Electronic Structure and Optical Properties of BiOI as a Photocatalyst Driven by Visible Light
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimized Structure
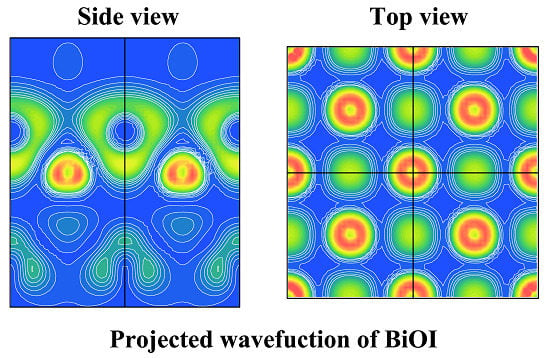
2.2. Electronic Structure
2.3. Optical Properties
3. Computational Methods
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Mills, A.; Le Hunte, S. An overview of semiconductor photocatalysis. J. Photochem. Photobiol. A 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Choi, W.; Termin, A.; Hoffmann, M.R. The role of metal ion dopants in quantum-sized TiO2: Correlation between photoreactivity and charge carrier recombination dynamics. J. Phys. Chem. 1994, 98, 13669–13679. [Google Scholar] [CrossRef]
- Yu, J.-G.; Jimmy, C.Y.; Cheng, B.; Hark, S.K.; Iu, K. The effect of F−-doping and temperature on the structural and textural evolution of mesoporous TiO2 powders. J. Solid State Chem. 2003, 174, 372–380. [Google Scholar] [CrossRef]
- Maeda, K.; Takata, T.; Hara, M.; Saito, N.; Inoue, Y.; Kobayashi, H.; Domen, K. GaN:ZnO solid solution as a photocatalyst for visible-light-driven overall water splitting. J. Am. Chem. Soc. 2005, 127, 8286–8287. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xie, R.; Shang, J.K.; Mintz, E.A. Effect of precursor ratio on synthesis and optical absorption of TiON photocatalytic nanoparticles. J. Am. Cera. Soc. 2007, 90, 1045–1050. [Google Scholar] [CrossRef]
- Lin, H.-P.; Chen, C.-C.; Lee, W.W.; Lai, Y.-Y.; Chen, J.-Y.; Chen, Y.-Q.; Fu, J.-Y. Synthesis of a SrFeO3−x/g-C3N4 heterojunction with improved visible-light photocatalytic activities in chloramphenicol and crystal violet degradation. RSC Adv. 2016, 6, 2323–2336. [Google Scholar] [CrossRef]
- Yang, C.-T.; Lee, W.W.; Lin, H.-P.; Dai, Y.-M.; Chi, H.-T.; Chen, C.-C. A novel heterojunction photocatalyst, Bi2SiO5/g-C3N4: Synthesis, characterization, photocatalytic activity, and mechanism. RSC Adv. 2016, 6, 40664–40675. [Google Scholar] [CrossRef]
- Feng, Y.; Li, L.; Li, J.; Wang, J.; Liu, L. Synthesis of mesoporous BiOBr 3D microspheres and their photodecomposition for toluene. J. hazard. Mater. 2011, 192, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Zhang, J.; Miao, M.; Jin, Y. Solvothermal synthesis of flower-like BiOBr microspheres with highly visible-light photocatalytic performances. App. Catal. B 2012, 111, 334–341. [Google Scholar] [CrossRef]
- Zhang, K.-L.; Liu, C.-M.; Huang, F.-Q.; Zheng, C.; Wang, W.-D. Study of the electronic structure and photocatalytic activity of the BiOCl photocatalyst. App. Catal. B 2006, 68, 125–129. [Google Scholar] [CrossRef]
- Shenawi-Khalil, S.; Uvarov, V.; Kritsman, Y.; Menes, E.; Popov, I.; Sasson, Y. A new family of BiO(ClxBr1−x) visible light sensitive photocatalysts. Catal. Commun. 2011, 12, 1136–1141. [Google Scholar] [CrossRef]
- Zhang, X.; Ai, Z.; Jia, F.; Zhang, L. Generalized one-pot synthesis, characterization, and photocatalytic activity of hierarchical BiOX (X = Cl, Br, I) nanoplate microspheres. J. Phys. Chem. C 2008, 112, 747–753. [Google Scholar] [CrossRef]
- Xiao, X.; Hao, R.; Liang, M.; Zuo, X.-X.; Nan, J.-M.; Li, L.-S.; Zhang, W.-D. One-pot solvothermal synthesis of three-dimensional (3D) BiOI/BiOCl composites with enhanced visible-light photocatalytic activities for the degradation of bisphenol-A. J. Hazard. Mater. 2012, 122–130, 233–234. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-R.; Chou, S.-Y.; Chang, J.-L.; Huang, S.-T.; Lin, H.-P.; Chen, C.-C. Hydrothermal synthesis of bismuth oxybromide-bismuth oxyiodide composites with high visible light photocatalytic performance for the degradation of CV and phenol. RSC Adv. 2015, 5, 30851–30860. [Google Scholar] [CrossRef]
- Chou, S.-Y.; Chen, C.-C.; Dai, Y.-M.; Lin, J.-H.; Lee, W.W. Novel synthesis of bismuth oxyiodide/graphitic carbon nitride nanocomposites with enhanced visible-light photocatalytic activity. RSC Adv. 2016, 6, 33478–33491. [Google Scholar] [CrossRef]
- Lin, H.-P.; Lee, W.W.; Huang, S.-T.; Chen, L.-W.; Yeh, T.-W.; Fu, J.-Y.; Chen, C.-C. Controlled hydrothermal synthesis of PbBiO2Br/BiOBr heterojunction with enhanced visible-driven-light photocatalytic activities. J. Mol. Catal. A: Chem. 2016, 417, 168–183. [Google Scholar] [CrossRef]
- Huang, S.-T.; Jiang, Y.-R.; Chou, S.-Y.; Dai, Y.-M.; Chen, C.-C. Synthesis, characterization, photocatalytic activity of visible-light-responsive photocatalysts BiOxCly/BiOmBrn by controlled hydrothermal method. J. Mol. Catal. A: Chem. 2014, 391, 105–120. [Google Scholar] [CrossRef]
- Jiang, Y.-R.; Lin, H.-P.; Chung, W.-H.; Dai, Y.-M.; Lin, W.-Y.; Chen, C.-C. Controlled hydrothermal synthesis of BiOxCly/BiOmIn composites exhibiting visible-light photocatalytic degradation of crystal violet. J. Hazard. Mater. 2015, 283, 787–805. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.W.; Lu, C.-S.; Chuang, C.-W.; Chen, Y.-J.; Fu, J.-Y.; Siao, C.-W.; Chen, C.-C. Synthesis of bismuth oxyiodides and their composites: Characterization, photocatalytic activity, and degradation mechanisms. RSC Adv. 2015, 5, 23450–23463. [Google Scholar] [CrossRef]
- Zhao, Z.-Y.; Dai, W.-W. Structural, Electronic, and Optical Properties of Eu-Doped BiOX (X = F, Cl, Br, I): A DFT+U Study. Inorg. Chem. 2014, 53, 13001–13011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, L. Electronic and Band Structure Tuning of Ternary Semiconductor Photocatalysts by Self Doping: The Case of BiOI. J. Phys. Chem. C 2010, 114, 18198–18206. [Google Scholar] [CrossRef]
- Min, Y.; Zhou, M.; Wen, L.-Y.; Zhao, H.-P.; Lei, Y. A highly efficient visible-light driven photocatalyst: Two dimensional square-like bismuth oxyiodine nanosheets. Dalton Trans. 2014, 43, 9549–9556. [Google Scholar]
- Pan, M.; Zhang, H.; Gao, G.; Liu, L.; Chen, W. Facet-Dependent Catalytic Activity of Nanosheet-Assembled Bismuth Oxyiodide Microspheres in Degradation of Bisphenol A. Environ. Sci. Technol. 2015, 49, 6240–6248. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cao, W.; Su, Y.; Wang, Y.; Wang, X. Synthesis, characterization and photocatalytic performance of novel visible-light-induced Ag/BiOI. Appl. Catal. B 2012, 111–112, 71–279. [Google Scholar] [CrossRef]
- Reddy, K.H.; Martha, S.; Parida, K.M. Fabrication of Novel p-BiOI/n-ZnTiO3 Heterojunction for Degradation of Rhodamine 6G under Visible Light Irradiation. Inorg. Chem. 2013, 52, 6390–6401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, L.; Xie, T.; Wang, D. Low-Temperature Synthesis and High Visible-Light-Induced Photocatalytic Activity of BiOI/TiO2 Heterostructures. J. Phys. Chem. C 2009, 113, 7371–7378. [Google Scholar] [CrossRef]
- Wang, W.; Huang, F.; Lin, X.; Yang, J. Visible-light-responsive photocatalysts xBiOBr–(1−x) BiOI. Catal. Commun. 2008, 9, 8–12. [Google Scholar] [CrossRef]
- Chen, L.; Huang, R.; Xiong, M.; Yuan, Q.; He, J.; Jia, J.; Yao, M.-Y.; Luo, S.-L.; Au, C.-T.; Yin, S.-F. Room-Temperature Synthesis of Flower-Like BiOX (X = Cl, Br, I) Hierarchical Structures and their Visible-Light Photocatalytic Activity. Inorg. Chem. 2013, 52, 11118–11125. [Google Scholar] [CrossRef] [PubMed]
- Keller, E.; Krämer, V. A Strong Deviation from Vegard's Rule: X-Ray Powder Investigations of the Three Quasi-Binary Phase Systems BiOX-BiOY (X, Y = Cl, Br, I). Z. Naturforsch. B 2005, 60, 1255–1263. [Google Scholar]
- Sato, J.; Kobayashi, H.; Inoue, Y. Photocatalytic activity for water decomposition of indates with octahedrally coordinated d10 configuration. II. Roles of geometric and electronic structures. J. Phys. Chem. B 2003, 107, 7970–7975. [Google Scholar] [CrossRef]
- Huang, W.L. Electronic structures and optical properties of BiOX (X = F, Cl, Br, I) via DFT calculations. J. Comput. Chem. 2009, 30, 1882–1891. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, L.; Zhou, Z. Towards better photocatalysts: First-principles studies of the alloying effects on the photocatalytic activities of bismuth oxyhalides under visible light. Phys. Chem. Chem. Phys. 2012, 14, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Henle, J.; Simon, P.; Frenzel, A.; Scholz, S.; Kaskel, S. Nanosized BiOX (X= Cl, Br, I) particles synthesized in reverse microemulsions. Chem. Mater. 2007, 19, 366–373. [Google Scholar] [CrossRef]
- Wang, W.; Huang, F.; Lin, X. xBiOI–(1 − x) BiOCl as efficient visible-light-driven photocatalysts. Scr. Mater. 2007, 56, 669–672. [Google Scholar] [CrossRef]
- Perdew, J.P.; Levy, M. Physical content of the exact Kohn-Sham orbital energies: Band gaps and derivative discontinuities. Phys. Rev. Lett. 1983, 51, 1884–1887. [Google Scholar] [CrossRef]
- Stampfl, C.; Van de Walle, C. Density-functional calculations for III-V nitrides using the local-density approximation and the generalized gradient approximation. Phys. Rev. B 1999, 59. [Google Scholar] [CrossRef]
- Huang, W.L.; Zhu, Q. DFT calculations on the electronic structures of BiOX (X = F, Cl, Br, I) photocatalysts with and without semicore Bi 5d states. J. Comput. Chem. 2009, 30, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Prasad, K.; Sanjines, R.; Schmid, P.E.; Levy, F. Electrical and optical properties of TiO2 anatase thin films. J. Appl. Phys. 1994, 75, 2042–2047. [Google Scholar] [CrossRef]
- Pascual, J.; Camassel, J.; Mathieu, H. Resolved Quadrupolar Transition in TiO2. Phys. Rev. Lett. 1977, 39, 1490–1493. [Google Scholar] [CrossRef]
- Frederikse, H.P.R. Recent Studies on Rutile (TiO2). J. Appl. Phys. 1961, 32, 2211–2215. [Google Scholar] [CrossRef]
- Walsh, A.; Da Silva, J.L.F.; Wei, S.-H. Origins of band-gap renormalization in degenerately doped semiconductors. Phys. Rev. B 2008, 78, 075211. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, K.; Zhang, L. Visible Light Photocatalysis of BiOI and its Photocatalytic Activity Enhancement by in Situ Ionic Liquid Modification. J. Phys. Chem. C 2011, 115, 14300–14308. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, X.; Sun, P.; Zhang, L. ZnO/BiOI Heterostructures: Photoinduced Charge-Transfer Property and Enhanced Visible-Light Photocatalytic Activity. J. Phys. Chem. C 2011, 115, 20555–20564. [Google Scholar] [CrossRef]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Kristallog. 2005, 220, 567–570. [Google Scholar] [CrossRef] [Green Version]
- Pfrommer, B.G.; Côté, M.; Louie, S.G.; Cohen, M.L. Relaxation of crystals with the quasi-Newton method. J. Comput. Phys. 1997, 131, 233–240. [Google Scholar] [CrossRef]
- Tkatchenko, A.; Scheffler, M. Accurate Molecular Van Der Waals Interactions from Ground-State Electron Density and Free-Atom Reference Data. Phys. Rev. Lett. 2009, 102. [Google Scholar] [CrossRef] [PubMed]









| Method | Crystallographic Parameters | Fractional Coordinates of Atoms | Bond Lengths (Å) | |||||
|---|---|---|---|---|---|---|---|---|
| a and b (Å) | c (Å) | V (Å 3) | O | I | Bi | Bi–O | Bi–I a | |
| GGA | 3.9900 | 9.4984 | 151.2155 | (0, 0, 0) | (0, 0.5, 0.6760) | (0, 0.5, 0.1294) | 2.3431 | 3.3370, 5.1920 |
| GGA + U | 3.9199 | 9.3099 | 143.0519 | (0, 0, 0) | (0, 0.5, 0.6761) | (0, 0.5, 0.1291) | 2.2990 | 3.3130, 5.0930 |
| HSE06 | 3.7556 | 7.8692 | 110.9911 | (0, 0, 0) | (0, 0.5, 0.6466) | (0, 0.5, 0.1440) | 2.1931 | 3.1260, 3.9550 |
| Exp.b | 3.885 (2) | 9.151 (5) | 138.12 (2) | (0, 0, 0) | (0, 0.5, 0.6671) | (0, 0.5, 0.1338) | 2.3430 | 3.3615, 4.8795 |
| Method | O | I | Bi | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| s | p | Total | Charge | s | p | Total | Charge | s | p | Total | Charge | |
| GGA | 1.91 | 4.98 | 6.90 | −0.90 | 1.96 | 5.37 | 7.33 | −0.33 | 1.97 | 1.80 | 3.78 | 1.22 |
| GGA + U | 1.88 | 5.08 | 6.97 | −0.97 | 1.98 | 5.37 | 7.35 | −0.35 | 1.93 | 1.75 | 3.68 | 1.32 |
| HSE06 | 1.89 | 5.13 | 7.02 | −1.02 | 1.81 | 5.39 | 7.20 | −0.20 | 2.11 | 1.67 | 13.78 | 1.22 |
| k-point (direction) | mh* (m0) | k-point (direction) | me* (m0) | ||||
|---|---|---|---|---|---|---|---|
| GGA | GGA + U | HSE06 | GGA | GGA + U | HSE06 | ||
| Z (Z → A) | 0.795 | 0.809 | 0.658 | Z (Z → A) | 0.204 | 0.194 | 0.217 |
| (Z → A) | 0.454 | 0.675 | 0.464 | (A → Z) | 0.318 | 0.319 | 0.270 |
| (Γ → M) | 0.254 | 0.235 | 0.187 | M (Γ → M) | 0.460 | 0.393 | 0.204 |
| ZR (Z → R) | 1.158 | 0.977 | 0.663 | (Γ → M) | 0.768 | 0.674 | 0.430 |
| - | - | - | - | (Z → R) | 0.424 | 0.421 | 0.277 |
| - | - | - | - | Γ (Γ → X) | 0.185 | 0.188 | 0.162 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, W.-W.; Zhao, Z.-Y. Electronic Structure and Optical Properties of BiOI as a Photocatalyst Driven by Visible Light. Catalysts 2016, 6, 133. https://doi.org/10.3390/catal6090133
Dai W-W, Zhao Z-Y. Electronic Structure and Optical Properties of BiOI as a Photocatalyst Driven by Visible Light. Catalysts. 2016; 6(9):133. https://doi.org/10.3390/catal6090133
Chicago/Turabian StyleDai, Wen-Wu, and Zong-Yan Zhao. 2016. "Electronic Structure and Optical Properties of BiOI as a Photocatalyst Driven by Visible Light" Catalysts 6, no. 9: 133. https://doi.org/10.3390/catal6090133