Synthesis of Soluble Star-Shaped Polymers via In and Out Approach by Ring-Opening Metathesis Polymerization (ROMP) of Norbornene: Factors Affecting the Synthesis
Abstract
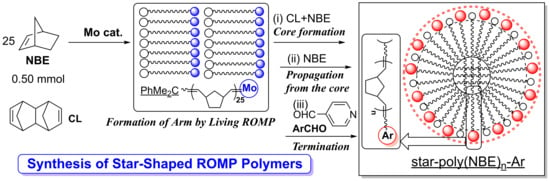
:1. Introduction
2. Results and Discussion
Synthesis of Pyridine Modified Star-Shaped Ring-Opened Poly(norbornene)
3. Concluding Remarks
4. Experimental Section
4.1. General Procedure
4.2. General Polymerization Procedure
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Matyjaszewski, K. Macromolecular Engineering: From Precise Macromolecular Synthesis to Macroscopic Materials Properties and Applications; Matyjaszewski, K., Gnanou, Y., Leibler, L., Eds.; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Hadjichristidis, N.; Pitsikalis, M.; Pispas, S.; Iatrou, H. Polymers with complex architecture by living anionic polymerization. Chem. Rev. 2001, 101, 3747–3792. [Google Scholar] [CrossRef] [PubMed]
- Hirao, A.; Hayashi, M.; Loykulnant, S.; Sugiyama, K.; Ryu, S.W.; Haraguchi, N.; Matsuo, A.; Higashihara, T. Precise syntheses of chain-multi-functionalized polymers, star-branched polymers, star-linear block polymers, densely branched polymers, and dendritic branched polymers based on iterative approach using functionalized 1,1-diphenylethylene derivatives. Prog. Polym. Sci. 2005, 30, 111–182. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Tsarevsky, N.V. Macromolecular engineering by atom transfer radical polymerization. J. Am. Chem. Soc. 2014, 136, 6513–6533. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Tsarevsky, N.V. Nanostructured functional materials prepared by atom transfer radical polymerization. Nat. Chem. 2009, 1, 276–288. [Google Scholar] [CrossRef]
- Khana, K.; Varshney, S.; Kakkar, A. Miktoarm star polymers: Advances in synthesis, self-assembly, and applications. Polym. Chem. 2010, 1, 1171–1185. [Google Scholar] [CrossRef]
- Ren, J.M.; McKenzie, T.G.; Fu, Q.; Wong, E.H.H.; Xu, J.; An, Z.; Shanmugam, S.; Davis, T.P.; Boyer, C.; Qiao, G.G. Star polymers. Chem. Rev. 2016, 116, 6743–6836. [Google Scholar] [CrossRef]
- Buchmeiser, M.R. Homogeneous metathesis polymerization by well-defined group VI and group VIII transition-metal alkylidenes: Fundamentals and applications in the preparation of advanced materials. Chem. Rev. 2000, 100, 1565–1604. [Google Scholar] [CrossRef] [PubMed]
- Grubbs, R.H. (Ed.) Handbook of Metathesis; Wiley-VCH: Weinheim, Germany, 2003; Volume 3. [Google Scholar]
- Buchmeiser, M. (Ed.) Metathesis Polymerisation; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Hilf, S.; Kilbinger, A.F.M. Functional end groups for polymers prepared using ring-opening metathesis polymerization. Nat. Chem. 2009, 1, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Abdellatif, M.M. Precise synthesis of polymers containing functional end groups by living ring-opening metathesis polymerization (ROMP): Efficient tools for synthesis of block/graft copolymers. Polymer 2010, 51, 1861–1881. [Google Scholar] [CrossRef]
- Leitgeb, A.; Wappel, J.; Slugovc, C. The ROMP toolbox upgraded. Polymer 2010, 51, 2927–2946. [Google Scholar] [CrossRef]
- Grubbs, R.H. Handbook of Metathesis, 2nd ed.; Grubbs, R.H., Khosravi, E., Eds.; Wiley-VCH: Weinheim, Germany, 2015; Volume 3. [Google Scholar]
- Lunn, D.J.; Discekici, E.H.; de Alaniz, J.R.; Gutekunst, W.R.; Hawker, C.J. Established and emerging strategies for polymer chain-end modification. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 2903–2914. [Google Scholar] [CrossRef]
- Chen, Y.; Abdellatif, M.M.; Nomura, K. Olefin metathesis polymerization: Some recent developments in the precise polymerizations for synthesis of advanced materials (by ROMP, ADMET). Tetrahedron 2018, 74, 619–643. [Google Scholar] [CrossRef]
- Nomura, K.; Watanabe, Y.; Fujita, S.; Fujiki, M.; Otani, H. Facile controlled synthesis of soluble star shape polymers by ring-opening metathesis polymerization (ROMP). Macromolecules 2009, 42, 899–901. [Google Scholar] [CrossRef]
- Otani, H.; Fujita, S.; Watanabe, Y.; Fujiki, M.; Nomura, K. A facile, controlled synthesis of soluble star polymers containing a sugar residue by ring-opening metathesis polymerization (ROMP). Macromol. Symp. 2010, 293, 53–57. [Google Scholar] [CrossRef]
- Takamizu, K.; Nomura, K. Synthesis of oligo(thiophene) coated star shape ROMP polymers: Unique emission properties by the precise integration of functionality. J. Am. Chem. Soc. 2012, 134, 7892–7895. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Tanaka, K.; Fujita, S. Use of pyridine coated star shape ROMP polymers as the supported ligand for Ru-catalyzed chemoselective hydrogen transfer reduction of ketones. Organometallics 2012, 31, 5074–5080. [Google Scholar] [CrossRef]
- Sun, Z.; Nomura, K. One-pot synthesis of end-functionalised soluble star-shaped polymers by living ring-opening metathesis polymerisation using molybdenum-alkylidene catalyst. RSC Adv. 2018, 8, 27703–27708. [Google Scholar] [CrossRef]
- Saunders, R.S.; Cohen, R.E.; Wong, S.J.; Schrock, R.R. Synthesis of amphiphilic star block copolymers using ring-opening metathesis polymerization. Macromolecules 1992, 25, 2055–2057. [Google Scholar] [CrossRef]
- Liu, J.; Burts, A.O.; Li, Y.; Zhukhovitskiy, A.V.; Ottaviani, M.F.; Turro, N.J.; Johnson, J.A. “Brush-first” method for the parallel synthesis of photocleavable, nitroxide-labeled poly (ethylene glycol) star polymers. J. Am. Chem. Soc. 2012, 134, 16337–16344. [Google Scholar] [CrossRef]
- Liao, L.; Liu, J.; Dreaden, E.C.; Morton, S.W.; Shopsowitz, K.E.; Hammond, P.T.; Johnson, J.A. A convergent synthetic platform for single-nanoparticle combination cancer therapy: Ratiometric loading and controlled release of cisplatin, doxorubicin, and camptothecin. J. Am. Chem. Soc. 2014, 136, 5896–5899. [Google Scholar] [CrossRef]
- Burts, A.O.; Gao, A.X.; Johnson, J.A. Brush-first synthesis of core-photodegradable miktoarm star polymers via ROMP: Towards photoresponsive self-assemblies. Macromol. Rapid Commun. 2014, 35, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.C.; Bruno, P.M.; Nguyen, H.V.-T.; Liao, L.; Liu, J.; Hemann, M.T.; Johnson, J.A. Using an RNAi signature assay to guide the design of three-drug-conjugated nanoparticles with validated mechanisms, in vivo efficacy, and low toxicity. J. Am. Chem. Soc. 2016, 138, 12494–12501. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.V.-T.; Chen, Q.; Paletta, J.T.; Harvey, P.; Jiang, Y.; Zhang, H.; Boska, M.D.; Ottaviani, M.F.; Jasanoff, A.; Rajca, A.; et al. Nitroxide-based macromolecular contrast agents with unprecedented transverse relaxivity and stability for magnetic resonance imaging of tumors. ACS Cent. Sci. 2017, 3, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, Y.; Nguyen, H.V.-T.; Johnson, J.A. Mikto-brush-arm star polymers via cross-linking of dissimilar bottlebrushes: Synthesis and solution morphologies. ACS Macro Lett. 2017, 6, 963–968. [Google Scholar] [CrossRef]
- Nguyen, H.V.-T.; Gallagher, N.M.; Vohidov, F.; Jiang, Y.; Kawamoto, K.; Zhang, H.; Park, J.V.; Huang, Z.; Ottaviani, M.F.; Rajca, A.; et al. Scalable Synthesis of Multivalent Macromonomers for ROMP. ACS Macro Lett. 2018, 7, 472–476. [Google Scholar] [CrossRef]
- Kalow, J.A.; Swager, T.M. Synthesis of miktoarm branched conjugated copolymers by ROMPing in and out. ACS Macro Lett. 2015, 4, 1229–1233. [Google Scholar] [CrossRef] [PubMed]
- Buchmeiser, M.R. Ring-opening metathesis polymerization-derived materials for separation science, heterogeneous catalysis and tissue engineering. Macromol. Symp. 2010, 298, 17–24. [Google Scholar] [CrossRef]
- Lubbad, S.; Buchmeiser, M.R. A new approach to high-capacity functionalized monoliths via post-synthesis grafting. Macromol. Rapid Commun. 2003, 24, 580–584. [Google Scholar] [CrossRef]
- Mayr, M.; Wang, D.; Kröll, R.; Schuler, N.; Prühs, S.; Fürstner, A.; Buchmeiser, M.R. Monolithic disk-supported metathesis catalysts for use in combinatorial chemistry. Adv. Synth. Catal. 2005, 347, 484–492. [Google Scholar] [CrossRef]
- Buchmeiser, M.R. Metathesis polymerization: A versatile tool for the synthesis of surface-functionalized supports and monolithic materials. In Handbook of Metathesis; Grubbs, R.H., Ed.; Wiley-VCH: Weinheim, Germany, 2003; Volume 3, pp. 226–254. [Google Scholar]
- Buchmeiser, M.R. Polymer-supported well-defined metathesis catalysts. Chem. Rev. 2009, 109, 303–321. [Google Scholar] [CrossRef]
- Schrock, R.R. The discovery and development of high oxidation state Mo and W imido alkylidene complexes for alkene metathesis. In Handbook of Metathesis; Grubbs, R.H., Ed.; Wiley-VCH: Weinheim, Germany, 2003; Volume 1, pp. 8–32. [Google Scholar]
- Schrock, R.R. Recent Advances in High Oxidation State Mo and W Imido Alkylidene Chemistry. Chem. Rev. 2009, 109, 3211–3226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, J.J.; Furusho, H.; Paton, R.M.; Nomura, K. Precise synthesis of poly(macromonomer)s containing sugars by repetitive ROMP and their attachments to poly(ethylene glycol): Synthesis, TEM analysis and their properties as amphiphilic block fragments. Chem. Eur. J. 2007, 13, 8985–8997. [Google Scholar] [CrossRef] [PubMed]
- Gestwicki, J.E.; Cairo, C.W.; Strong, L.E.; Oetjen, K.A.; Kiessling, L.L. Influencing receptor-ligand binding mechanisms with multivalent ligand architecture. J. Am. Chem. Soc. 2002, 124, 14922–14933. [Google Scholar] [CrossRef] [PubMed]
- Schrock, R.R.; Murdzek, J.S.; Bazan, G.C.; Robbins, J.; Dimare, M.; O’Regan, M.B. Synthesis of molybdenum imido alkylidene complexes and some reactions involving acyclic olefins. J. Am. Chem. Soc. 1990, 112, 3875–3886. [Google Scholar] [CrossRef]
- Stille, J.K.; Frey, D.A. Tetracyclic dienes. I. The Diels-Alder adduct of norbornadiene and cyclopentadiene. J. Am. Chem. Soc. 1959, 81, 4273–4275. [Google Scholar] [CrossRef]




| Run | Toluene b/g | 2nd | 3rd | Mne | Mw/Mne | Yield f | |||
|---|---|---|---|---|---|---|---|---|---|
| x/y/z | CL c /equiv | NBE c /equiv | conc. d /×10−2 M | time /min | NBE c /equiv | /×10−5 | /% | ||
| 1 g | 3.0/4.0/4.0 | 10 | -- | 8.67 | 50 | 25 | 0.88 | 1.19 | 96 |
| 2 g | 3.0/4.0/4.0 | 15 | -- | 9.91 | 50 | 25 | 1.34 | 1.30 | 96 |
| 3 g | 3.0/4.0/4.0 | 15 | -- | 9.91 | 70 | 25 | 2.08 | 2.88 h | 94 |
| 4 | 3.0/4.0/4.0 | 15 | 5 | 11.1 | 50 | 25 | 1.75 | 1.97 h | 95 |
| 5 | 3.0/4.0/4.0 | 15 | 5 | 11.1 | 70 | 25 | 2.13 | 2.22 h | 90 |
| 6 | 3.0/4.0/4.0 | 15 | 5 | 11.1 | 90 | 25 | 3.10 | 6.96 h | 99 |
| 7 | 3.0/4.0/4.0 | 15 | 5 | 11.1 | 120 | 25 | 3.45 | 4.74 h | 94 |
| 8 | 3.0/4.0/4.0 | 15 | 5 | 11.1 | 120 | 25 | 3.66 | 5.56 h | 90 |
| 9 g | 5.0/4.0/6.0 | 15 | -- | 7.71 | 50 | 25 | 1.37 | 1.44 | 97 |
| 10 g | 5.0/4.0/6.0 | 15 | -- | 7.71 | 70 | 25 | 1.44 | 1.46 | 99 |
| 11 | 5.0/4.0/6.0 | 15 | 5 | 8.67 | 50 | 25 | 1.45 | 1.73 h | 93 |
| 12 | 5.0/4.0/6.0 | 15 | 5 | 8.67 | 70 | 25 | 1.57 | 1.57 h | 95 |
| 13 | 5.0/4.0/6.0 | 15 | 5 | 8.67 | 90 | 25 | 1.93 | 2.05 h | 93 |
| 14 | 5.0/4.0/6.0 | 15 | 5 | 8.67 | 120 | 25 | 2.40 | 2.22 h | 93 |
| 15 g | 11.0/4.0/5.0 | 15 | -- | 4.62 | 50 | 25 | 1.37 | 1.22 | 90 |
| 16 g | 11.0/4.0/5.0 | 15 | 5 | 5.20 | 50 | 25 | 1.45 | 1.28 | 91 |
| 17 g | 11.0/4.0/5.0 | 15 | -- | 4.62 | 50 | 50 | 1.56 | 1.17 h | 96 |
| 18 g | 11.0/4.0/5.0 | 15 | 5 | 5.20 | 50 | 50 | 1.91 | 1.36 h | 98 |
| 19 g | 11.0/4.0/5.0 | 15 | -- | 4.62 | 70 | 25 | 1.49 | 1.37 | 94 |
| 20 g | 11.0/4.0/5.0 | 15 | 5 | 5.20 | 70 | 25 | 1.53 | 1.39 | 96 |
| 21 g | 11.0/4.0/5.0 | 15 | -- | 4.62 | 70 | 50 | 1.64 | 1.28 h | 94 |
| 22 g | 11.0/4.0/5.0 | 15 | 5 | 5.20 | 70 | 50 | 2.02 | 1.45 h | 99 |
| Run | Toluene b/g | 2nd | 3rd | Mne | Mw/Mne | Yield f | |||
|---|---|---|---|---|---|---|---|---|---|
| x/y/z | CL c /equiv | NBE c /equiv | conc. d /×10−2 M | time /min | NBE c /equiv | /×10−5 | /% | ||
| 23 g | 3.0/4.0/4.0 | 20 | -- | 11.1 | 50 | 25 | 2.86 | 2.51 h | 95 |
| 24 g | 3.0/4.0/4.0 | 20 | -- | 11.1 | 70 | 25 | 3.49 | 4.31 h | 98 |
| 25 g | 3.0/4.0/4.0 | 20 | -- | 11.1 | 90 | 25 | 2.86 | 2.19 h | 90 |
| 26 | 3.0/4.0/4.0 | 20 | 5 | 12.4 | 50 | 25 | 2.98 | 5.91 h | 94 |
| 27 | 3.0/4.0/4.0 | 20 | 5 | 12.4 | 90 | 25 | 2.99 | 2.29 h | 98 |
| 28 | 5.0/4.0/6.0 | 20 | 5 | 9.63 | 50 | 25 | 2.36 | 2.26 h | 99 |
| 29 | 5.0/4.0/6.0 | 20 | 5 | 9.63 | 70 | 25 | 2.93 | 2.56 h | 94 |
| 30 | 5.0/4.0/6.0 | 20 | 5 | 9.63 | 90 | 25 | 2.64 | 2.34 h | 99 |
| 31 | 5.0/4.0/6.0 | 20 | 5 | 9.63 | 120 | 25 | 2.74 | 2.04 h | 99 |
| 32 g | 11.0/4.0/5.0 | 20 | -- | 5.20 | 50 | 25 | 1.49 | 1.44 h | 90 |
| 33 g | 11.0/4.0/5.0 | 20 | -- | 5.20 | 70 | 25 | 1.78 | 1.54 h | 91 |
| 34 | 11.0/4.0/5.0 | 20 | 5 | 5.78 | 50 | 25 | 1.40 | 1.58 h | 99 |
| 35 | 11.0/4.0/5.0 | 20 | 5 | 5.78 | 70 | 25 | 1.64 | 1.91 h | 98 |
| 36 | 11.0/4.0/5.0 | 20 | 5 | 5.78 | 90 | 25 | 1.81 | 2.20 h | 98 |
| 37 | 11.0/4.0/5.0 | 20 | 5 | 5.78 | 120 | 25 | 2.14 | 2.22 h | 94 |
| 38 | 11.0/8.0/5.0 | 20 | 5 | 4.56 | 50 | 25 | 1.53 | 1.52 | 99 |
| 39 | 11.0/8.0/5.0 | 20 | 5 | 4.56 | 70 | 25 | 1.68 | 1.51 h | 97 |
| 40 | 15.0/8.0/5.0 | 20 | 5 | 3.77 | 50 | 25 | 1.52 | 1.43 | 99 |
| 41 | 15.0/8.0/5.0 | 20 | 5 | 3.77 | 70 | 25 | 1.64 | 1.46 h | 98 |
| Run | Toluene b/g | 2nd | 3rd | Mnf | Mw/Mnf | Yield g | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| x/y/z | CL(1) c /equiv | NBE c /equiv | CL(2) c /equiv | conc. d /×10−2 M | Time e /min | NBE c /equiv | /×10−5 | /% | ||
| 10 h | 5.0/4.0/6.0 | 15 | -- | -- | 7.71 | 70 | 25 | 1.44 | 1.46 | 99 |
| 12 | 5.0/4.0/6.0 | 15 | 5 | -- | 8.67 | 70 | 25 | 1.57 | 1.57 | 95 |
| 13 | 5.0/4.0/6.0 | 15 | 5 | -- | 8.67 | 90 | 25 | 1.93 | 2.05 | 93 |
| 28 | 5.0/4.0/6.0 | 20 | 5 | -- | 9.63 | 50 | 25 | 2.36 | 2.26 i | 99 |
| 29 | 5.0/4.0/6.0 | 20 | 5 | -- | 9.63 | 70 | 25 | 2.93 | 2.56 i | 94 |
| 30 | 5.0/4.0/6.0 | 20 | 5 | -- | 9.63 | 90 | 25 | 2.64 | 2.34 i | 99 |
| 31 | 5.0/4.0/6.0 | 20 | 5 | -- | 9.63 | 120 | 25 | 2.74 | 2.04 i | 99 |
| 42 | 5.0/6.0/4.0 | 10 | 5 | 10 | 7.88 | 40 | 25 | 1.15 | 1.88 i | 91 |
| 43 | 5.0/6.0/4.0 | 10 | 5 | 10 | 7.88 | 60 | 25 | 1.43 | 4.42 i | 92 |
| 44 | 5.0/6.0/4.0 | 10 | 5 | 10 | 7.88 | 80 | 25 | 1.44 | 2.11 i | 91 |
| 45 | 5.0/6.0/4.0 | 10 | 5 | 10 | 7.88 | 110 | 25 | 3.01 | 3.42 i | 94 |
| 46 | 5.0/6.0/4.0 | 10 | 5 | 10 | 7.88 | 60 | 50 | 2.41 | 2.83 i | 94 |
| 47 | 5.0/6.0/4.0 | 10 | 5 | 10 | 7.88 | 80 | 50 | 2.63 | 2.35 i | 92 |
| 48 | 5.0/6.0/4.0 | 10 | 5 | 10 | 7.88 | 110 | 50 | 3.66 | 2.81 i | 93 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Morishita, K.; Nomura, K. Synthesis of Soluble Star-Shaped Polymers via In and Out Approach by Ring-Opening Metathesis Polymerization (ROMP) of Norbornene: Factors Affecting the Synthesis. Catalysts 2018, 8, 670. https://doi.org/10.3390/catal8120670
Sun Z, Morishita K, Nomura K. Synthesis of Soluble Star-Shaped Polymers via In and Out Approach by Ring-Opening Metathesis Polymerization (ROMP) of Norbornene: Factors Affecting the Synthesis. Catalysts. 2018; 8(12):670. https://doi.org/10.3390/catal8120670
Chicago/Turabian StyleSun, Zelin, Kazuya Morishita, and Kotohiro Nomura. 2018. "Synthesis of Soluble Star-Shaped Polymers via In and Out Approach by Ring-Opening Metathesis Polymerization (ROMP) of Norbornene: Factors Affecting the Synthesis" Catalysts 8, no. 12: 670. https://doi.org/10.3390/catal8120670