Google Scholar] [CrossRef]
- Luxbacher, T. The Zeta Guide: Principles of the Streaming Potential Technique; Anton Paar GmbH: Graz, Austria, 2014. [Google Scholar]
- Silverstein, R.M.; Webster, F.; Kiemle, D. Spectrophotometric Identification of Organic Compounds; John Wiley and Sons: New York, NY, USA, 2005. [Google Scholar]
- Reddy, L.S.; Ko, Y.H.; Yu, J.S. Hydrothermal Synthesis and Photocatalytic Property of β-Ga2O3 Nanorods. Nanoscale Res. Lett. 2015, 10, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Jędrzak, A.; Rębiś, T.; Kuznowicz, M.; Jesionowski, T. Bio-inspired magnetite/lignin/polydopamine-glucose oxidase biosensing nanoplatform. From synthesis, via sensing assays to comparison with others glucose testing techniques. Int. J. Biol. Macromol. 2019, 127, 677–682. [Google Scholar] [CrossRef]
- Ramon-Marquez, T.; Medina-Castillo, A.L.; Fernandez-Sanchez, J.F.; Fernandez-Guti’errez, A. Evaluation of different functional groups for covalent immobilization of enzymes in the development of biosensors with oxygen optical transduction. Anal. Methods 2015, 7, 2943–2949. [Google Scholar] [CrossRef] [Green Version]
- Mohammad, R.; Mojtaba, A. Immobilization of glucose oxidase on meso-porous glassceramic with the skeleton of CaTi4(PO4)6. J. Adv. Mater. Process. 2013, 2, 57–65. [Google Scholar]
- Abbasi, M.; Amiri, R.; Bordbar, A.K.; Ranjbakhsh, E.; Khosropour, A.R. Improvement of the Stability and Activity of Immobilized Glucose Oxidase on Modified Iron Oxide Magnetic Nanoparticles. Appl. Surf. Sci. 2016, 364, 752–757. [Google Scholar] [CrossRef]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Biochemistry; W. H. Freeman: New York, NY, USA, 2002. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation microgram quantitiesmof protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 254, 248–254. [Google Scholar] [CrossRef]
- Klapiszewski, Ł.; Zdarta, J.; Jesionowski, T. Titania/lignin hybrid materials as a novel support for -amylase immobilization: A comprehensive study. Colloids Surf. B Biointerfaces 2018, 162, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.J.; Khan, F.H.; Husain, Q.N. Application of Immobilized Ipomoea batata β Amylase in the Saccharification of Starch. J. Appl. Biol. Sci. 2011, 5, 33. [Google Scholar]





















| Sample | Content of Elements by Weight (%) | ||||||
|---|---|---|---|---|---|---|---|
| Ga | Zr | O | C | S | Na | I | |
| Ga2O3 | 72.9 | - | 27.1 | - | - | - | - |
| ZrO2 | - | 67.6 | 32.4 | - | - | - | - |
| Ga2O3/Lig | 29.0 | - | 22.3 | 46.8 | 0.7 | 0.4 | 0.7 |
| ZrO2/Lig | - | 27.3 | 25.5 | 42.9 | 0.7 | 1.3 | 0.7 |
| Sample | Zeta Potential (mV) | Mean Hydrodynamic Diameter (nm) | PdI |
|---|---|---|---|
| Ga2O3 | −32.2 | 930–1472 | 0.35 |
| ZrO2 | −33.5 | 269–283 | 0.29 |
| Ga2O3 (modified) | −28.2 | 1408–1932 | 0.41 |
| ZrO2 (modified) | −29.4 | 348–380 | 0.37 |
| Lignin | −42.1 | 280–348 | 0.19 |
| Ga2O3/lignin | −35.8 | 1566–2251 | 0.69 |
| ZrO2/lignin | −36.1 | 656–785 | 0.57 |
| Sample | Elemental Content (%) | |||
|---|---|---|---|---|
| N | C | H | S | |
| kraft lignin | 50.36 ± 0.13 | 5.79 ± 0.04 | 3.15 ± 0.08 | |
| kraft lignin (activated) * | 52.27 ± 0.15 | 5.09 ± 0.02 | 2.77 ± 0.05 | |
| Ga2O3 | ||||
| ZrO2 | ||||
| Ga2O3 (modified) ** | 0.34 ± 0.01 | 5.82 ± 0.04 | 0.66 ± 0.01 | |
| ZrO2 (modified) ** | 0.32 ± 0.01 | 5.64 ± 0.03 | 0.62 ± 0.01 | |
| Ga2O3/Lig | 0.23 ± 0.01 | 19.97 ± 0.01 | 2.38 ± 0.02 | 2.22 ± 0.01 |
| ZrO2/Lig | 0.22 ± 0.01 | 19.38 ± 0.04 | 2.34 ± 0.02 | 1.09 ± 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jędrzak, A.; Rębiś, T.; Kuznowicz, M.; Kołodziejczak-Radzimska, A.; Zdarta, J.; Piasecki, A.; Jesionowski, T. Advanced Ga2O3/Lignin and ZrO2/Lignin Hybrid Microplatforms for Glucose Oxidase Immobilization: Evaluation of Biosensing Properties by Catalytic Glucose Oxidation. Catalysts 2019, 9, 1044. https://doi.org/10.3390/catal9121044
Jędrzak A, Rębiś T, Kuznowicz M, Kołodziejczak-Radzimska A, Zdarta J, Piasecki A, Jesionowski T. Advanced Ga2O3/Lignin and ZrO2/Lignin Hybrid Microplatforms for Glucose Oxidase Immobilization: Evaluation of Biosensing Properties by Catalytic Glucose Oxidation. Catalysts. 2019; 9(12):1044. https://doi.org/10.3390/catal9121044
Chicago/Turabian StyleJędrzak, Artur, Tomasz Rębiś, Maria Kuznowicz, Agnieszka Kołodziejczak-Radzimska, Jakub Zdarta, Adam Piasecki, and Teofil Jesionowski. 2019. "Advanced Ga2O3/Lignin and ZrO2/Lignin Hybrid Microplatforms for Glucose Oxidase Immobilization: Evaluation of Biosensing Properties by Catalytic Glucose Oxidation" Catalysts 9, no. 12: 1044. https://doi.org/10.3390/catal9121044