Simultaneous Optimal Production of Flavonol Aglycones and Degalloylated Catechins from Green Tea Using a Multi-Function Food-Grade Enzyme
Abstract
:1. Introduction
2. Results
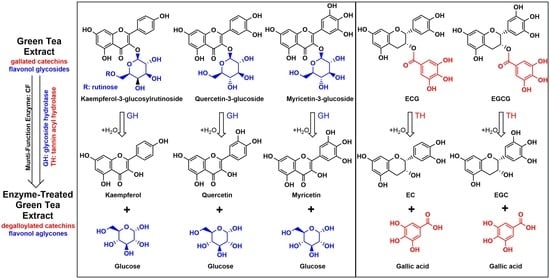
2.1. Composition Changes of GTE by Various Food–Grade Enzymes
2.2. Optimum Condition of Enzyme CF for Producing Flavonol Aglycones and GA
2.3. Investigation of Enzyme Activities by Fractionation
2.4. Kinetic Analysis of Enzyme CF for Producing Flavonol Aglycones
2.5. Hydrolysis of GTE in Enzymatic and Acid Hydrolysis Conditions
2.6. Rationale of the Enzymatic Reactions from Molecular Docking Simulation
3. Discussion
4. Materials and Methods
4.1. Chemicals and Enzymes
4.2. Preparation of GTE
4.3. Assay of Multi-Functional Activities of Food-Grade Enzymes
4.4. Screening of Food-Grade Enzymes for Producing Flavonol Aglycones from GTE
4.5. Investigation of Optimal Enzymatic Conditions for Producing Flavonol Aglycones and GA from GTE
4.6. Size Distribution, Separation, and Determination of the Core Activities of CF
4.7. Kinetic Analysis of CF for Producing Flavonol Aglycones
4.8. Time-Course of Enzyme CF and Hydrochloride Hydrolysis of GTE for Producing Flavonol Aglycones
4.9. HPLC Analysis of Catechins and Flavonols
4.10. Molecular Docking Simulations
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Assay of Multi-Functional Activities of Commercial Enzymes
References
- Monobe, M.; Nomura, S.; Ema, K.; Matsunaga, A.; Nesumi, A.; Yoshida, K.; Maeda-Yamamoto, M.; Horie, H. Quercetin glycosides-rich tea cultivars (Camellia sinensis L.) in Japan. Food Sci. Technol. Res. 2015, 21, 333–340. [Google Scholar] [CrossRef]
- Peterson, J.; Dwyer, J.; Bhagwat, S.; Haytowitz, D.; Holden, J.; Eldridge, A.L.; Beecher, G.; Aladesanmi, J. Major flavonoids in dry tea. J. Food Compos. Anal. 2005, 18, 487–501. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea polyphenols for health promotion. Life Sci. Adv. Exp. Clin. Endocrinol. 2007, 81, 519–533. [Google Scholar] [CrossRef] [Green Version]
- Cabrera, C.; Artacho, R.; Gimenez, R. Beneficial effects of green tea—A review. J. Am. Coll. Nutr. 2006, 25, 79–99. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Xu, H.; Heritier, J.; Andlauer, W. Determination of catechins and flavonol glycosides in Chinese tea varieties. Food Chem. 2012, 132, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Engelhardt, U.H.; Thräne, C.; Maiwald, B.; Stark, J. Determination of flavonol glycosides in green tea, oolong tea and black tea by UHPLC compared to HPLC. Food Chem. 2015, 183, 30–35. [Google Scholar] [CrossRef]
- Iwashina, T. The structure and distribution of the flavonoids in plants. J. Plant Res. 2000, 113, 287–299. [Google Scholar] [CrossRef]
- Walle, T.; Otake, Y.; Walle, U.K.; Wilson, F.A. Quercetin glucosides are completely hydrolyzed in ileostomy patients before absorption. J. Nutr. 2000, 130, 2658–2661. [Google Scholar] [CrossRef]
- Olthof, M.R.; Hollman, P.C.; Vree, T.B.; Katan, M.B. Bioavailabilities of quercetin-3-glucoside and quercetin-4′-glucoside do not differ in humans. J. Nutr. 2000, 130, 1200–1203. [Google Scholar] [CrossRef]
- Hollman, P.C.H.; Bijsman, M.N.C.P.; van Gameren, Y.; Cnossen, E.P.J.; de Vries, J.H.M.; Katan, M.B. The sugar moiety is a major determinant of the absorption of dietary flavonoid glycosides in man. Free Radic. Res. 1999, 31, 569–573. [Google Scholar] [CrossRef]
- Xiao, J.; Cao, H.; Wang, Y.; Zhao, J.; Wei, X. Glycosylation of dietary flavonoids decreases the affinities for plasma protein. J. Agric. Food. Chem. 2009, 57, 6642–6648. [Google Scholar] [CrossRef] [PubMed]
- Hostetler, G.; Riedl, K.; Cardenas, H.; Diosa-Toro, M.; Arango, D.; Schwartz, S.; Doseff, A.I. Flavone deglycosylation increases their anti-inflammatory activity and absorption. Mol. Nutr. Food Res. 2012, 56, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Semwal, D.K.; Semwal, R.B.; Combrinck, S.; Viljoen, A. Myricetin: A dietary molecule with diverse biological activities. Nutrients 2016, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Lea, M.A. Flavonol regulation in tumor cells. J. Cell. Biochem. 2015, 116, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.; Monobe, M.; Ema, K.; Matsunaga, A.; Maeda-Yamamoto, M.; Horie, H. Effects of flavonol-rich green tea cultivar (Camellia sinensis L.) on plasma oxidized LDL levels in hypercholesterolemic mice. Biosci. Biotechnol. Biochem. 2016, 80, 360–362. [Google Scholar] [CrossRef]
- Menezes, R.; Rodriguez-Mateos, A.; Kaltsatou, A.; Gonzalez-Sarrias, A.; Greyling, A.; Giannaki, C.; Andres-Lacueva, C.; Milenkovic, D.; Gibney, E.R.; Dumont, J.; et al. Impact of flavonols on cardiometabolic biomarkers: A meta-analysis of randomized controlled human trials to explore the role of inter-individual variability. Nutrients 2017, 9, 117. [Google Scholar] [CrossRef]
- Plumb, G.W.; Price, K.R.; Williamson, G. Antioxidant properties of flavonol glycosides from tea. Redox Rep. 1999, 4, 13–16. [Google Scholar] [CrossRef]
- Hur, S.J.; Lee, S.Y.; Kim, Y.C.; Choi, I.; Kim, G.B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Georgetti, S.R.; Vicentini, F.T.; Yokoyama, C.Y.; Borin, M.F.; Spadaro, A.C.; Fonseca, M.J. Enhanced in vitro and in vivo antioxidant activity and mobilization of free phenolic compounds of soybean flour fermented with different β-glucosidase-producing fungi. J. Appl. Microbiol. 2009, 106, 459–466. [Google Scholar] [CrossRef]
- Ni, H.; Chen, F.; Jiang, Z.D.; Cai, M.Y.; Yang, Y.F.; Xiao, A.F.; Cai, H.N. Biotransformation of tea catechins using Aspergillus niger tannase prepared by solid state fermentation on tea byproduct. LWT Food Sci. Technol. 2015, 60, 1206–1213. [Google Scholar] [CrossRef]
- Macedo, J.A.; Ferreira, L.R.; Camara, L.E.; Santos, J.C.; Gambero, A.; Macedo, G.A.; Ribeiro, M.L. Chemopreventive potential of the tannase-mediated biotransformation of green tea. Food Chem. 2012, 133, 358–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baik, J.H.; Shin, K.S.; Park, Y.; Yu, K.W.; Suh, H.J.; Choi, H.S. Biotransformation of catechin and extraction of active polysaccharide from green tea leaves via simultaneous treatment with tannase and pectinase. J. Sci. Food Agric. 2015, 95, 2337–2344. [Google Scholar] [CrossRef] [PubMed]
- Farrell, T.L.; Ellam, S.L.; Forrelli, T.; Williamson, G. Attenuation of glucose transport across Caco-2 cell monolayers by a polyphenol-rich herbal extract: Interactions with SGLT1 and GLUT2 transporters. BioFactors 2013, 39, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N. Diet-derived phenols in plasma and tissues and their implications for health. Planta Med. 2004, 70, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Walle, T. Absorption and metabolism of flavonoids. Free Radic. Biol. Med. 2004, 36, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Van Duynhoven, J.; Vaughan, E.E.; Jacobs, D.M.; Kemperman, R.A.; van Velzen, E.J.J.; Gross, G.; Roger, L.C.; Possemiers, S.; Smilde, A.K.; Dore, J.; et al. Metabolic fate of polyphenols in the human superorganism. Proc. Natl. Acad. Sci. USA 2010, 108, 4531–4538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itan, Y.; Jones, B.L.; Ingram, C.J.; Swallow, D.M.; Thomas, M.G. A worldwide correlation of lactase persistence phenotype and genotypes. BMC Evol. Biol. 2010, 10, 36. [Google Scholar] [CrossRef]
- Nemeth, K.; Plumb, G.W.; Berrin, J.G.; Juge, N.; Jacob, R.; Naim, H.Y.; Williamson, G.; Swallow, D.M.; Kroon, P.A. Deglycosylation by small intestinal epithelial cell β-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur. J. Nutr. 2003, 42, 29–42. [Google Scholar] [CrossRef]
- Choi, E.H.; Rha, C.S.; Balusamy, S.R.; Kim, D.O.; Shim, S.M. Impact of bioconversion of gallated catechins and flavonol glycosides on bioaccessibility and intestinal cellular uptake of catechins. J. Agric. Food. Chem. 2019, 67, 2331–2339. [Google Scholar] [CrossRef]
- Rha, C.-S.; Jeong, H.W.; Park, S.; Lee, S.; Jung, Y.S.; Kim, D.-O. Antioxidative, anti-inflammatory, and anticancer effects of purified flavonol glycosides and aglycones in green tea. Antioxidants 2019, 8, 278. [Google Scholar] [CrossRef]
- van Pouderoyen, G.; Snijder, H.J.; Benen, J.A.E.; Dijkstra, B.W. Structural insights into the processivity of endopolygalacturonase I from Aspergillus niger. FEBS Lett. 2003, 554, 462–466. [Google Scholar] [CrossRef]
- Ren, B.; Wu, M.; Wang, Q.; Peng, X.; Wen, H.; McKinstry, W.J.; Chen, Q. Crystal structure of tannase from Lactobacillus plantarum. J. Mol. Biol. 2013, 425, 2737–2751. [Google Scholar] [CrossRef] [PubMed]
- AFMB. Glycoside Hydrolase Family Classification. Available online: http://www.cazy.org/Glycoside-Hydrolases.html (accessed on 25 November 2018).
- Dube, A.; Ng, K.; Nicolazzo, J.A.; Larson, I. Effective use of reducing agents and nanoparticle encapsulation in stabilizing catechins in alkaline solution. Food Chem. 2010, 122, 662–667. [Google Scholar] [CrossRef]
- Kirk, O.; Borchert, T.V.; Fuglsang, C.C. Industrial enzyme applications. Curr. Opin. Biotechnol. 2002, 13, 345–351. [Google Scholar] [CrossRef]
- Yao, J.; Fan, X.J.; Lu, Y.; Liu, Y.H. Isolation and characterization of a novel tannase from a metagenomic library. J. Agric. Food. Chem. 2011, 59, 3812–3818. [Google Scholar] [CrossRef]
- Lu, M.-J.; Chen, C. Enzymatic modification by tannase increases the antioxidant activity of green tea. Food Res. Int. 2008, 41, 130–137. [Google Scholar] [CrossRef]
- Matsumoto, T.; Shimada, S.; Hata, Y.; Tanaka, T.; Kondo, A. Multi-functional glycoside hydrolase: Blon_0625 from Bifidobacterium longum subsp. infantis ATCC 15697. Enzym. Microb. Technol. 2015, 68, 10–14. [Google Scholar] [CrossRef]
- Baik, J.H.; Suh, H.J.; Cho, S.Y.; Park, Y.; Choi, H.S. Differential activities of fungi-derived tannases on biotransformation and substrate inhibition in green tea extract. J. Biosci. Bioeng. 2014, 118, 546–553. [Google Scholar] [CrossRef]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef] [Green Version]
- Flipphi, M.J.; Visser, J.; van der Veen, P.; de Graaff, L.H. Arabinase gene expression in Aspergillus niger: Indications for coordinated regulation. Microbiology 1994, 140, 2673–2682. [Google Scholar] [CrossRef]
- Gallego, M.V.; Piñaga, F.; Ramón, D.; Vallés, S. Purification and characterization of an α-L-rhamnosidase from Aspergillus terreus of interest in winemaking. J. Food Sci. 2001, 66, 204–209. [Google Scholar] [CrossRef]
- Kim, B.H.; Park, S.K. Enhancement of volatile aromatic compounds in black raspberry wines via enzymatic treatment. J. Inst. Brew. 2017, 123, 277–283. [Google Scholar] [CrossRef] [Green Version]
- McCleary, B.V.; McKie, V.A.; Draga, A.; Rooney, E.; Mangan, D.; Larkin, J. Hydrolysis of wheat flour arabinoxylan, acid-debranched wheat flour arabinoxylan and arabino-xylo-oligosaccharides by β-xylanase, α-L-arabinofuranosidase and β-xylosidase. Carbohydr. Res. 2015, 407, 79–96. [Google Scholar] [CrossRef] [PubMed]
- Codex, F.C. Food Chemicals Codex, 10th ed.; Available online: http://www.foodchmicalscodex.org (accessed on 11 March 2019).
- Iibuchi, S.; Minoda, Y.; Yamada, K. Studies on tannin acyl hydrolase of microorganisms: Part II. A new method determining the enzyme activity using the change of ultra violet absorption. Agric. Biol. Chem. 1967, 31, 513–518. [Google Scholar] [CrossRef]







| Name of Enzyme a (abbreviation) | Cellulyve-AN (AN) | Cellulase-KN (KN) | Glucosidase-BT (BT) | Plantase-CF (CF) | Plantase-UF (UF) | Peclyve ARA-NS (NS) | Sumizyme-AC (AC) | Tannase-KTFHR (TN) | |
|---|---|---|---|---|---|---|---|---|---|
| EC number | 3.2.1.4 | 3.2.1.4 | 3.2.1.21 | 3.2.1.15 | 3.2.1.6.; 3.2.1.99 | 3.2.1.99 | 3.2.1.4 | 3.1.1.20 | |
| Class of glycosidase b | C | C | B | P | G; A | A | C | T | |
| Activity (U c) | arabinase | 2 | 0.2 | N/D d | 62 | 55 | 175 | N/D | N/D |
| α-arabinofuranosidase | 36 | 124 | N/D | 3406 | 1058 | 473 | 69 | N/Ae | |
| α-arabinopyranosidase | 313 | 36 | N/D | 260 | 235 | 51 | 567 | N/A | |
| β-glucosidase | 32 | 6 | 280 | 28 | 40 | 12 | 36 | N/A | |
| β-galactosidase | 0 | 17 | 2 | 10 | 9 | 14 | 3 | N/A | |
| β-mannosidase | 40 | 2 | 3 | 58 | 1 | 1 | 130 | N/A | |
| α-rhamnosidase | 4 | 245 | N/D | 3 | 1 | 6 | 4 | N/A | |
| tannin acyl hydrolase | N/D | 3.9 | 0.7 | 14.8 | N/D | N/D | 3.4 | 520.4 | |
| β-xylosidase | 12 | 14 | 40 | 74 | 3 | 1 | 55 | N/A | |
| Test pH (optimum f) | 5.0 (3.0–5.0) | 5.0 (3.0–5.0) | 5.0 (3.0–6.0) | 5.0 (3.0–5.5) | 5.0 (4.0–6.5) | 5.0 (4.0–6.5) | 5.0 (3.5–5.0) | 5.0 (3.0–6.0) | |
| Test temp. (°C; optimum f) | 50 (30–50) | 50 (40–50) | 50 (35–65) | 50 (50–60) | 50 (40–60) | 50 (40–60) | 60 (40–65) | 40(40) | |
| Applied amount (% v/v) of enzyme | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| Regression a | Gallic acid | Myricetin | Quercetin | Kaempferol | Sum of flavonols | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | Temperature (°C) | Temperature (°C) | Temperature (°C) | Temperature (°C) | |||||||||||
| 35 | 50 | 50/35 b | 35 | 50 | 50/35 | 35 | 50 | 50/35 | 35 | 50 | 50/35 | 35 | 50 | 50/35 | |
| Vm (μg∙mL−1∙h−1) | |||||||||||||||
| HW | N.A.c | 169.5 d | N.A. | 21.9 | 279.6 | 12.8 | 14.4 | 42.7 | 3.0 | 5.7 | 13.7 | 2.4 | 34.9 | 195.7 | 5.6 |
| NLe | 95.1 | 153.3 | 1.6 | 17.0 | 82.9 | 4.9 | 12.5 | 32.8 | 2.6 | 4.6 | 11.8 | 2.6 | 35.5 | 131.6 | 3.7 |
| K′m (mg∙mL−1) | |||||||||||||||
| HW | N.A. | 2.9 | N.A. | 14.3 | 286.3 | 20.0 | 7.4 | 31.9 | 4.3 | 5.1 | 15.0 | 2.9 | 6.3 | 68.2 | 10.8 |
| NL | 2.8 | 3.8 | 1.3 | 8.3 | 46.3 | 5.6 | 6.4 | 20.2 | 3.2 | 4.2 | 11.4 | 2.7 | 7.4 | 87.5 | 11.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rha, C.-S.; Kim, S.-W.; Byoun, K.H.; Hong, Y.D.; Kim, D.-O. Simultaneous Optimal Production of Flavonol Aglycones and Degalloylated Catechins from Green Tea Using a Multi-Function Food-Grade Enzyme. Catalysts 2019, 9, 861. https://doi.org/10.3390/catal9100861
Rha C-S, Kim S-W, Byoun KH, Hong YD, Kim D-O. Simultaneous Optimal Production of Flavonol Aglycones and Degalloylated Catechins from Green Tea Using a Multi-Function Food-Grade Enzyme. Catalysts. 2019; 9(10):861. https://doi.org/10.3390/catal9100861
Chicago/Turabian StyleRha, Chan-Su, Shin-Woo Kim, Kyoung Hee Byoun, Yong Deog Hong, and Dae-Ok Kim. 2019. "Simultaneous Optimal Production of Flavonol Aglycones and Degalloylated Catechins from Green Tea Using a Multi-Function Food-Grade Enzyme" Catalysts 9, no. 10: 861. https://doi.org/10.3390/catal9100861