Catalytic Systems for 5-Hydroxymethylfurfural Preparation from Different Biomass Feedstocks: A Review
Abstract
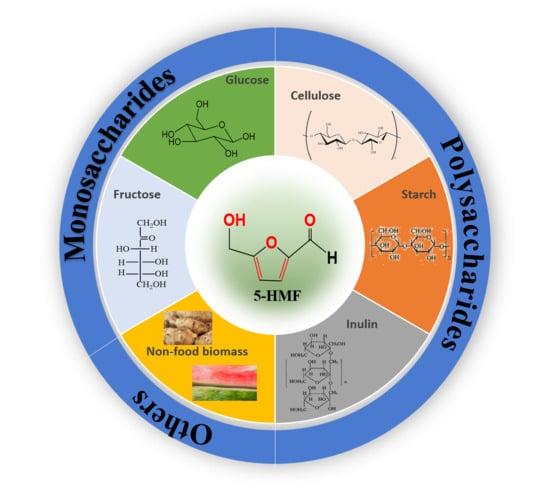
:1. Introduction
2. Catalytic System for HMF Synthesis from Different Raw Materials
2.1. Monosaccharide-Based Catalytic Systems
2.1.1. Fructose as a Raw Material
| Saccharides | Catalyst | Solvent | Temperature/°C | Time/min | Yield/% | Ref. |
|---|---|---|---|---|---|---|
| Fructose | [BMIM]OH | DMSO | 160 | 480 | 91.6 | [21] |
| Fructose | [CMIm]Cl | DMSO | 120 | 120 | 95.7 | [22] |
| Fructose | LF | DMSO | 393K | 90 | ~90 | [23] |
| Fructose | N3P3Cl6 | [Bmim]Cl | 80 | 20 | 92.8 | [24] |
| Fructose | CM-SO3H | [BMIM]Cl | 80 | 30 | 83.5 | [25] |
| Fructose | P-TiO2 | water-THF | 175 | 30 | 98.6 | [26] |
| Fructose | C16-SO3H-γ-Al2O3-650 °C | DMSO/H2O | 110 | 240 | 84 | [27] |
| Fructose | GO | DMSO | 120 | 240 | 60.8 | [28] |
2.1.2. Glucose as a Raw Material
2.1.3. High-Fructose Corn Syrup
2.2. Polysaccharide-Based Catalytic Systems
2.2.1. Cellulose-Based Raw Materials
2.2.2. Starch-Based Raw Materials
2.2.3. Inulin as a Raw Material
2.3. Catalytic Systems for Non-Food Biomass Feedstocks
3. Isolation and Purification of 5-HMF
4. Conclusions and Outlook
- (1)
- For the conversion of cellulose-based polysaccharides to HMF via a hydrolysis–isomerisation–dehydration cascade, multifunctional catalytic systems for improving the overall reaction efficiency should be further enriched, and the design of new catalysts with adjustable B and L acids is of significance for improving the efficiency of the reaction.
- (2)
- Solvent systems also have a potential impact on the synthesis of HMF. A suitable solvent system can inhibit the production of by-products and facilitate the conversion of raw materials, thus improving the selectivity of the reaction. Aqueous solutions are the most economical and green reaction solvents, but they reduce product yields due to easy evaporation during the reaction compared to expensive ionic liquids. Therefore, there is a need to continue to explore efficient and inexpensive reaction solvent systems.
- (3)
- Future research should still focus on exploring green, economic and sustainable conversion strategies that are compatible with the environment. Emphasis will be placed on the feasibility of industrial production while focusing on environmental issues to harmonise economic development with environmental protection. There is a need to design and develop high-performance, non-homogeneous catalytic systems and enhance their catalyst stability to allow for multiple reuses and cost savings.
- (4)
- The by-products from the synthesis of HMF can be resourcefully utilised and considered for conversion into valuable materials or fuels. HMF has very good application prospects and is one of the important platform compounds with great research value and significance.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tilman, D.; Socolow, R.; Foley, J.A.; Hill, J.; Larson, E.; Lynd, L.; Pacala, S.; Reilly, J.; Searchinger, T.; Somerville, C.; et al. Beneficial Biofuels—The Food, Energy, and Environment Trilemma. Science 2009, 325, 270–271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z. Synthesis of γ-Valerolactone from Carbohydrates and its Applications. ChemSusChem 2016, 9, 156–171. [Google Scholar] [CrossRef] [PubMed]
- George, W.; Huber, S.I.; Corma, A. Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef]
- Zhu Chenjie, D.F.; Hanjie, Y.; Pingkai, O. Catalytic production of liquid hydrocarbon fuels and fuel additives from lignocellulosic platform molecules. CIESC J. 2015, 66, 2785–2792. [Google Scholar] [CrossRef]
- Weng, R.; Lu, X.; Ji, N.; Fukuoka, A.; Shrotri, A.; Li, X.; Zhang, R.; Zhang, M.; Xiong, J.; Yu, Z. Taming the butterfly effect: Modulating catalyst nanostructures for better selectivity control of the catalytic hydrogenation of biomass-derived furan platform chemicals. Catal. Sci. Technol. 2021, 11, 7785–7806. [Google Scholar] [CrossRef]
- Van Putten, R.-J.; van der Waal, J.C.; de Jong, E.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, A Versatile Platform Chemical Made from Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef]
- Yong, G.; Zhang, Y.; Ying, J.Y. Efficient Catalytic System for the Selective Production of 5-Hydroxymethylfurfural from Glucose and Fructose. Angew. Chem. Int. Ed. 2008, 47, 9345–9348. [Google Scholar] [CrossRef]
- Lee, S.-B.; Jeong, G.-T. Catalytic Conversion of Chitosan to 5-Hydroxymethylfurfural Under Low Temperature Hydrothermal Process. Appl. Biochem. Biotechnol. 2015, 176, 1151–1161. [Google Scholar] [CrossRef]
- Oh, S.J.; Park, J.; Na, J.G.; Oh, Y.K.; Chang, Y.K. Production of 5-hydroxymethylfurfural from agarose by using a solid acid catalyst in dimethyl sulfoxide. RSC Adv. 2015, 5, 47983–47989. [Google Scholar] [CrossRef]
- Jia, S.; He, Y.; Wang, G. Dimethylsulfoxide/Water Mixed Solvent Mediated Synthesis of 5-Hydroxymethylfurfural from Galactose with Aluminum Salt Catalyst. ChemistrySelect 2017, 2, 2356–2362. [Google Scholar] [CrossRef]
- Liu, D.; Xiao, R.; Ying, H.; Chen, Y.; Zhu, C.; Du, F.; Zhang, H.; Ouyang, P. Research progress in catalytic valorization of lignocellulose. Sci. Sin. Chim. 2015, 45, 454–478. [Google Scholar] [CrossRef]
- Marcotullio, G.; De Jong, W. Chloride ions enhance furfural formation from d-xylose in dilute aqueous acidic solutions. Green Chem. 2010, 12, 1739–1746. [Google Scholar] [CrossRef]
- Xin, L.; Gaofeng, S.; Zhao, W.; Fenfang, L.; Yawen, H.; Kaiqiang, Y. Research progress on the preparation of 5-hydroxymethylfurfural by converting biomass resources. Mod. Chem. Ind. 2020, 40, 22–26. [Google Scholar] [CrossRef]
- Asghari, F.S.; Yoshida, H. Dehydration of fructose to 5-hydroxymethylfurfural in sub-critical water over heterogeneous zirconium phosphate catalysts. Carbohydr. Res. 2006, 341, 2379–2387. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, X.; Yuan, L.; Liu, X. Fructose decomposition kinetics in organic acids-enriched high temperature liquid water. Biomass Bioenergy 2009, 33, 1182–1187. [Google Scholar] [CrossRef]
- Asghari, F.S.; Yoshida, H. Kinetics of the Decomposition of Fructose Catalyzed by Hydrochloric Acid in Subcritical Water: Formation of 5-Hydroxymethylfurfural, Levulinic, and Formic Acids. Ind. Eng. Chem. Res. 2007, 46, 7703–7710. [Google Scholar] [CrossRef]
- Yoshida, F.S.A.H. Acid-Catalyzed Production of 5-Hydroxymethyl Furfural from D-Fructose in Subcritical Water. Ind. Eng. Chem. Res. 2006, 45, 2163–2173. [Google Scholar]
- Chheda, J.N.; Huber, G.W.; Dumesic, J.A. Liquid-Phase Catalytic Processing of Biomass-Derived Oxygenated Hydrocarbons to Fuels and Chemicals. Angew. Chem. Int. Ed. 2007, 46, 7164–7183. [Google Scholar] [CrossRef]
- Hansen, T.S.; Woodley, J.M.; Riisager, A. Efficient microwave-assisted synthesis of 5-hydroxymethylfurfural from concentrated aqueous fructose. Carbohydr. Res. 2009, 344, 2568–2572. [Google Scholar] [CrossRef]
- Lopes, M.; Dussan, K.; Leahy, J.J.; da Silva, V.T. Conversion of d -glucose to 5-hydroxymethylfurfural using Al2O3-promoted sulphated tin oxide as catalyst. Catal. Today 2017, 279, 233–243. [Google Scholar] [CrossRef]
- Qu, Y.S.; Song, Y.L.; Huang, C.P.; Zhang, J.; Chen, B.H. Dehydration of Fructose to 5-Hydroxymethylfurfural Catalyzed by Alkaline Ionic Liquid. Adv. Mater. Res. 2011, 287–290, 1585–1590. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, B.; Zhang, Z.; Chen, L. Conversion of carbohydrates into 5-hydroxymethylfurfural catalyzed by acidic ionic liquids in dimethyl sulfoxide. Ind. Crops Prod. 2013, 50, 264–269. [Google Scholar] [CrossRef]
- Tang, H.; Li, N.; Li, G.; Wang, W.; Wang, A.; Cong, Y.; Wang, X. Dehydration of Carbohydrates to 5-Hydroxymethylfurfural over Lignosulfonate-Based Acidic Resin. ACS Sustain. Chem. Eng. 2018, 6, 5645–5652. [Google Scholar] [CrossRef]
- Song, J.; Zhang, B.; Shi, J.; Ma, J.; Yang, G.; Han, B. Dehydration of Carbohydrates to 5-Hydroxymethylfurfural in Ionic Liquids Catalyzed by Hexachlorotriphosphazene. Chin. J. Chem. 2012, 30, 2079–2084. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Xie, X.; Huang, C.; Yang, S. Dehydration of fructose, sucrose and inulin to 5-hydroxymethylfurfural over yeast-derived carbonaceous microspheres at low temperatures. RSC Adv. 2019, 9, 9041–9048. [Google Scholar] [CrossRef]
- Atanda, L.; Shrotri, A.; Mukundan, S.; Ma, Q.; Konarova, M.; Beltramini, J. Direct Production of 5-Hydroxymethylfurfural via Catalytic Conversion of Simple and Complex Sugars over Phosphated TiO2. ChemSusChem 2015, 8, 2907–2916. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Wang, K.; Gao, L.; Guo, X. Efficient conversion of fructose to 5-hydroxymethylfurfural by functionalized γ-Al2O3 beads. Appl. Organomet. Chem. 2019, 33, e4821. [Google Scholar] [CrossRef]
- Nie, G.; Tong, X.; Zhang, Y.; Xue, S. Efficient Production of 5-Hydroxymethylfurfural (HMF) from d-Fructose and Inulin with Graphite Derivatives as the Catalysts. Catal. Lett. 2014, 144, 1759–1765. [Google Scholar] [CrossRef]
- Yang, L.; Tsilomelekis, G.; Caratzoulas, S.; Vlachos, D.G. Mechanism of Brønsted Acid-Catalyzed Glucose Dehydration. ChemSusChem 2015, 8, 1334–1341. [Google Scholar] [CrossRef]
- Mimura, N.; Sato, O.; Shirai, M.; Yamaguchi, A. 5-Hydroxymethylfurfural Production from Glucose, Fructose, Cellulose, or Cellulose–based Waste Material by Using a Calcium Phosphate Catalyst and Water as a Green Solvent. ChemistrySelect 2017, 2, 1305–1310. [Google Scholar] [CrossRef]
- Hongyan Yang, J.L.; Lu, J.; Lan, P.; Zhang, H. Study on Catalytic Conversion of Cellulose to 5-Hydroxymethyl Furfural by Directional Degradation in Deep Eutectic Solvents. BioResources 2020, 15, 3344–3355. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Williams, L.D.; Ebede, C.C. Mechanism of the dehydration of d-fructose to 5-hydroxymethylfurfural in dimethyl sulfoxide at 150 °C: An NMR study. Carbohydr. Res. 2008, 343, 3021–3024. [Google Scholar] [CrossRef] [PubMed]
- Antal, M.J., Jr.; Mok, W.S.; Richards, G.N. Mechanism of formation of 5-(hydroxymethyl)-2-furaldehyde from D-fructose and sucrose. Carbohydr. Res. 1990, 199, 91–109. [Google Scholar] [CrossRef] [PubMed]
- Moreau, C.; Finiels, A.; Vanoye, L. Dehydration of fructose and sucrose into 5-hydroxymethylfurfural in the presence of 1-H-3-methyl imidazolium chloride acting both as solvent and catalyst. J. Mol. Catal. A Chem. 2006, 253, 165–169. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, C.-w.; Abu-Omar, M.M. Conversion of carbohydrates and lignocellulosic biomass into 5-hydroxymethylfurfural using AlCl3·6H2O catalyst in a biphasic solvent system. Green Chem. 2012, 14, 509–513. [Google Scholar] [CrossRef]
- Wang, T.; Pagán-Torres, Y.J.; Combs, E.J.; Dumesic, J.A.; Shanks, B.H. Water-Compatible Lewis Acid-Catalyzed Conversion of Carbohydrates to 5-Hydroxymethylfurfural in a Biphasic Solvent System. Top. Catal. 2012, 55, 657–662. [Google Scholar] [CrossRef]
- Shen, Y.; Sun, J.; Yi, Y.; Wang, B.; Xu, F.; Sun, R. 5-Hydroxymethylfurfural and levulinic acid derived from monosaccharides dehydration promoted by InCl 3 in aqueous medium. J. Mol. Catal. A Chem. 2014, 394, 114–120. [Google Scholar] [CrossRef]
- Jia, S.; Xu, Z.; Zhang, Z.C. Catalytic conversion of glucose in dimethylsulfoxide/water binary mix with chromium trichloride: Role of water on the product distribution. Chem. Eng. J. 2014, 254, 333–339. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, Z.; Song, J.; Zhou, Y.; Han, B. Efficient conversion of glucose into 5-hydroxymethylfurfural catalyzed by a common Lewis acid SnCl4 in an ionic liquid. Green Chem. 2009, 11, 1746–1749. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Z.; Liu, B.; Zhou, Q.; Wang, S.; Deng, K. Catalytic conversion of fructose into furans using FeCl3 as catalyst. J. Ind. Eng. Chem. 2014, 20, 644–649. [Google Scholar] [CrossRef]
- Feng, Y.; Zuo, M.; Wang, T.; Jia, W.; Zhao, X.; Zeng, X.; Sun, Y.; Tang, X.; Lei, T.; Lin, L. Efficient synthesis of glucose into 5-hydroxymethylfurfural with SO42−/ZrO2 modified H+ zeolites in different solvent systems. J. Taiwan Inst. Chem. Eng. 2019, 96, 431–438. [Google Scholar] [CrossRef]
- Pumrod, S.; Kaewchada, A.; Roddecha, S.; Jaree, A. 5-HMF production from glucose using ion exchange resin and alumina as a dual catalyst in a biphasic system. RSC Adv. 2020, 10, 9492–9498. [Google Scholar] [CrossRef] [PubMed]
- Chung, N.H.; Oanh, V.T.; Thoa, L.K.; Hoang, P.H. Catalytic Conversion of Glucose into 5-Hydroxymethyl Furfural Over Cu–Cr/ZSM-5 Zeolite. Catal. Lett. 2019, 150, 170–177. [Google Scholar] [CrossRef]
- Li, Z.; Su, K.; Ren, J.; Yang, D.; Cheng, B.; Kim, C.K.; Yao, X. Direct catalytic conversion of glucose and cellulose. Green Chem. 2018, 20, 863–872. [Google Scholar] [CrossRef]
- Wang, J.; Ren, J.; Liu, X.; Xi, J.; Xia, Q.; Zu, Y.; Lu, G.; Wang, Y. Direct conversion of carbohydrates to 5-hydroxymethylfurfural using Sn-Mont catalyst. Green Chem. 2012, 14, 2506–2512. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, W.; Wang, N.; Wang, H.; Li, W.; Song, Z. Effect of Different Ionic Liquids on 5-Hydroxymethylfurfural Preparation from Glucose in DMA over AlCl3: Experimental and Theoretical Study. Chin. J. Chem. 2015, 33, 583–588. [Google Scholar] [CrossRef]
- Casey, J.P. High Fructose Corn Syrup. A Case History of Innovation. Starch—Stärke 2006, 29, 196–204. [Google Scholar] [CrossRef]
- Lo, C.; Li, S.; Wang, Y.; Tan, D.; Pan, M.; Sang, S.; Ho, C. Reactive dicarbonyl compounds and 5-(hydroxymethyl)-2-furfural in carbonated beverages containing high fructose corn syrup. Food Chem. 2008, 107, 1099–1105. [Google Scholar] [CrossRef]
- Jeong, J.; Antonyraj, C.A.; Shin, S.; Kim, S.; Kim, B.; Lee, K.-Y.; Cho, J.K. Commercially attractive process for production of 5-hydroxymethyl-2-furfural from high fructose corn syrup. J. Ind. Eng. Chem. 2013, 19, 1106–1111. [Google Scholar] [CrossRef]
- Rao, K.T.V.; Souzanchi, S.; Yuan, Z.; Xu, C. One-pot sol–gel synthesis of a phosphated TiO2 catalyst for conversion of monosaccharide, disaccharides, and polysaccharides to 5-hydroxymethylfurfural. New J. Chem. 2019, 43, 12483–12493. [Google Scholar] [CrossRef]
- Lin, C.; Wu, H.; Wang, J.; Huang, J.; Cao, F.; Zhuang, W.; Lu, Y.; Chen, J.; Jia, H.; Ouyang, P. Preparation of 5-Hydroxymethylfurfural from High Fructose Corn Syrup Using Organic Weak Acid in Situ as Catalyst. Ind. Eng. Chem. Res. 2020, 59, 4358–4366. [Google Scholar] [CrossRef]
- Souzanchi, S.; Nazari, L.; Rao, K.T.V.; Yuan, Z.; Tan, Z.; Xu, C. 5-HMF production from industrial grade sugar syrups derived from corn and wood using niobium phosphate catalyst in a biphasic continuous-flow tubular reactor. Catal. Today 2023, 407, 274–280. [Google Scholar] [CrossRef]
- Zhang, Y.-R.; Li, N.; Li, M.-F.; Fan, Y.-M. Highly efficient conversion of microcrystalline cellulose to 5-hydroxymethyl furfural in a homogeneous reaction system. RSC Adv. 2016, 6, 21347–21351. [Google Scholar] [CrossRef]
- Yan, L.; Ma, R.; Wei, H.; Li, L.; Zou, B.; Xu, Y. Ruthenium trichloride catalyzed conversion of cellulose into 5-hydroxymethylfurfural in biphasic system. Bioresour. Technol. 2019, 279, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Du, Y.; Zhang, W.; Cheng, X.; Wang, J. Catalytic conversion of cellulose into 5-hydroxymethylfurfural over chromium trichloride in ionic liquid. Korean J. Chem. Eng. 2014, 31, 1786–1791. [Google Scholar] [CrossRef]
- Nogueira, J.S.M.; Santana, V.T.; Henrique, P.V.; Aguiar, L.G.d.; Silva, J.P.A.; Mussatto, S.I.; Carneiro, L.M. Production of 5-Hydroxymethylfurfural from Direct Conversion of Cellulose Using Heteropolyacid/Nb2O5 as Catalyst. Catalysts 2020, 10, 1417. [Google Scholar] [CrossRef]
- Cao, Z.; Fan, Z.; Chen, Y.; Li, M.; Shen, T.; Zhu, C.; Ying, H. Efficient preparation of 5-hydroxymethylfurfural from cellulose in a biphasic system over hafnyl phosphates. Appl. Catal. B Environ. 2019, 244, 170–177. [Google Scholar] [CrossRef]
- Tang, Z.; Su, J. Direct conversion of cellulose to 5-hydroxymethylfurfural (HMF) using an efficient and inexpensive boehmite catalyst. Carbohydr. Res. 2019, 481, 52–59. [Google Scholar] [CrossRef]
- Wen, Z.; Yu, L.; Mai, F.; Ma, Z.; Chen, H.; Li, Y. Catalytic Conversion of Microcrystalline Cellulose to Glucose and 5-Hydroxymethylfurfural over a Niobic Acid Catalyst. Ind. Eng. Chem. Res. 2019, 58, 17675–17681. [Google Scholar] [CrossRef]
- Chheda, J.N.; Román-Leshkov, Y.; Dumesic, J.A. Production of 5-hydroxymethylfurfural and furfural by dehydration of biomass-derived mono- and poly-saccharides. Green Chem. 2007, 9, 342–350. [Google Scholar] [CrossRef]
- Chun, J.A.; Lee, J.W.; Yi, Y.B.; Hong, S.S.; Chung, C.H. Direct conversion of starch to hydroxymethylfurfural in the presence of an ionic liquid with metal chloride. Starch—Stärke 2010, 62, 326–330. [Google Scholar] [CrossRef]
- Yi, Y.-B.; Ha, M.-G.; Lee, J.-W.; Chung, C.-H. New role of chromium fluoride: Its catalytic action on the synthesis of hydroxymethylfurfural in ionic liquid using raw plant biomass and characterization of biomass hydrolysis. Chem. Eng. J. 2012, 180, 370–375. [Google Scholar] [CrossRef]
- Zhang, Y.; Guan, W.; Song, H.; Wei, Y.; Jin, P.; Li, B.; Yan, C.; Pan, J.; Yan, Y. Coupled acid and base UiO-66-type MOFs supported on g-C3N4 as a bi-functional catalyst for one-pot production of 5-HMF from glucose. Microporous Mesoporous Mater. 2020, 305, 110328. [Google Scholar] [CrossRef]
- Cao, J.; Ma, M.; Liu, J.; Yang, Y.; Liu, H.; Xu, X.; Huang, J.; Yue, H.; Tian, G.; Feng, S. Highly effective transformation of carbohydrates to 5-Hydroxymethylfurfural with Al-montmorillonite as catalyst. Appl. Catal. A Gen. 2019, 571, 96–101. [Google Scholar] [CrossRef]
- Fachri, B.A.; Abdilla, R.M.; Rasrendra, C.B.; Heeres, H.J. Experimental and modeling studies on the acid-catalyzed conversion of inulin to 5-hydroxymethylfurfural in water. Chem. Eng. Res. Des. 2016, 109, 65–75. [Google Scholar] [CrossRef]
- Menegazzo, F.; Ghedini, E.; Signoretto, M. 5-Hydroxymethylfurfural (HMF) Production from Real Biomasses. Molecules 2018, 23, 2201. [Google Scholar] [CrossRef]
- Qi, X.; Watanabe, M.; Aida, T.M.; Smith, R.L., Jr. Efficient one-pot production of 5-hydroxymethylfurfural from inulin in ionic liquids. Green Chem. 2010, 12, 1855–1860. [Google Scholar] [CrossRef]
- Heo, J.B.; Lee, Y.-S.; Chung, C.-H. Raw plant-based biorefinery: A new paradigm shift towards biotechnological approach to sustainable manufacturing of HMF. Biotechnol. Adv. 2019, 37, 107422. [Google Scholar] [CrossRef]
- Portillo Perez, G.; Mukherjee, A.; Dumont, M.-J. Insights into HMF catalysis. J. Ind. Eng. Chem. 2019, 70, 1–34. [Google Scholar] [CrossRef]
- Yang, F.; Liu, Q.; Bai, X.; Du, Y. Conversion of biomass into 5-hydroxymethylfurfural using solid acid catalyst. Bioresour. Technol. 2011, 102, 3424–3429. [Google Scholar] [CrossRef]
- Jiang, N.; Qi, W.; Wu, Z.; Su, R.; He, Z. “One-pot” conversions of carbohydrates to 5-hydroxymethylfurfural using Sn-ceramic powder and hydrochloric acid. Catal. Today 2018, 302, 94–99. [Google Scholar] [CrossRef]
- Tang, H.; Li, N.; Chen, F.; Li, G.; Wang, A.; Cong, Y.; Wang, X.; Zhang, T. Highly efficient synthesis of 5-hydroxymethylfurfural with carbohydrates over renewable cyclopentanone-based acidic resin. Green Chem. 2017, 19, 1855–1860. [Google Scholar] [CrossRef]
- Walia, M.; Sharma, U.; Agnihotri, V.K.; Singh, B. Silica-supported boric acid assisted conversion of mono- and poly-saccharides to 5-hydroxymethylfurfural in ionic liquid. RSC Adv. 2014, 4, 14414–14418. [Google Scholar] [CrossRef]
- Cao, L.; Yu, I.K.M.; Tsang, D.C.W.; Zhang, S.; Ok, Y.S.; Kwon, E.E.; Song, H.; Poon, C.S. Phosphoric acid-activated wood biochar for catalytic conversion of starch-rich food waste into glucose and 5-hydroxymethylfurfural. Bioresour. Technol. 2018, 267, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Pan, D.; Wu, Y.; Xu, N.; Yang, H.; Gao, L.; Li, W.; Xiao, G. Direct Conversion of Wheat Straw Components into Furan Compounds Using a Highly Efficient and Reusable SnCl2-PTA/β Zeolite Catalyst. Ind. Eng. Chem. Res. 2019, 58, 9276–9285. [Google Scholar] [CrossRef]
- Shao, Y.; Long, Y.; Zhou, Y.; Jin, Z.; Zhou, D.; Shen, D. 5-Hydroxymethylfurfural production from watermelon peel by microwave hydrothermal liquefaction. Energy 2019, 174, 198–205. [Google Scholar] [CrossRef]
- Pińkowska, H.; Krzywonos, M.; Wolak, P.; Seruga, P.; Górniak, A.; Złocińska, A.; Ptak, M. Sustainable Production of 5-Hydroxymethylfurfural from Pectin-Free Sugar Beet Pulp in a Simple Aqueous Phase System-Optimization with Doehlert Design. Energies 2020, 13, 5649. [Google Scholar] [CrossRef]
- Mo, H.-b.; Chen, X.-p.; Liao, X.-y.; Zhou, T. Sustainable synthesis of 5-hydroxymethylfurfural from waste cotton stalk catalyzed by solid superacid-SO42−/ZrO2. J. Cent. South Univ. 2017, 24, 1745–1753. [Google Scholar] [CrossRef]
- Ito, R.; Miyafuji, H.; Miyazaki, Y.; Kawai, T. Production of 5-hydroxymethylfurfural from wood by ionic liquid treatment. J. Wood Sci. 2016, 62, 349–355. [Google Scholar] [CrossRef]
- Slak, J.; Pomeroy, B.; Kostyniuk, A.; Grilc, M.; Likozar, B. A review of bio-refining process intensification in catalytic conversion reactions, separations and purifications of hydroxymethylfurfural (HMF) and furfural. Chem. Eng. J. 2022, 429, 132325. [Google Scholar] [CrossRef]
- Kougioumtzis, M.A.; Marianou, A.; Atsonios, K.; Michailof, C.; Nikolopoulos, N.; Koukouzas, N.; Triantafyllidis, K.; Lappas, A.; Kakaras, E. Production of 5-HMF from Cellulosic Biomass: Experimental Results and Integrated Process Simulation. Waste Biomass Valorization 2018, 9, 2433–2445. [Google Scholar] [CrossRef]
- Zhang, Y.-B.; Luo, Q.-X.; Lu, M.-H.; Luo, D.; Liu, Z.-W.; Liu, Z.-T. Controllable and scalable synthesis of hollow-structured porous aromatic polymer for selective adsorption and separation of HMF from reaction mixture of fructose dehydration. Chem. Eng. J. 2019, 358, 467–479. [Google Scholar] [CrossRef]
- Dietz, C.H.J.T.; Kroon, M.C.; Di Stefano, M.; van Sint Annaland, M.; Gallucci, F. Selective separation of furfural and hydroxymethylfurfural from an aqueous solution using a supported hydrophobic deep eutectic solvent liquid membrane. Faraday Discuss. 2018, 206, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; He, X.; Cai, C.; Xiao, J.; Liu, Y.; Chen, Z.; Pan, B.; Lin, X. Adsorption isotherm, kinetics simulation and breakthrough analysis of 5-hydroxymethylfurfural adsorption/desorption behavior of a novel polar-modified post-cross-linked poly (divinylbenzene-co-ethyleneglycoldimethacrylate) resin. Chemosphere 2020, 239, 124732. [Google Scholar] [CrossRef]





| Physical Properties Items | Values |
|---|---|
| Molecular weight | 126.11 |
| Colour | Yellow |
| State | Solid |
| Odour | The odour of chamomile flowers |
| Density | 1.243 at 25 °C |
| Boiling point | 114–116 °C at 1 hPa |
| Melting point | 31.5 °C |
| Solubility | Soluble |
| Refractive index | 1.5627 at 18 °C |
| Flash point | 79 °C |
| UV absorption maximum | 283 nm |
| Saccharides | Catalyst | Solvent | Temperature/°C | Time/min | Yield/% | Ref. |
|---|---|---|---|---|---|---|
| Glucose | RCP160M | H2O-NMP-NaCl/MIBK | 120 | 480 | 84.92 | [42] |
| Glucose | Cu-Cr/ZSM-5 | DMSO | 140 | 240 | 50.4 | [43] |
| Glucose | SPPS | ILs | 140 | 240 | 87.2 | [44] |
| Glucose | Sn-Mont | THF/DMSO | 160 | 180 | 53.5 | [45] |
| Glucose | AlCl3 + [NMP]Br | DMA | 120 | 120 | 57 | [46] |
| Saccharides | Catalyst | Solvent | Temperature/°C | Time/min | Yield/% | Ref. |
|---|---|---|---|---|---|---|
| MCC (microcrystalline cellulose) | HCl | ZnCl2aq | 150 | 40 | 80.6 | [53] |
| Microcrystalline cellulose | RuCl3 | NaClaq-butanol | 220 | 30 | 83.3 | [54] |
| Cellulose | CrCl3·6H2O | TBAC | 140 | 90 | 43.7 | [55] |
| Cellulose | HPW/Nb2O5 | Acetone/H2O | 200 | 30 | 20.6 | [56] |
| Microcrystalline cellulose | HfO(PO4)2 | NaCl-H2O/THF | 190 | 240 | 69.8 | [57] |
| Cellulose | γ-AlOOH | (BmimCl) + DMSO | 160 | 120 | 58.4 | [58] |
| Microcrystalline cellulose | NBO | H2O | 230 | 120 | 7 | [59] |
| Saccharides | Catalyst | Solvent | Temperature/°C | Time/min | Yield/% | Ref. |
|---|---|---|---|---|---|---|
| Inulin | Sn-CP/HCl | H2O/DMSO | 170 | 240 | 55.9 | [71] |
| Inulin (enzymatically hydrolysed) | NA-p | Water-2-butanol | 433K | 80 | 74 | [70] |
| Inulin | CM-SO3H | [BMIM][Cl] | 80 | 60 | 59.2 | [25] |
| Inulin | GO | DMSO | 160 | 100 | 58.2 | [28] |
| Inulin | GO | THF-H2O | 160 | 100 | 61.2 | [28] |
| Inulin | SCFC | DMSO | 393K | 180 | 80.1 | [72] |
| Inulin | SBBA | [bmim]HSO4 | 120 | 300 | 88 | [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, J.; Pan, Y.; Zhou, H.; Tang, Y.; Ren, G.; Yu, Z.; Li, J.; Zhang, R.; Li, X.; Qiao, Y.; et al. Catalytic Systems for 5-Hydroxymethylfurfural Preparation from Different Biomass Feedstocks: A Review. Catalysts 2024, 14, 30. https://doi.org/10.3390/catal14010030
Tao J, Pan Y, Zhou H, Tang Y, Ren G, Yu Z, Li J, Zhang R, Li X, Qiao Y, et al. Catalytic Systems for 5-Hydroxymethylfurfural Preparation from Different Biomass Feedstocks: A Review. Catalysts. 2024; 14(1):30. https://doi.org/10.3390/catal14010030
Chicago/Turabian StyleTao, Jiao, Yunchuan Pan, Haiyang Zhou, Yufei Tang, Guoquan Ren, Zhihao Yu, Jiaxuan Li, Rui Zhang, Xiaoyun Li, Yina Qiao, and et al. 2024. "Catalytic Systems for 5-Hydroxymethylfurfural Preparation from Different Biomass Feedstocks: A Review" Catalysts 14, no. 1: 30. https://doi.org/10.3390/catal14010030