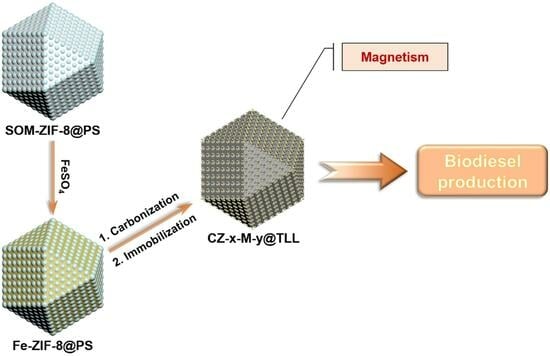
Preparation of Ordered Macroporous ZIF-8-Derived Magnetic Carbon Materials and Its Application for Lipase Immobilization
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of CZ-x-M-y and CZ-x
2.2. Immobilization Properties of CZ-x-M-y@TLL and CZ-x@TLL
2.3. The Methanolysis Reactions of Soybean Oil Catalyzed by CZ-x-M-y@TLL and CZ-x@TLL
3. Materials and Methods
3.1. Materials
3.2. Preparation of CZ-x-M-y and CZ-x
3.3. Immobilization of Lipase
3.4. Measurement of Enzyme Activity
3.5. Lipase-Catalyzed Methanolysis of Soybean Oil
3.6. Reusability of the Immobilized Lipases
3.7. Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Monteiro, R.R.; Arana-Peña, S.; da Rocha, T.N.; Miranda, L.P.; Berenguer-Murcia, Á.; Tardioli, P.W.; dos Santos, J.C.; Fernandez-Lafuente, R. Liquid lipase preparations designed for industrial production of biodiesel. Is it really an optimal solution? Renew. Energy 2021, 164, 1566–1587. [Google Scholar] [CrossRef]
- Basso, A.; Serban, S. Industrial applications of immobilized enzymes—A review. Mol. Catal. 2019, 479, 110607. [Google Scholar] [CrossRef]
- Chandra, P.; Enespa; Singh, R.; Arora, P.K. Microbial lipases and their industrial applications: A comprehensive review. Microb. Cell Fact. 2020, 19, 169. [Google Scholar] [CrossRef] [PubMed]
- Gotor-Fernández, V.; Brieva, R.; Gotor, V. Lipases: Useful biocatalysts for the preparation of pharmaceuticals. J. Mol. Catal. B-Enzym. 2006, 40, 111–120. [Google Scholar] [CrossRef]
- Sarmah, N.; Revathi, D.; Sheelu, G.; Yamuna Rani, K.; Sridhar, S.; Mehtab, V.; Sumana, C. Recent advances on sources and industrial applications of lipases. Biotechnol. Prog. 2018, 34, 5–28. [Google Scholar] [CrossRef]
- Cavalcante, F.T.T.; Neto, F.S.; Rafael de Aguiar Falcão, I.; Da Erick Silva Souza, J.; de Moura Junior, L.S.; da Silva Sousa, P.; Rocha, T.G.; de Sousa, I.G.; de Lima Gomes, P.H.; de Souza, M.C.M.; et al. Opportunities for improving biodiesel production via lipase catalysis. Fuel 2021, 288, 119577. [Google Scholar] [CrossRef]
- Guldhe, A.; Singh, B.; Mutanda, T.; Permaul, K.; Bux, F. Advances in synthesis of biodiesel via enzyme catalysis: Novel and sustainable approaches. Renew. Sustain. Energy Rev. 2015, 41, 1447–1464. [Google Scholar] [CrossRef]
- Ortiz, C.; Ferreira, M.L.; Barbosa, O.; dos Santos, J.C.S.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Briand, L.E.; Fernandez-Lafuente, R. Novozym 435: The “perfect” lipase immobilized biocatalyst? Catal. Sci. Technol. 2019, 9, 2380–2420. [Google Scholar] [CrossRef]
- Remonatto, D.; Miotti, R.H., Jr.; Monti, R.; Bassan, J.C.; de Paula, A.V. Applications of immobilized lipases in enzymatic reactors: A review. Process Biochem. 2022, 114, 1–20. [Google Scholar] [CrossRef]
- Lian, X.; Fang, Y.; Joseph, E.; Wang, Q.; Li, J.; Banerjee, S.; Lollar, C.; Wang, X.; Zhou, H.-C. Enzyme-MOF (metal-organic framework) composites. Chem. Soc. Rev. 2017, 46, 3386–3401. [Google Scholar] [CrossRef]
- Liang, W.; Wied, P.; Carraro, F.; Sumby, C.J.; Nidetzky, B.; Tsung, C.-K.; Falcaro, P.; Doonan, C.J. Metal-Organic Framework-Based Enzyme Biocomposites. Chem. Rev. 2021, 121, 1077–1129. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zou, Z.Q.; Dai, L.M.; Liu, D.H.; Du, W. Ordered Macro-Microporous ZIF-8 with Different Macropore Sizes and Their Stable Derivatives for Lipase Immobilization in Biodiesel Production. ACS Sustain. Chem. Eng. 2022, 10, 14503–14514. [Google Scholar] [CrossRef]
- Wang, D.; Jana, D.; Zhao, Y. Metal-Organic Framework Derived Nanozymes in Biomedicine. Acc. Chem. Res. 2020, 53, 1389–1400. [Google Scholar] [CrossRef]
- Song, B.; Ren, D.; Wang, Z.; Huang, Y.; Zhang, X.; Zhang, S.; Gong, X.; Chen, W. Laccase immobilization on bimetallic MOF-derived porous carbon materials for the removal of bisphenol A. J. Chem. Technol. Biotechnol. 2023, 98, 919–931. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rathod, V.K. Magnetic-metal organic framework (magnetic-MOF): A novel platform for enzyme immobilization and nanozyme applications. Int. J. Biol. Macromol. 2018, 120, 2293–2302. [Google Scholar] [CrossRef]
- Gao, X.; Zhai, Q.; Hu, M.; Li, S.; Jiang, Y. Hierarchically porous magnetic Fe3O4/Fe-MOF used as an effective platform for enzyme immobilization: A kinetic and thermodynamic study of structure–activity. Catal. Sci. Technol. 2021, 11, 2446–2455. [Google Scholar] [CrossRef]
- Ji, Y.; Wu, Z.Z.; Zhang, P.; Qiao, M.; Hu, Y.; Shen, B.X.; Li, B.Z.; Zhang, X. Enzyme-functionalized magnetic framework composite fabricated by one-pot encapsulation of lipase and Fe3O4 nanoparticle into metal-organic framework. Biochem. Eng. J. 2021, 169, 107962. [Google Scholar] [CrossRef]
- Li, Q.; Pan, Y.; Li, H.; Alhalhooly, L.; Li, Y.; Chen, B.; Choi, Y.; Yang, Z. Size-Tunable Metal-Organic Framework-Coated Magnetic Nanoparticles for Enzyme Encapsulation and Large-Substrate Biocatalysis. ACS Appl. Mater. Interfaces 2020, 12, 41794–41801. [Google Scholar] [CrossRef]
- Suo, H.; Geng, H.; Zhang, L.; Liu, G.; Yan, H.; Cao, R.; Zhu, J.; Hu, Y.; Xu, L. Covalent immobilization of lipase on an ionic liquid-functionalized magnetic Cu-based metal-organic framework with boosted catalytic performance in flavor ester synthesis. J. Mat. Chem. B 2023, 11, 1302–1311. [Google Scholar] [CrossRef]
- Fu, C.-W.; Lirio, S.; Shih, Y.-H.; Liu, W.-L.; Lin, C.-H.; Huang, H.-Y. The Cooperativity of Fe3O4 and Metal-Organic Framework as Multifunctional Nanocomposites for Laser Desorption Ionization Process. Chemistry 2018, 24, 9598–9605. [Google Scholar] [CrossRef]
- Ke, F.; Qiu, L.-G.; Yuan, Y.-P.; Jiang, X.; Zhu, J.-F. Fe3O4@MOF core–shell magnetic microspheres with a designable metal–organic framework shell. J. Mater. Chem. 2012, 22, 9497. [Google Scholar] [CrossRef]
- Zhao, X.; Qi, F.; Yuan, C.; Du, W.; Liu, D. Lipase-catalyzed process for biodiesel production: Enzyme immobilization, process simulation and optimization. Renew. Sustain. Energy Rev. 2015, 44, 182–197. [Google Scholar] [CrossRef]
- Shen, K.; Zhang, L.; Chen, X.; Liu, L.; Zhang, D.; Han, Y.; Chen, J.; Long, J.; Luque, R.; Li, Y.; et al. Ordered macro-microporous metal-organic framework single crystals. Science 2018, 359, 206–210. [Google Scholar] [CrossRef]
- Hu, Y.; Dai, L.; Liu, D.; Du, W. Rationally designing hydrophobic UiO-66 support for the enhanced enzymatic performance of immobilized lipase. Green Chem. 2018, 20, 4500–4506. [Google Scholar] [CrossRef]





| Material | BET Surface Areas (m2·g−1) | DFT Pore Volumes (cm3·g−1) |
|---|---|---|
| CZ-600-M-0.5 | 157.6 | 0.211 |
| CZ-700-M-0.5 | 148.4 | 0.404 |
| CZ-800-M-0.5 | 88.1 | 0.256 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Zhou, H.; Dai, L.; Liu, D.; Du, W. Preparation of Ordered Macroporous ZIF-8-Derived Magnetic Carbon Materials and Its Application for Lipase Immobilization. Catalysts 2024, 14, 55. https://doi.org/10.3390/catal14010055
Shi Y, Zhou H, Dai L, Liu D, Du W. Preparation of Ordered Macroporous ZIF-8-Derived Magnetic Carbon Materials and Its Application for Lipase Immobilization. Catalysts. 2024; 14(1):55. https://doi.org/10.3390/catal14010055
Chicago/Turabian StyleShi, Yongheng, Hao Zhou, Lingmei Dai, Dehua Liu, and Wei Du. 2024. "Preparation of Ordered Macroporous ZIF-8-Derived Magnetic Carbon Materials and Its Application for Lipase Immobilization" Catalysts 14, no. 1: 55. https://doi.org/10.3390/catal14010055