High Yield Synthesis of Hydroxyapatite (HAP) and Palladium Doped HAP via a Wet Chemical Synthetic Route
Abstract
:1. Introduction
2. Results and Discussion
2.1. Hydroxyapatite (HAP) Synthesis
2.2. Palladium Doped HAP
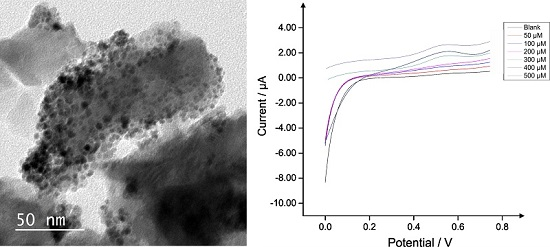
2.3. Application of Pd0 Doped HAP
3. Experimental Procedures
3.1. Materials and Chemicals
3.2. Synthesis of HAP
3.3. Synthesis of Pd0 Doped HAP
3.4. Electrochemical Measurements
3.5. Characterisation of the HAP and Pd0 Doped HAP
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Feng, Y.; Yin, H.; Gao, D.; Wang, A.; Shen, L.; Meng, M. Selective oxidation of 1,2-propanediol to lactic acid catalyzed by hydroxylapatite nanorod-supported au/pd bimetallic nanoparticles under atmospheric pressure. J. Catal. 2014, 316, 67–77. [Google Scholar] [CrossRef]
- Bellucci, D.; Sola, A.; Gazzarri, M.; Chiellini, F.; Cannillo, V. A new hydroxyapatite-based biocomposite for bone replacement. Mater. Sci. Eng. C 2013, 33, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-C.; Tsou, H.-K.; Hsu, H.-C.; Hsu, S.-K.; Liou, S.-P.; Ho, W.-F. A hydrothermal synthesis of eggshell and fruit waste extract to produce nanosized hydroxyapatite. Ceram. Int. 2013, 39, 8183–8188. [Google Scholar] [CrossRef]
- Loca, D.; Locs, J.; Dubnika, A.; Zalite, V.; Berzina-Cimdina, L. 9-porous hydroxyapatite for drug delivery. In Hydroxyapatite (hap) for biomedical applications; Mucalo, M., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 189–209. [Google Scholar]
- Kongsri, S.; Janpradit, K.; Buapa, K.; Techawongstien, S.; Chanthai, S. Nanocrystalline hydroxyapatite from fish scale waste: Preparation, characterization and application for selenium adsorption in aqueous solution. Chem. Eng. J. 2013, 215–216, 522–532. [Google Scholar] [CrossRef]
- Vila, M.; Sánchez-Salcedo, S.; Vallet-Regí, M. Hydroxyapatite foams for the immobilization of heavy metals: From waters to the human body. Inorg. Chim. Acta 2012, 393, 24–35. [Google Scholar] [CrossRef]
- Gupta, N.; Kushwaha, A.K.; Chattopadhyaya, M.C. Adsorptive removal of Pb2+, Co2+ and Ni2+ by hydroxyapatite/chitosan composite from aqueous solution. J. Taiwan Inst. Chem. Eng. 2012, 43, 125–131. [Google Scholar] [CrossRef]
- Salah, T.A.; Mohammad, A.M.; Hassan, M.A.; El-Anadouli, B.E. Development of nano-hydroxyapatite/chitosan composite for cadmium ions removal in wastewater treatment. J. Taiwan Inst. Chem. Eng. 2014, 45, 1571–1577. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Ji, X.; Banks, C.E.; Zhang, W. Conversion of natural egg-shell to 3d flower-like hydroxyapatite agglomerates for highly sensitive detection of As3+ ions. Mater. Lett. 2012, 78, 120–123. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Y.; Zhang, Y.; Li, H.; Tan, Y.; Luo, L.; Duan, J.; Li, K.; Banks, C.E. Facile and controllable synthesis of hydroxyapatite/graphene hybrid materials with enhanced sensing performance towards ammonia. Analyst 2015, 140, 5235–5242. [Google Scholar] [CrossRef] [PubMed]
- Kanchana, P.; Sekar, C. Edta assisted synthesis of hydroxyapatite nanoparticles for electrochemical sensing of uric acid. Mater. Sci. Eng. C 2014, 42, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Roy, S.K.; Kundu, B.; Datta, S.; Basu, D. Synthesis of nano-sized hydroxyapatite powders through solution combustion route under different reaction conditions. Mater. Sci. Eng. B 2011, 176, 14–21. [Google Scholar] [CrossRef]
- Padmanabhan, S.K.; Balakrishnan, A.; Chu, M.C.; Lee, Y.J.; Kim, T.N.; Cho, S.J. Sol–gel synthesis and characterization of hydroxyapatite nanorods. Particuology 2009, 7, 466–470. [Google Scholar] [CrossRef]
- Mori, K.; Hara, T.; Mizugaki, T.; Ebitani, K.; Kaneda, K. Hydroxyapatite-supported palladium nanoclusters: A highly active heterogeneous catalyst for selective oxidation of alcohols by use of molecular oxygen. J. Am. Chem. Soc. 2004, 126, 10657–10666. [Google Scholar] [CrossRef] [PubMed]
- Indra, A.; Gopinath, C.S.; Bhaduri, S.; Kumar Lahiri, G. Hydroxyapatite supported palladium catalysts for suzuki-miyaura cross-coupling reaction in aqueous medium. Catal. Sci. Technol. 2013, 3, 1625–1633. [Google Scholar] [CrossRef]
- Özhava, D.; Özkar, S. Rhodium(0) nanoparticles supported on hydroxyapatite nanospheres and further stabilized by dihydrogen phosphate ion: A highly active catalyst in hydrogen generation from the methanolysis of ammonia borane. Int. J. Hydrogen Energy 2015, 40, 10491–10501. [Google Scholar] [CrossRef]
- Andrasekhar, A.; Sagadevan, S.; Dakshnamoorthy, A. Synthesis and characterisation of nano-hydroxyapatite (n-hap) using the wet chemical technique. Int. J. Phys. Sci. 2013, 8, 1639–1645. [Google Scholar]
- Kamalanathan, P.; Ramesh, S.; Bang, L.T.; Niakan, A.; Tan, C.Y.; Purbolaksono, J.; Chandran, H.; Teng, W.D. Synthesis and sintering of hydroxyapatite derived from eggshells as a calcium precursor. Ceram. Int. 2014, 40, 16349–16359. [Google Scholar] [CrossRef]
- Wang, P.; Li, C.; Gong, H.; Jiang, X.; Wang, H.; Li, K. Effects of synthesis conditions on the morphology of hydroxyapatite nanoparticles produced by wet chemical process. Powder Technol. 2010, 203, 315–321. [Google Scholar] [CrossRef]
- Bahrololoom, M.E.; Javidi, M.; Javadpour, S.; Ma, J. Characterisation of natural hydroxyapatite extracted from bovine cortical bone ash. J. Ceram. Process. Res. 2009, 10, 129–138. [Google Scholar]
- Liu, J.; Li, K.; Wang, H.; Zhu, M.; Yan, H. Rapid formation of hydroxyapatite nanostructures by microwave irradiation. Chem. Phys. Lett. 2004, 396, 429–432. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, X.; Ma, X.; Su, W.; Zhang, Y.; Sun, X.; Li, X. Alginate-intervened hydrothermal synthesis of hydroxyapatite nanocrystals with nanopores. Cryst. Growth Des. 2015, 15, 1949–1956. [Google Scholar] [CrossRef]
- Ji, X.; Su, P.; Liu, C.; Song, J.; Liu, C.; Li, J.; Tan, H.; Wu, F.; Yang, L.; Fu, R.; et al. A novel ethanol induced and stabilized hierarchical nanorods: Hydroxyapatite nanopeanut. J. Am. Ceram. Soc. 2015, 98, 1702–1705. [Google Scholar] [CrossRef]
- Kavitha, M.; Subramanian, R.; Narayanan, R.; Udhayabanu, V. Solution combustion synthesis and characterization of strontium substituted hydroxyapatite nanocrystals. Powder Technol. 2014, 253, 129–137. [Google Scholar] [CrossRef]
- Dhand, V.; Rhee, K.Y.; Park, S.J. The facile and low temperature synthesis of nanophase hydroxyapatite crystals using wet chemistry. Mater. Sci. Eng. C 2014, 36, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ning, X.; Bai, Y.; Jia, W. A scalable synthesis of non-agglomerated and low-aspect ratio hydroxyapatite nanocrystals using gelatinized starch matrix. Mater. Lett. 2013, 113, 142–145. [Google Scholar] [CrossRef]
- Öner, M.; Uysal, U. Synthesis of hydroxyapatite crystals using carboxymethyl inulin for use as a delivery of ibuprofen. Mater. Sci. Eng. C 2013, 33, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Kothapalli, C.; Wei, M.; Vasiliev, A.; Shaw, M.T. Influence of temperature and concentration on the sintering behavior and mechanical properties of hydroxyapatite. Acta Mater. 2004, 52, 5655–5663. [Google Scholar] [CrossRef]
- Ramesh, S.; Tan, C.Y.; Tolouei, R.; Amiriyan, M.; Purbolaksono, J.; Sopyan, I.; Teng, W.D. Sintering behavior of hydroxyapatite prepared from different routes. Mater. Des. 2012, 34, 148–154. [Google Scholar] [CrossRef]
- Landi, E.; Celotti, G.; Logroscino, G.; Tampieri, A. Carbonated hydroxyapatite as bone substitute. J. Eur. Ceram. Soc. 2003, 23, 2931–2937. [Google Scholar] [CrossRef]
- Okada, M.; Furuzono, T. Low-temperature synthesis of nanoparticle-assembled, transparent, and low-crystallized hydroxyapatite blocks. J. Colloid Interface Sci. 2011, 360, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Abert, J.; Bergmann, C.; Fischer, H. Wet chemical synthesis of strontium-substituted hydroxyapatite and its influence on the mechanical and biological properties. Ceram. Int. 2014, 40, 9195–9203. [Google Scholar] [CrossRef]
- Yamini, D.; Devanand Venkatasubbu, G.; Kumar, J.; Ramakrishnan, V. Raman scattering studies on peg functionalized hydroxyapatite nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 117, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.P.; Franca, A.S.; Irudayaraj, J. 7-vibrational spectroscopy for food quality and safety screening. In High throughput Screening for Food Safety Assessment; Bhunia, A.K., Kim, M.S., Taitt, C.R., Eds.; Woodhead Publishing: Cambridge, UK, 2015; pp. 165–194. [Google Scholar]
- Mohandes, F.; Salavati-Niasari, M.; Fathi, M.; Fereshteh, Z. Hydroxyapatite nanocrystals: Simple preparation, characterization and formation mechanism. Mater. Sci. Eng. C 2014, 45, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, S.R.; Venkatesh, R. A study of hydroxyapatite fibers prepared via sol–gel route. Mater. Lett. 2004, 58, 3320–3323. [Google Scholar] [CrossRef]
- Sargin, Y.; Kizilyalli, M.; Telli, C.; Güler, H. A new method for the solid-state synthesis of tetracalcium phosphate, a dental cement: X-ray powder diffraction and ir studies. J. Eur. Ceram. Soc. 1997, 17, 963–970. [Google Scholar] [CrossRef]
- Javadi, A.; Shockravi, A.; Koohgard, M.; Malek, A.; Shourkaei, F.A.; Ando, S. Nitro-substituted polyamides: A new class of transparent and highly refractive materials. Eur. Polym. J. 2015, 66, 328–341. [Google Scholar] [CrossRef]
- Arul, K.T.; Ramya, J.R.; Bhalerao, G.M.; Kalkura, S.N. Physicochemical characterization of the superhydrophilic, magnesium and silver ions co-incorporated nanocrystalline hydroxyapatite, synthesized by microwave processing. Ceram. Int. 2014, 40, 13771–13779. [Google Scholar] [CrossRef]
- Nazir, R.; Iqbal, N.; Khan, A.S.; Akram, A.; Asif, A.; Chaudhry, A.A.; Rehman, I.U.; Hussain, R. Rapid synthesis of thermally stable hydroxyapaptite. Ceram. Int. 2012, 38, 457–462. [Google Scholar] [CrossRef]
- Gutowska, I.; Machoy, Z.; Machalinski, B. The role of bivalent metals in hydroxyapatite structures as revealed by molecular modeling with the hyperchem software. J. Biomed. Mater. Res. A 2005, 75, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.A.; Tai, N.H.; Chen, S.K.; Tsai, T.Y. Enhancing the electrical conductivity of carbon-nanotube-based transparent conductive films using functionalized few-walled carbon nanotubes decorated with palladium nanoparticles as fillers. ACS Nano 2011, 5, 6500–6506. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Chao, S.; Bai, Z.; Yan, H.; Wang, K.; Yang, L. Based on a new support for synthesis of highly efficient palladium/hydroxyapatite catalyst for ethanol electrooxidation. Electrochim. Acta 2014, 132, 31–36. [Google Scholar] [CrossRef]
- Tang, Y.Y.; Kao, C.L.; Chen, P.Y. Electrochemical detection of hydrazine using a highly sensitive nanoporous gold electrode. Anal. Chim. Acta 2012, 711, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Zelnick, S.D.; Mattie, D.R.; Stepaniak, P.C. Occupational exposure to hydrazines: Treatment of acute central nervous system toxicity. Aviat. Space Environ. Med. 2003, 74, 1285–1291. [Google Scholar] [PubMed]
- Choudhary, G.; Hansen, H. Human health perspective of environmental exposure to hydrazines: A review. Chemosphere 1998, 37, 801–843. [Google Scholar] [CrossRef]
- Panchompoo, J.; Aldous, L.; Downing, C.; Crossley, A.; Compton, R.G. Facile synthesis of pd nanoparticle modified carbon black for electroanalysis: Application to the detection of hydrazine. Electroanalysis 2011, 23, 1568–1578. [Google Scholar] [CrossRef]
- Rakap, M.; Özkar, S. Hydroxyapatite-supported cobalt(0) nanoclusters as efficient and cost-effective catalyst for hydrogen generation from the hydrolysis of both sodium borohydride and ammonia-borane. Catal. Today 2012, 183, 17–25. [Google Scholar] [CrossRef]
- Chen, X.; Liu, W.; Tang, L.; Wang, J.; Pan, H.; Du, M. Electrochemical sensor for detection of hydrazine based on au@pd core–shell nanoparticles supported on amino-functionalized tio2 nanotubes. Mater. Sci. Eng. C 2014, 34, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhu, M.; Zheng, M.; Tang, Y.; Chen, Y.; Lu, T. Electrocatalytic oxidation and detection of hydrazine at carbon nanotube-supported palladium nanoparticles in strong acidic solution conditions. Electrochim. Acta 2011, 56, 4930–4936. [Google Scholar] [CrossRef]
- Ivanov, S.; Lange, U.; Tsakova, V.; Mirsky, V.M. Electrocatalytically active nanocomposite from palladium nanoparticles and polyaniline: Oxidation of hydrazine. Sens. Actuators B Chem. 2010, 150, 271–278. [Google Scholar] [CrossRef]
- Ji, X.; Banks, C.E.; Holloway, A.F.; Jurkschat, K.; Thorogood, C.A.; Wildgoose, G.G.; Compton, R.G. Palladium sub-nanoparticle decorated ‘bamboo’ multi-walled carbon nanotubes exhibit electrochemical metastability: Voltammetric sensing in otherwise inaccessible ph ranges. Electroanalysis 2006, 18, 2481–2485. [Google Scholar] [CrossRef]
- Batchelor-McAuley, C.; Banks, C.E.; Simm, A.O.; Jones, T.G.J.; Compton, R.G. The electroanalytical detection of hydrazine: A comparison of the use of palladium nanoparticles supported on boron-doped diamond and palladium plated bdd microdisc array. Analyst 2006, 131, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.; Šljukić, B.; Salter, C.; Crossley, A.; Compton, R.G. Development of an electrochemical sensor nanoarray for hydrazine detection using a combinatorial approach. Electroanalysis 2007, 19, 1062–1068. [Google Scholar] [CrossRef]
- Ejaz, A.; Ahmed, M.S.; Jeon, S. Highly efficient benzylamine functionalized graphene supported palladium for electrocatalytic hydrazine determination. Sens. Actuators B Chem. 2015, 221, 1256–1263. [Google Scholar] [CrossRef]
- Rastogi, P.K.; Ganesan, V.; Krishnamoorthi, S. Palladium nanoparticles decorated gaur gum based hybrid material for electrocatalytic hydrazine determination. Electrochim. Acta 2014, 125, 593–600. [Google Scholar] [CrossRef]
- Wan, Q.; Liu, Y.; Wang, Z.; Wei, W.; Li, B.; Zou, J.; Yang, N. Graphene nanoplatelets supported metal nanoparticles for electrochemical oxidation of hydrazine. Electrochem. Commun. 2013, 29, 29–32. [Google Scholar] [CrossRef]
- Ahmar, H.; Keshipour, S.; Hosseini, H.; Fakhari, A.R.; Shaabani, A.; Bagheri, A. Electrocatalytic oxidation of hydrazine at glassy carbon electrode modified with ethylenediamine cellulose immobilized palladium nanoparticles. J. Electroanal. Chem. 2013, 690, 96–103. [Google Scholar] [CrossRef]
- Gauthard, F.; Epron, F.; Barbier, J. Palladium and platinum-based catalysts in the catalytic reduction of nitrate in water: Effect of copper, silver, or gold addition. J. Catal. 2003, 220, 182–191. [Google Scholar] [CrossRef]
- Rakap, M.; Özkar, S. Hydroxyapatite-supported palladium(0) nanoclusters as effective and reusable catalyst for hydrogen generation from the hydrolysis of ammonia-borane. Int. J. Hydrogen Energy 2011, 36, 7019–7027. [Google Scholar] [CrossRef]
- Galdino, F.E.; Foster, C.W.; Bonacin, J.A.; Banks, C.E. Exploring the electrical wiring of screen-printed configurations utilised in electroanalysis. Anal. Methods 2015, 7, 1208–1214. [Google Scholar] [CrossRef]






| Solvent Used to Maintain pH during the Synthesis | Yield % | Comments | Time | Temperature of Synthesis/°C | References |
|---|---|---|---|---|---|
| Tris hydroxyl methyl amino methane | 27–36 | - | Not stated | 300–500 | [24] |
| Ammonia | Not stated | Yield stated as high, however no specific value mentioned | ~30 h | 37 | [25] |
| Ammonia | >75 | Starch used to prevent agglomeration of nanoparticles | ~40 h | 85 | [26] |
| Potassium hydroxide | Not stated | Yield stated as high, however no specific value mentioned | ~30 h | 70 | [27] |
| Ammonia | Not stated | Yield not stated | ~8 days | 25–100 | [28] |
| Orthophosphoric acid | Not stated | Yield not stated | ~30 h | 18–22 | [29] |
| Ammonia and deionised water | Not stated | Yield not stated | >70 h | 18–22 | [13] |
| Ammonia | Not stated | Yield not stated | ~50 h | 95 | [30] |
| Ammonia | Not stated | Yield not stated | ~40 h | 18–22 | [31] |
| Phosphoric acid | Not stated | Yield not stated | ~80 h | 18–22 | [32] |
| Sodium hydroxide | 84 | - | <24 h | 18–22 | This work |
| Metal | Pd Loading/wt % | Support | Electrode | Electrochemical Method | Linear Range/µM | LOD (3σ)/µM | References |
|---|---|---|---|---|---|---|---|
| Au/Pd | Not stated | TiO2 nanotubes | GCE | CV | 0.06–700 | 12 | [49] |
| Pd | 20.6 | Carbon nanotubes | GCE | CV | 2.5–700 | 1 | [50] |
| Pd | 100 | PANI | GCE | CV | 10–300 | 0.5 | [51] |
| Pd | 0.6 | Carbon black | GCE | CV | 5–50 | 8.8 | [47] |
| Pd | 100 | Not stated | MWCNT | LSV | 56–157 | 10 | [52] |
| Pd | 100 | Not stated | BDD | LSV | 27.2–85 | 2.6 | [53] |
| Pd/Au/Ag | Not stated | Glassy Carbon Microspheres | BPPG | CV | 0–300 | 4 | [54] |
| Pd | Not stated | Graphene | GCE | CV | 1–740 | 17 | [55] |
| Pd | Not stated | Guar gum | GCE | CV | 50–600 | 4.1 | [56] |
| Au/Pd | Not stated | Graphene nanoplatelets | GCE | CV | Not stated | Not stated | [57] |
| Pd | 7.3 | EDAC | GCE | CV | 5–150 | 1.5 | [58] |
| Pd | 4 | HAP | SPE | LSV | 50–400 | 30 | This work |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamieniak, J.; Bernalte, E.; Foster, C.W.; Doyle, A.M.; Kelly, P.J.; Banks, C.E. High Yield Synthesis of Hydroxyapatite (HAP) and Palladium Doped HAP via a Wet Chemical Synthetic Route. Catalysts 2016, 6, 119. https://doi.org/10.3390/catal6080119
Kamieniak J, Bernalte E, Foster CW, Doyle AM, Kelly PJ, Banks CE. High Yield Synthesis of Hydroxyapatite (HAP) and Palladium Doped HAP via a Wet Chemical Synthetic Route. Catalysts. 2016; 6(8):119. https://doi.org/10.3390/catal6080119
Chicago/Turabian StyleKamieniak, Joanna, Elena Bernalte, Christopher W. Foster, Aidan M. Doyle, Peter J. Kelly, and Craig E. Banks. 2016. "High Yield Synthesis of Hydroxyapatite (HAP) and Palladium Doped HAP via a Wet Chemical Synthetic Route" Catalysts 6, no. 8: 119. https://doi.org/10.3390/catal6080119