Uniformly Dispersed Nano Pd-Ni Oxide Supported on Polyporous CeO2 and Its Application in Methane Conversion of Tail Gas from Dual-Fuel Engine
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Property of Metal Nanoparticles and Supported Samples
2.2. The Dispersion of Pd and Ni Species on CeO2 of PdNiO-H Sample
2.3. Reducibility of CeO2 Supported Bimetallic Oxide Samples
2.4. Oxygen Vacancy of CeO2 Supported Samples
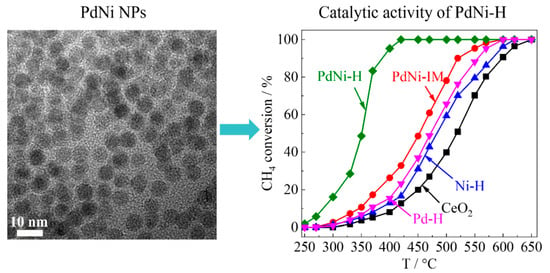
2.5. The Catalytic Performance of CeO2 Supported Bimetallic Catalysts in Methane Combustion Reaction
3. Experimental Section
3.1. Preparation of Catalysts
3.1.1. Synthesis of PdNi-H Catalysts
3.1.2. Synthesis of PdNi-IM
3.2. Catalytic Performance Test
3.3. Characterization Techniques
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cargnello, M.; Jaén, J.J.D.; Garrido, J.C.H.; Bakhmutsky, K.; Montini, T.; Gámez, J.J.C.; Gorte, R.J.; Fornasiero, P. Exceptional activity for methane combustion over modular Pd@CeO2 subunits on functionalized Al2O3. Science 2012, 337, 713–717. [Google Scholar] [CrossRef]
- Yang, X.; Gao, Q.; Zhao, Z.; Guo, Y.; Guo, Y.; Wang, L.; Wang, Y.; Zhan, W. Surface tuning of noble metal doped perovskite oxide by synergistic effect of thermal treatment and acid etching: A new path to high-performance catalysts for methane combustion. Appl. Catal. B 2018, 239, 373–382. [Google Scholar] [CrossRef]
- Willis, J.J.; Gallo, A.; Sokaras, D.; Aljama, H.; Nowak, S.H.; Goodman, E.D.; Wu, L.; Tassone, C.J.; Jaramillo, T.F.; Abild-Pedersen, F.; et al. Systematic structure–property relationship studies in palladium-catalyzed methane complete combustion. ACS Catal. 2017, 7, 7810–7821. [Google Scholar] [CrossRef]
- Losch, P.; Huang, W.; Vozniuk, O.; Goodman, E.D.; Schmidt, W.; Cargnello, M. Modular Pd/zeolite composites demonstrating the key role of support hydrophobic/hydrophilic character in methane catalytic combustion. ACS Catal. 2019, 9, 4742–4753. [Google Scholar] [CrossRef]
- Huang, F.; Chen, J.; Hu, W.; Li, G.; Wu, Y.; Yuan, S.; Zhong, L.; Chen, Y. Pd or PdO: Catalytic active site of methane oxidation operated close to stoichiometric air-to-fuel for natural gas vehicles. Appl. Catal. B 2017, 219, 73–81. [Google Scholar] [CrossRef]
- Colussi, S.; Gayen, A.; Farnesi Camellone, M.; Boaro, M.; Llorca, J.; Fabris, S.; Trovarelli, A. Nanofaceted Pd-O sites in Pd-Ce surface superstructures: Enhanced activity in catalytic combustion of methane. Angew. Chem. Int. Ed. 2009, 48, 8481–8484. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kreft, S.; Georgi, G.; Fulda, G.; Pohl, M.-M.; Seeburg, D.; Berger-Karin, C.; Kondratenko, E.V.; Wohlrab, S. Improved catalytic methane combustion of Pd/CeO2 catalysts via porous glass integration. Appl. Catal. B 2015, 179, 313–320. [Google Scholar] [CrossRef]
- Mayernick, A.D.; Janik, M.J. Methane oxidation on Pd–Ceria: A DFT study of the mechanism over PdxCe1−xO2, Pd, and PdO. J. Catal. 2011, 278, 16–25. [Google Scholar] [CrossRef]
- Satsuma, A.; Tojo, T.; Okuda, K.; Yamamoto, Y.; Arai, S.; Oyama, J. Effect of preparation method of Co-promoted Pd/alumina for methane combustion. Catal. Today 2015, 242, 308–314. [Google Scholar] [CrossRef]
- Elias, J.S.; Risch, M.; Giordano, L.; Mansour, A.N.; Shao-Horn, Y. Structure, bonding, and catalytic activity of monodisperse, transition-metal-substituted CeO2 nanoparticles. J. Am. Chem. Soc. 2014, 136, 17193–17200. [Google Scholar] [CrossRef]
- Qi, J.; Zhao, K.; Li, G.; Gao, Y.; Zhao, H.; Yu, R.; Tang, Z. Multi-shelled CeO2 hollow microspheres as superior photocatalysts for water oxidation. Nanoscale 2014, 6, 4072–4077. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, Q.; Yuan, S.; Zhang, M.; Ohno, T. Morphology control and characterization of broom-like porous CeO2. Chem. Eng. J. 2015, 260, 126–132. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, Y.; Huang, F.; Xiao, Y.; Cai, G.; Zhang, Y.; Zheng, Y.; Jiang, L. Catalytic activity and stability over nanorod-like ordered mesoporous phosphorus-doped alumina supported palladium catalysts for methane combustion. ACS Catal. 2018, 8, 11016–11028. [Google Scholar] [CrossRef]
- Duan, H.; You, R.; Xu, S.; Li, Z.; Qian, K.; Cao, T.; Huang, W.; Bao, X. Pentacoordinated Al(3+)-stabilized active Pd structures on Al2O3-coated palladium catalysts for methane combustion. Angew. Chem. Int. Ed. 2019, 58, 12043–12048. [Google Scholar] [CrossRef]
- Shen, J.; Hayes, R.E.; Wu, X.; Semagina, N. 100° temperature reduction of wet methane combustion: Highly active Pd–Ni/Al2O3 Catalyst versus Pd/NiAl2O4. ACS Catal. 2015, 5, 2916–2920. [Google Scholar] [CrossRef]
- Zou, X.; Rui, Z.; Ji, H. Core–shell NiO@PdO nanoparticles supported on alumina as an advanced catalyst for methane oxidation. ACS Catal. 2017, 7, 1615–1625. [Google Scholar] [CrossRef]
- Pan, X.; Zhang, Y.; Miao, Z.; Yang, X. A novel PdNi/Al2O3 catalyst prepared by galvanic deposition for low temperature methane combustion. J. Energy Chem. 2013, 22, 610–616. [Google Scholar] [CrossRef]
- Persson, K.; Ersson, A.; Jansson, K.; Fierro, J.; Jaras, S. Influence of molar ratio on Pd–Pt catalysts for methane combustion. J. Catal. 2006, 243, 14–24. [Google Scholar] [CrossRef]
- Mussio, A.; Danielis, M.; Divins, N.J.; Llorca, J.; Colussi, S.; Trovarelli, A. Structural evolution of bimetallic PtPd/CeO2 methane oxidation catalysts prepared by dry milling. ACS Appl. Mater. Interfaces 2021, 13, 31614–31623. [Google Scholar] [CrossRef]
- Göksu, H.; Ho, S.F.; Metin, Ö.; Korkmaz, K.; Mendoza Garcia, A.; Gültekin, M.S.; Sun, S. Tandem dehydrogenation of ammonia borane and hydrogenation of nitro/nitrile compounds catalyzed by graphene-supported NiPd alloy nanoparticles. ACS Catal. 2014, 4, 1777–1782. [Google Scholar] [CrossRef]
- Feng, L.; Chong, H.; Li, P.; Xiang, J.; Fu, F.; Yang, S.; Yu, H.; Sheng, H.; Zhu, M. Pd–Ni alloy nanoparticles as effective catalysts for miyaura–heck coupling reactions. J. Phys. Chem. C 2015, 119, 11511–11515. [Google Scholar] [CrossRef]
- Tang, C.; Li, J.; Yao, X.; Sun, J.; Cao, Y.; Zhang, L.; Gao, F.; Deng, Y.; Dong, L. Mesoporous NiO–CeO2 catalysts for CO oxidation: Nickel content effect and mechanism aspect. Appl. Catal. A 2015, 494, 77–86. [Google Scholar] [CrossRef]
- Zou, Q.; Zhao, Y.; Jin, X.; Fang, J.; Li, D.; Li, K.; Lu, J.; Luo, Y. Ceria-nano supported copper oxide catalysts for CO preferential oxidation: Importance of oxygen species and metal-support interaction. Appl. Surf. Sci. 2019, 494, 1166–1176. [Google Scholar] [CrossRef]
- Pal, P.; Singha, R.K.; Saha, A.; Bal, R.; Panda, A.B. Defect-induced efficient partial oxidation of methane over nonstoichiometric Ni/CeO2 nanocrystals. J. Phys. Chem. C 2015, 119, 13610–13618. [Google Scholar] [CrossRef]
- Meng, L.; Jia, A.-P.; Lu, J.-Q.; Luo, L.-F.; Huang, W.-X.; Luo, M.-F. Synergetic effects of PdO species on CO oxidation over PdO–CeO2 catalysts. J. Phys. Chem. C 2011, 115, 19789–19796. [Google Scholar] [CrossRef]
- Shinde, V.M.; Madras, G. Nanostructured Pd modified Ni/CeO2 catalyst for water gas shift and catalytic hydrogen combustion reaction. Appl. Catal. B 2013, 132–133, 28–38. [Google Scholar] [CrossRef]
- Hilli, Y.; Kinnunen, N.M.; Suvanto, M.; Savimäki, A.; Kallinen, K.; Pakkanen, T.A. Preparation and characterization of Pd–Ni bimetallic catalysts for CO and C3H6 oxidation under stoichiometric conditions. Appl. Catal. A 2015, 497, 85–95. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z.; Akmal, Z.S. Direct decomposition of methane over Pd promoted Ni/SBA-15 catalysts. Appl. Surf. Sci. 2015, 353, 127–136. [Google Scholar] [CrossRef]
- Daniel, M.; Loridant, S. Probing reoxidation sites by in situ Raman spectroscopy: Differences between reduced CeO2 and Pt/CeO2. J. Raman Spectrosc. 2012, 43, 1312–1319. [Google Scholar] [CrossRef]
- Gulyaev, R.V.; Kardash, T.Y.; Malykhin, S.E.; Stonkus, O.A.; Ivanova, A.S.; Boronin, A.I. The local structure of Pd(x)Ce(1−x)O(2−x−delta) solid solutions. Phys. Chem. Chem. Phys. 2014, 16, 13523–13539. [Google Scholar] [CrossRef]
- Gulyaev, R.V.; Slavinskaya, E.M.; Novopashin, S.A.; Smovzh, D.V.; Zaikovskii, A.V.; Osadchii, D.Y.; Bulavchenko, O.A.; Korenev, S.V.; Boronin, A.I. Highly active PdCeO composite catalysts for low-temperature CO oxidation, prepared by plasma-arc synthesis. Appl. Catal. B 2014, 147, 132–143. [Google Scholar] [CrossRef]
- Rotaru, C.G.; Postole, G.; Florea, M.; Matei-Rutkovska, F.; Pârvulescu, V.I.; Gelin, P. Dry reforming of methane on ceria prepared by modified precipitation route. Appl. Catal. A 2015, 494, 29–40. [Google Scholar] [CrossRef]
- Zhang, Z.; Han, D.; Wei, S.; Zhang, Y. Determination of active site densities and mechanisms for soot combustion with O2 on Fe-doped CeO2 mixed oxides. J. Catal. 2010, 276, 16–23. [Google Scholar] [CrossRef]
- Mahammadunnisa, S.; Manoj Kumar Reddy, P.; Lingaiah, N.; Subrahmanyam, C. NiO/Ce1−xNixO2−δ as an alternative to noble metal catalysts for CO oxidation. Catal. Sci. Technol. 2013, 3, 730–736. [Google Scholar] [CrossRef]
- Holgado, J.P.; Alvarez, R.; Munuera, G. Study of CeO XPS spectra by factor analysis: Reduction of CeO2. Appl. Surf. Sci. 2000, 161, 301–315. [Google Scholar] [CrossRef]
- Karpenko, A.; Leppelt, R.; Cai, J.; Plzak, V.; Chuvilin, A.; Kaiser, U.; Behm, R. Deactivation of a Au/CeO2 catalyst during the low-temperature water–gas shift reaction and its reactivation: A combined TEM, XRD, XPS, DRIFTS, and activity study. J. Catal. 2007, 250, 139–150. [Google Scholar] [CrossRef]
- Yao, X.; Xiong, Y.; Zou, W.; Zhang, L.; Wu, S.; Dong, X.; Gao, F.; Deng, Y.; Tang, C.; Chen, Z.; et al. Correlation between the physicochemical properties and catalytic performances of CexSn1−xO2 mixed oxides for NO reduction by CO. Appl. Catal. B 2014, 144, 152–165. [Google Scholar] [CrossRef]
- Nguyen, T.-S.; Postole, G.; Loridant, S.; Bosselet, F.; Burel, L.; Aouine, M.; Massin, L.; Gélin, P.; Morfin, F.; Piccolo, L. Ultrastable iridium–ceria nanopowders synthesized in one step by solution combustion for catalytic hydrogen production. J. Mater. Chem. A 2014, 2, 19822–19832. [Google Scholar] [CrossRef]
- Cong, Q.; Chen, L.; Wang, X.; Ma, H.; Zhao, J.; Li, S.; Hou, Y.; Li, W. Promotional effect of nitrogen-doping on a ceria unary oxide catalyst with rich oxygen vacancies for selective catalytic reduction of NO with NH3. Chem. Eng. J. 2020, 379, 122302. [Google Scholar] [CrossRef]
- Gardner, T.H.; Spivey, J.J.; Kugler, E.L.; Campos, A.; Hissam, J.C.; Roy, A.D. Structural characterization of Ni-substituted hexaaluminate catalysts using EXAFS, XANES, XPS, XRD, and TPR. J. Phys. Chem. C 2010, 114, 7888–7894. [Google Scholar] [CrossRef]
- Yashnik, S.A.; Chesalov, Y.A.; Ishchenko, A.V.; Kaichev, V.V.; Ismagilov, Z.R. Effect of Pt addition on sulfur dioxide and water vapor tolerance of Pd-Mn-hexaaluminate catalysts for high-temperature oxidation of methane. Appl. Catal. B 2017, 204, 89–106. [Google Scholar] [CrossRef]
- Goodman, E.D.; Dai, S.; Yang, A.-C.; Wrasman, C.; Gallo, A.; Bare, S.R.; Hoffman, A.S.; Jaramillo, T.F.; Graham, G.W.; Pan, X.; et al. Monodisperse Pt/Pd bimetallic nanocrystals demonstrate platinum effect on palladium methane combustion activity and stability. ACS Catal. 2017, 7, 4372–4380. [Google Scholar] [CrossRef]
- Ahmad, Y.H.; Mohamed, A.T.; Mahmoud, K.A.; Aljaber, A.S.; Al-Qaradawi, S.Y. Natural clay-supported palladium catalysts for methane oxidation reaction: Effect of alloying. RSC Adv. 2019, 9, 32928–32935. [Google Scholar] [CrossRef]
- Lin, J.; Chen, Y.; Liu, X.; Chen, X.; Zheng, Y.; Huang, F.; Xiao, Y.; Zheng, Y.; Jiang, L. Microstructural property regulation and performance in methane combustion reaction of ordered mesoporous alumina supported palladium-cobalt bimetallic catalysts. Appl. Catal. B 2020, 263, 118269. [Google Scholar] [CrossRef]








| Sample | Lattice Constant (nm) a | SBET (m2/g) |
|---|---|---|
| CeO2 | 0.05444 | 154.7 |
| Ni-H | 0.05414 | 121.7 |
| Pd-H | 0.05437 | 120.4 |
| PdNi -H | 0.05396 | 120.2 |
| PdNi-IM | 0.05429 | 121.8 |
| Catalysts | O″ /(O″ + O′) a | Ce3+ (%) b | Pd4+/Pd2+ c |
|---|---|---|---|
| CeO2 | 0.42 | 12.11 | - |
| Pd-H | 0.43 | 14.92 | 0.10 |
| Ni-H | 0.47 | 17.75 | - |
| PdNi-IM | 0.48 | 21.89 | 0.17 |
| PdNi-H | 0.53 | 30.59 | 0.20 |
| Catalysts | Pd wt.% | Feed Concentration | GHSV (h−1) | T50 (°C) | Reference |
|---|---|---|---|---|---|
| PdCo/Al2O3 | 0.5 | 0.4%CH4/10%O2/N2 | 300,000 | 500 | [9] |
| PdNi/Al2O3 | 0.029 | 4100 ppm methane, 5 mol % water | 3128 | 400 | [15] |
| PtPd/ MnLaAl11O19 | 0.67 | 5%CH4 in air | 10,000 | 355 | [41] |
| PtPd/Al2O3 | 0.5 | 0.5%CH4/2.0%O2/Ar | 200,000 | 450 | [42] |
| PdNi@Hal | 1 | 1%CH4/20%O2/Ar | 72,000 | 355 | [43] |
| Pd/6CoDEG/OMA | 0.5 | 1%CH4/10%O2/N2 | 30,000 | 385 | [44] |
| PdNi/H | 0.5 | 1%CH4/5%O2/N2 | 30,000 | 351 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, C.; Chen, L.; Alodhayb, A.N.; Wu, J.; Tan, M.; Yang, Y. Uniformly Dispersed Nano Pd-Ni Oxide Supported on Polyporous CeO2 and Its Application in Methane Conversion of Tail Gas from Dual-Fuel Engine. Catalysts 2024, 14, 24. https://doi.org/10.3390/catal14010024
Luo C, Chen L, Alodhayb AN, Wu J, Tan M, Yang Y. Uniformly Dispersed Nano Pd-Ni Oxide Supported on Polyporous CeO2 and Its Application in Methane Conversion of Tail Gas from Dual-Fuel Engine. Catalysts. 2024; 14(1):24. https://doi.org/10.3390/catal14010024
Chicago/Turabian StyleLuo, Chunlian, Luwei Chen, Abdullah N. Alodhayb, Jianhua Wu, Mingwu Tan, and Yanling Yang. 2024. "Uniformly Dispersed Nano Pd-Ni Oxide Supported on Polyporous CeO2 and Its Application in Methane Conversion of Tail Gas from Dual-Fuel Engine" Catalysts 14, no. 1: 24. https://doi.org/10.3390/catal14010024