Facile Synthesis of Bimetallic Pt-Ag/Graphene Composite and Its Electro-Photo-Synergistic Catalytic Properties for Methanol Oxidation
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. Materials
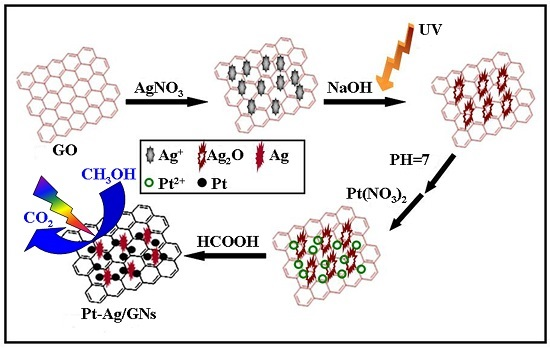
3.2. Preparation of Pt-Ag/GNs
3.3. Characterization Method
3.4. Electrochemical Measurements
3.5. Photo-Electrochemical Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jiang, S.P.; Liu, Z.; Hao, L.T.; Mu, P. Synthesis and characterization of PDDA-stabilized Pt nanoparticles for direct methanol fuel cells. Electrochim. Acta 2006, 51, 5721–5730. [Google Scholar] [CrossRef]
- Yu, L.H.; Xi, J.Y. TiO2 nanoparticles promoted Pt/C catalyst for ethanol electro-oxidation. Electrochim. Acta 2012, 67, 166–171. [Google Scholar] [CrossRef]
- Dutta, A.; Ouyang, J.Y. Ternary NiAuPt nanoparticles on reduced graphene oxide as catalysts toward the electrochemical oxidation reaction of ethanol. ACS Catal. 2015, 16, 1371–1380. [Google Scholar] [CrossRef]
- Bo, Z.; Hu, D.; Kong, J.; Yan, J.H.; Cen, K.F. Performance of vertically oriented graphene supported platinum–ruthenium bimetallic catalyst for methanol oxidation. J. Power Sources 2015, 273, 530–537. [Google Scholar] [CrossRef]
- Ammam, M.; Easton, E.B. PtCu/C and Pt(Cu)/C catalysts: Synthesis, characterization and catalytic activity towards ethanol electrooxidation. J. Power Sources 2013, 222, 79–87. [Google Scholar] [CrossRef]
- Guo, J.X.; Sun, Y.F.; Zhang, X.; Tang, L.; Liu, H.T. FePt nanoalloys anchored reduced graphene oxide as high-performance electrocatalysts for formic acid and methanol oxidation. J. Alloys Compd. 2014, 604, 286–291. [Google Scholar] [CrossRef]
- Hoseini, S.J.; Barzegar, Z.; Bahrami, M.; Roushani, M.; Rashidi, M. Organometallic precursor route for the fabrication of PtSn bimetallic nanotubes and Pt3Sn/reduced-graphene oxide nanohybrid thin films at oil–water interface and study of their electrocatalytic activity in methanol oxidation. J. Organomet. Chem. 2014, 769, 1–6. [Google Scholar] [CrossRef]
- Kepeniene, V.; Jablonskiene, J.; Vaiciuniene, J.; Kondrotas, R.; Juskenas, R. Investigation of graphene supported platinum-cobalt nanocomposites as electrocatalysts for ethanol oxidation. J. Electrochem. Soc. 2014, 59, 135–136. [Google Scholar]
- Shen, Y.; Zhang, M.Z.; Xiao, K.J.; Xi, Z.Y. Synthesis of Pt, PtRh, and PtRhNi alloys supported by pristine graphene nanosheets for ethanol electrooxidation. ChemCatChem 2014, 6, 3254–3261. [Google Scholar] [CrossRef]
- Lu, Y.Z.; Jiang, Y.T.; Wu, H.B.; Wei, C. Nano-PtPd cubes on graphene exhibit enhanced activity and durability in methanol electrooxidation after CO stripping-cleaning. J. Phys. Chem. C 2013, 117, 2926–2938. [Google Scholar] [CrossRef]
- Li, L.Z.; Chen, M.X.; Huang, G.B.; Yang, N.; Zhang, L.; Wang, H.; Liu, Y.; Wang, W.; Gao, J.P. A green method to prepare Pd–Ag nanoparticles supported on reduced graphene oxide and their electrochemical catalysis of methanol and ethanol oxidation. J. Power Sources 2014, 263, 13–21. [Google Scholar] [CrossRef]
- Kale, M.J.; Avanesian, T.; Christopher, P. Direct photocatalysis by plasmonic nanostructures. ACS Catal. 2013, 4, 116–128. [Google Scholar] [CrossRef]
- Hou, W.B.; Cronin, S.B. A review of surface plasmon resonance-enhanced photocatalysis. Adv. Funct. Mater. 2013, 23, 1612–1619. [Google Scholar] [CrossRef]
- Gao, L.N.; Yue, W.B.; Tao, S.S.; Fan, L.Z. Novel strategy for preparation of graphene-Pd, Pt composite, and its enhanced electrocatalytic activity for alcohol oxidation. Langmuir 2012, 29, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.Q.; Wu, Q.; Shi, G.Q. Graphene based new energy materials. Energy Environ. Sci. 2011, 4, 1113–1132. [Google Scholar] [CrossRef]
- Lei, F.L.; Li, Z.S.; Ye, L.T.; Wang, Y.L.; Lin, S. One-pot synthesis of Pt/SnO2/GNs and its electro-photo-synergistic catalysis for methanol oxidation. Int. J. Hydrogen Energy 2015, 41, 255–264. [Google Scholar] [CrossRef]
- Ye, L.T.; Li, Z.S.; Zhang, L.; Lei, F.L.; Lin, S. A green one-pot synthesis of Pt/TiO2/Graphene composites and its electro-photo-synergistic catalytic properties for methanol oxidation. J. Colloid Interface Sci. 2014, 433, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Hu, C.G.; He, X.S.; Liu, H.; Du, G.J.; Zhang, Y. Pt support of multidimensional active sites and radial channels formed by SnO2 flower-like crystals for methanol and ethanol oxidation. J. Power Sources 2011, 196, 4499–4505. [Google Scholar] [CrossRef]
- Wen, Z.L.; Yang, S.D.; Liang, Y.Y.; Wei, H.; Hao, T.; Liang, H.; Zhang, X.G.; Song, Q.J. The improved electrocatalytic activity of palladium/graphene nanosheets towards ethanol oxidation by tin oxide. Electrochim. Acta 2010, 56, 139–144. [Google Scholar] [CrossRef]
- Ma, S.S.; Xue, J.J.; Zhou, Y.M.; Zhang, Z.W. Photochemical synthesis of ZnO/Ag2O heterostructures with enhanced ultraviolet and visible photocatalytic activity. J. Mater. Chem. A 2014, 2, 7272–7280. [Google Scholar] [CrossRef]
- Li, S.S.; Lv, J.J.; Hu, Y.Y.; Zheng, J.N.; Chen, J.R.; Wang, A.J.; Feng, J.J. Facile synthesis of porous Pt–Pd nanospheres supported on reduced graphene oxide nanosheets for enhanced methanol electrooxidation. J. Power Sources 2014, 247, 213–218. [Google Scholar] [CrossRef]
- Zhang, L. A facile synthesis of flower-shaped TiO2/Ag microspheres and their application in photocatalysts. RSC Adv. 2014, 4, 54463–54468. [Google Scholar] [CrossRef]
- Sarkar, D.; Ghosh, C.K.; Mukherjee, S.; Chattopadhyay, K.K. Three dimensional Ag2O/TiO2 type-II (p–n) nanoheterojunctions for superior photocatalytic activity. ACS Appl. Mater. Interfaces 2013, 5, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Qian, H.H.; Hu, Y.; Dai, W.; Zhong, Y.J.; Chen, J.F.; Hu, X. Facile one-pot synthesis of uniform TiO2-Ag hybrid hollow spheres with enhanced photocatalytic activity. Dalton Trans. 2013, 42, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.Y.; Guo, M.W.; Wu, J.Y.; Xu, J.; Wang, W.C.; Chen, Z.D. Carbon-supported Ag@Pt core–shell nanoparticles with enhanced electrochemical activity for methanol oxidation and oxygen reduction reaction. J. Power Sources 2015, 277, 155–160. [Google Scholar] [CrossRef]
- Ganesan, P.; Prabu, M.; Sanetuntikul, J.; Shanmugam, S. Cobalt sulfide nanoparticles grown on nitrogen and sulfur codoped graphene oxide: An efficient electrocatalyst for oxygen reduction and rvolution reactions. ACS Catal. 2015, 5, 3625–3637. [Google Scholar] [CrossRef]
- Kim, Y.; Shanmugam, S. Polyoxometalate-reduced graphene oxide hybrid catalyst: Synthesis, structure, and electrochemical properties. ACS Appl. Mater. Interfaces 2013, 5, 12197–12204. [Google Scholar] [CrossRef] [PubMed]
- Graf, D.; Molitor, F.; Ensslin, K.; Stampfer, C.; Jungen, A.; Hierold, C.; Wirtz, L. Spatially resolved raman spectroscopy of single- and few-layer graphene. Nano Lett. 2007, 7, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Routh, P.; Das, S.; Shit, A.; Bairi, P.; Das, P.; Nandi, A.K. Graphene quantum dots from a facile sono-fenton reaction and its hybrid with a polythiophene graft copolymer toward photovoltaic application. ACS Appl. Mater. Interfaces 2013, 5, 12672–12680. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.W.; Duan, J.L.; Chen, H.L.; Zhang, Y.F.; Zhang, X.L. A carbon riveted Pt/graphene catalyst with high stability for direct methanol fuel cell. Microelectron. Eng. 2013, 110, 354–357. [Google Scholar] [CrossRef]
- Yan, Y.; Wei, W.; Liu, Y.Q.; Wang, F.X.; Zhe, Z.; Lei, Z.Q. Carbon supported heterostructured Pd–Ag nanoparticle: Highly active electrocatalyst for ethylene glycol oxidation. Int. J. Hydrogen Energy 2015, 40, 2225–2230. [Google Scholar]
- Wang, J.J.; Yin, G.P.; Zhang, J.; Wang, Z.B.; Gao, Y.Z. High utilization platinum deposition on single-walled carbon nanotubes as catalysts for direct methanol fuel cell. Electrochim. Acta 2007, 52, 7042–7050. [Google Scholar] [CrossRef]
- Yang, C.Z.; Laak, N.K.v.d.; Chan, K.Y.; Zhang, X. Microwave-assisted microemulsion synthesis of carbon supported Pt-WO3 nanoparticles as an electrocatalyst for methanol oxidation. Electrochim. Acta 2012, 75, 262–272. [Google Scholar] [CrossRef]
- Huang, H.L.; Liu, Y.J.; Gao, Q.Z.; Ruan, W.S.; Lin, X.M.; Xin, L. Rational construction of strongly coupled metal–metal oxide–graphene nanostructure with excellent electrocatalytic activity and durability. ACS Appl. Mater. Interfaces 2014, 6, 10258–10264. [Google Scholar] [CrossRef] [PubMed]
- Maiyalagan, T.; Alaje, T.O.; Scott, K. Highly stable Pt–Ru nanoparticles supported on three-dimensional cubic ordered mesoporous carbon (Pt–Ru/CMK-8) as promising electrocatalysts for methanol oxidation. J. Phys. Chem. C 2012, 116, 2630–2638. [Google Scholar] [CrossRef]
- Kashyout, A.B.; Bakr, A.; Nassr, A.B.A.A.; Giorgi, L.; Maiyalagan, T.; Youssef, B.A.B. Electrooxidation of methanol on carbon supported Pt-Ru nanocatalysts prepared by ethanol reduction method. Int. J. Electrochem. Sci. 2011, 6, 379–393. [Google Scholar]
- Lin, C.T.; Shiao, M.H.; Chang, M.N.; Chu, N.; Chen, Y.W.; Peng, Y.H.; Liao, B.H.; Huang, H.J.; Hsiao, C.N.; Tseng, F.G. A facile approach to prepare silicon-based Pt-Ag tubular dendritic nano-forests (tDNFs) for solar-light-enhanced methanol oxidation reaction. Nanoscale Res. Lett. 2015, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.S.; Huang, X.M.; Zhang, X.F.; Zhang, L.; Lin, S. The synergistic effect of graphene and polyoxometalates enhanced electrocatalytic activities of Pt-{PEI-GNs/[PMo12O40]3−}n composite films regarding methanol oxidation. J. Mater. Chem. 2012, 22, 23602–23607. [Google Scholar] [CrossRef]
- Zheng, J.N.; Lv, J.J.; Li, S.S.; Xue, M.W.; Wang, A.J.; Feng, J.J. One-pot synthesis of reduced graphene oxide supported hollow Ag@Pt core-shell nanospheres with enhanced electrocatalytic activity for ethylene glycol oxidation. J. Mater. Chem. 2014, 2, 3445–3451. [Google Scholar] [CrossRef]
- Maiyalagan, T.; Dong, X.C.; Chen, P.; Wang, X. Electrodeposited Pt on three-dimensional interconnected graphene as a free-standing electrode for fuel cell application. J. Mater. Chem. 2012, 22, 5286–5290. [Google Scholar] [CrossRef]
- Maiyalagan, T. Pt–Ru nanoparticles supported PAMAM dendrimer functionalized carbon nanofiber composite catalysts and their application to methanol oxidation. J. Solid State Electrochem. 2009, 13, 1561–1566. [Google Scholar] [CrossRef]
- Sanetuntikul, J.; Ketpang, K.; Shanmugam, S. Hierarchical nanostructured Pt8Ti-TiO2/C as an efficient and durable anode catalyst for direct methanol fuel cells. ACS Catal. 2015, 5, 7321–7327. [Google Scholar] [CrossRef]
- Guo, R.; Zhang, G.K.; Liu, J. Preparation of Ag/AgCl/BiMg2VO6 composite and its visible-light photocatalytic activity. Mater. Res. Bull. 2013, 48, 1857–1863. [Google Scholar] [CrossRef]
- Jin, R.; Cao, Y.; Mirkin, C.A.; Kelly, K.L.; Schatz, G.C.; Zheng, J.G. Photoinduced conversion of silver nanospheres to nanoprisms. Science 2001, 294, 1901–1903. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Fang, L.; Lu, H.D.; Li, Y.W.; Hu, C.Z.; Yu, H.G. One-pot pyridine-assisted synthesis of visible-light-driven photocatalyst Ag/Ag3PO4. Appl. Catal. B 2012, 115–116, 245–252. [Google Scholar] [CrossRef]
- Kovtyukhova, N.I.; Ollivier, P.J.; Martin, B.R.; Mallouk, T.E.; Chizhik, S.A.; Buzaneva, E.V.; Gorchinskiy, A.D. Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations. Chem. Mater. 1999, 11, 771–778. [Google Scholar] [CrossRef]
- Zeng, Q.; Cheng, J.S.; Tang, L.H.; Liu, X.F.; Liu, Y.Z.; Li, J.H.; Jiang, J.H. Self-assembled graphene–enzyme hierarchical nanostructures for electrochemical biosensing. Adv. Funct. Mater. 2010, 20, 3366–3372. [Google Scholar] [CrossRef]












© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Ye, L.; Li, Z.; Wang, Y.; Lei, F.; Lin, S. Facile Synthesis of Bimetallic Pt-Ag/Graphene Composite and Its Electro-Photo-Synergistic Catalytic Properties for Methanol Oxidation. Catalysts 2016, 6, 144. https://doi.org/10.3390/catal6090144
Xu S, Ye L, Li Z, Wang Y, Lei F, Lin S. Facile Synthesis of Bimetallic Pt-Ag/Graphene Composite and Its Electro-Photo-Synergistic Catalytic Properties for Methanol Oxidation. Catalysts. 2016; 6(9):144. https://doi.org/10.3390/catal6090144
Chicago/Turabian StyleXu, Shuhong, Lingting Ye, Zhongshui Li, Yanli Wang, Fengling Lei, and Shen Lin. 2016. "Facile Synthesis of Bimetallic Pt-Ag/Graphene Composite and Its Electro-Photo-Synergistic Catalytic Properties for Methanol Oxidation" Catalysts 6, no. 9: 144. https://doi.org/10.3390/catal6090144