Hydrolysis–Dehydration of Cellulose: Efficiency of NbZr Catalysts under Batch and Flow Conditions
Abstract
:1. Introduction
2. Results
2.1. Synthesis and Characterization of Catalysts
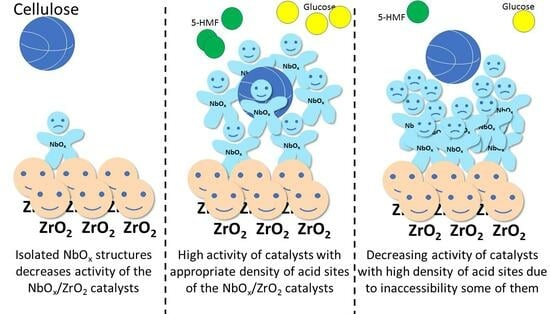
2.2. Catalytic Properties of NbZr Catalysts in a Batch Reactor
2.3. Catalytic Properties of NbZr Catalysts in a Flow Reactor
2.4. Efficiency of NbZr Catalysts
| № | Catalyst | T 1 (K) | Cell:H2O:Cat 1 (g:mL:g) | Yield of 5-HMF (%) | TOF 1 (mmol g−1·h−1) | Ref. |
|---|---|---|---|---|---|---|
| 1 | 0.5% NbOx/ZrO2 | 453 | 1:100:1 | 12.9 | 0.4 | [27] |
| 2 | 2.8% NbOx/ZrO2 | 453 | 1:100:1 | 16.1 | 1.6 | [27] |
| 3 | ZrO2 | 523 | 1:10:1 | 8.2 | 7.6 | [43] |
| 4 | TiO2 | 523 | 1:10:1 | 10.0 | 13.0 | [43] |
| 5 | TiO2-ZrO2 | 523 | 1:10:1 | 13.6 | 13.9 | [43] |
| 6 | γ-Al2O3 | 423 | 1:10:1 | - | 0.06 2 | [52] |
| 7 | SiO2 | 423 | 1:10:1 | - | 0.0 2 | [52] |
| 8 | SiO2-SO3H | 423 | 1:10:1 | - | 1.1 2 | [52] |
| 9 | Fe3O4-SiO2-SO3H | 423 | 1:10:1 | - | 0.5 2 | [52] |
| 10 | ZrO2 | 433 | 0.3:67:1 | - | 0.02 2 | [18] |
| 11 | TiO2 | 433 | 0.3:67:1 | - | 0.004 2 | [18] |
| 12 | Al2O3 | 433 | 0.3:67:1 | - | 0.002 2 | [18] |
| 13 | SiO2 | 433 | 0.3:67:1 | - | 0.09 2 | [18] |
| 14 | ZnO-ZrO2 | 463 | 1:100:1 | 5.8 | 0.09 | [50] |
| 15 | SO42−-ZrO2/montmorillonite | 473 | 5:50:1 | - | 10 2 | [21] |
| 16 | ZrO2-SO3H | 453 | 4:250:1 | 8.4 | 4 × 10−5 | [53] |
| 17 | ZrO2-SO3H | 423 | 0.9:100:1 | - | 0.03 2 | [49] |
| 18 | SiO2 | 453 | 20:2000:1 | - | 0.007 2 | [49] |
| 19 | γ-Al2O3 | 453 | 10:2000:1 | - | 0.007 2 | [49] |
| 20 | NbZr-W2 (4%) | 453 | 20:2000:1 | - | 16.8 | This work |
| 21 | NbZr-MAW1 (0.6%) | 453 | 10:2000:1 | - | 3.1 | This work |
3. Materials and Methods
3.1. Materials
3.2. Catalyst Characterization
3.3. Catalytic Test in a Batch Reactor
3.4. Catalytic Test in a Flow Reactor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tabassum, N.; Pothu, R.; Pattnaik, A.; Boddula, R.; Balla, P.; Gundeboyina, R.; Challa, P.; Rajesh, R.; Perugopu, V.; Mameda, N.; et al. Heterogeneous Catalysts for Conversion of Biodiesel-Waste Glycerol into High-Added-Value Chemicals. Catalysts 2022, 12, 767. [Google Scholar] [CrossRef]
- Pothu, R.; Mameda, N.; Mitta, H.; Boddula, R.; Gundeboyina, R.; Perugopu, V.; Radwan, A.B.; Abdullah, A.M.; Al-Qahtani, N. High Dispersion of Platinum Nanoparticles over Functionalized Zirconia for Effective Transformation of Levulinic Acid to Alkyl Levulinate Biofuel Additives in the Vapor Phase. J. Compos. Sci. 2022, 6, 300. [Google Scholar] [CrossRef]
- Delidovich, I.; Leonhard, K.; Palkovits, R. Cellulose and hemicellulose valorisation: An integrated challenge of catalysis and reaction engineering. Energy Environ. Sci. 2014, 7, 2803–2830. [Google Scholar] [CrossRef]
- Gallezot, P. Conversion of biomass to selected chemical products. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef]
- Gromov, N.V.; Medvedeva, T.B.; Rodikova, Y.A.; Babushkin, D.E.; Panchenko, V.N.; Timofeeva, M.N.; Zhizhina, E.G.; Taran, O.P.; Parmon, V.N. One-pot synthesis of formic acid via hydrolysis–oxidation of potato starch in the presence of cesium salts of heteropoly acid catalysts. RSC Adv. 2020, 10, 28856–28864. [Google Scholar] [CrossRef]
- Gromov, N.V.; Medvedeva, T.B.; Sorokina, K.N.; Samoylova, Y.V.; Rodikova, Y.A.; Parmon, V.N. Direct Conversion of Microalgae Biomass to Formic Acid under an Air Atmosphere with Soluble and Solid Mo–V–P Heteropoly Acid Catalysts. ACS Sustain. Chem. Eng. 2020, 8, 18947–18956. [Google Scholar] [CrossRef]
- Gromov, N.V.; Medvedeva, T.B.; Lukoyanov, I.A.; Panchenko, V.N.; Timofeeva, M.N.; Taran, O.P.; Parmon, V.N. Formic Acid Production via One-Pot Hydrolysis-Oxidation of Starch over Quaternary Ammonium Salts of Vanadium-Containing Keggin-Type Heteropoly Acids. Catalysts 2022, 12, 1252. [Google Scholar] [CrossRef]
- Valentini, F.; Kozell, V.; Petrucci, C.; Marrocchi, A.; Gu, Y.; Gelman, D.; Vaccaro, L. Formic acid, a biomass-derived source of energy and hydrogen for biomass upgrading. Energy Environ. Sci. 2019, 12, 2646–2664. [Google Scholar] [CrossRef]
- Van Putten, R.J.; van der Waal, J.C.; de Jong, E.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef]
- Ribeiro, L.S.; Órfão, J.J.d.M.; Pereira, M.F.R. Direct catalytic production of sorbitol from waste cellulosic materials. Bioresour. Technol. 2017, 232, 152–158. [Google Scholar] [CrossRef]
- Rout, P.K.; Nannaware, A.D.; Prakash, O.; Kalra, A.; Rajasekharan, R. Synthesis of hydroxymethylfurfural from cellulose using green processes: A promising biochemical and biofuel feedstock. Chem. Eng. Sci. 2016, 142, 318–346. [Google Scholar] [CrossRef]
- Siankevich, S.; Savoglidis, G.; Fei, Z.; Laurenczy, G.; Alexander, D.T.L.; Yan, N.; Dyson, P.J. A novel platinum nanocatalyst for the oxidation of 5-Hydroxymethylfurfural into 2,5-Furandicarboxylic acid under mild conditions. J. Catal. 2014, 315, 67–74. [Google Scholar] [CrossRef]
- Gallezot, P.; Kiennemann, A. Conversion of Biomass on Solid Catalysts. In Handbook of Heterogeneous Catalysis; Wiley: Weinheim, Germany, 2008; pp. 2447–2476. [Google Scholar] [CrossRef]
- Bruggink, A.; Schoevaart, R.; Kieboom, T. Concepts of Nature in Organic Synthesis: Cascade Catalysis and Multistep Conversions in Concert. Org. Process Res. Dev. 2003, 7, 622–640. [Google Scholar] [CrossRef]
- Gromov, N.V.; Medvedeva, T.B.; Taran, O.P.; Timofeeva, M.N.; Parmon, V.N. Hydrolysis of Cellulose in the Presence of Catalysts Based on Cesium Salts of Heteropoly Acids. Catal. Ind. 2021, 13, 73–80. [Google Scholar] [CrossRef]
- Aymonier, C.; Gromov, N.V.; Taran, O.P.; Parmon, V.N. Hydrolysis–dehydration of cellulose to glucose and 5-hydroxymethylfurfural over Sibunit solid acid carbon catalysts under semi-flow conditions. Wood Sci. Technol. 2021, 55, 607–624. [Google Scholar] [CrossRef]
- Nandiwale, K.Y.; Galande, N.D.; Thakur, P.; Sawant, S.D.; Zambre, V.P.; Bokade, V.V. One-Pot Synthesis of 5-Hydroxymethylfurfural by Cellulose Hydrolysis over Highly Active Bimodal Micro/Mesoporous H-ZSM-5 Catalyst. ACS Sustain. Chem. Eng. 2014, 2, 1928–1932. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, C.; He, H.; Wang, L. Glucose production from hydrolysis of cellulose over a novel silica catalyst under hydrothermal conditions. J. Environ. Sci. 2012, 24, 473–478. [Google Scholar] [CrossRef]
- Wattanapaphawong, P.; Reubroycharoen, P.; Yamaguchi, A. Conversion of cellulose into lactic acid using zirconium oxide catalysts. RSC Adv. 2017, 7, 18561–18568. [Google Scholar] [CrossRef]
- Joshi, S.S.; Zodge, A.D.; Pandare, K.V.; Kulkarni, B.D. Efficient Conversion of Cellulose to Levulinic Acid by Hydrothermal Treatment Using Zirconium Dioxide as a Recyclable Solid Acid Catalyst. Ind. Eng. Chem. Res. 2014, 53, 18796–18805. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, Y.; Tong, D.; Yang, M.; Fang, K.; Zhou, C.; Yu, W. Catalytic conversion of cellulose to reducing sugars over clay-based solid acid catalyst supported nanosized SO42−-ZrO2. Appl. Clay Sci. 2020, 185, 105376. [Google Scholar] [CrossRef]
- Ngee, E.L.S.; Gao, Y.; Chen, X.; Lee, T.M.; Hu, Z.; Zhao, D.; Yan, N. Sulfated Mesoporous Niobium Oxide Catalyzed 5-Hydroxymethylfurfural Formation from Sugars. Ind. Eng. Chem. Res. 2014, 53, 14225–14233. [Google Scholar] [CrossRef]
- Qiao, Y.; Feng, L.; Li, Z.; Zhang, Z.; Chen, J.; Na, H.; Zhu, J.; Chen, L. Effect of Adsorption of ZrO2 in Catalysts on the Efficiency of Hydrolysisof Cellulose to Sugar in Aqueous System under Microwave Radiation. Chin. J. Chem. 2020, 38, 399–405. [Google Scholar] [CrossRef]
- Yue, C.; Li, G.; Pidko, E.A.; Wiesfeld, J.J.; Rigutto, M.; Hensen, E.J.M. Dehydration of Glucose to 5-Hydroxymethylfurfural Using Nb-doped Tungstite. ChemSusChem 2016, 9, 2421–2429. [Google Scholar] [CrossRef]
- Kreissl, H.T.; Nakagawa, K.; Peng, Y.-K.; Koito, Y.; Zheng, J.; Tsang, S.C.E. Niobium oxides: Correlation of acidity with structure and catalytic performance in sucrose conversion to 5-hydroxymethylfurfural. J. Catal. 2016, 338, 329–339. [Google Scholar] [CrossRef]
- Carniti, P.; Gervasini, A.; Bossola, F.; Dal Santo, V. Cooperative action of Brønsted and Lewis acid sites of niobium phosphate catalysts for cellobiose conversion in water. Appl. Catal. B Environ. 2016, 193, 93–102. [Google Scholar] [CrossRef]
- Gromov, N.V.; Taran, O.P.; Semeykina, V.S.; Danilova, I.G.; Pestunov, A.V.; Parkhomchuk, E.V.; Parmon, V.N. Solid Acidic NbOx/ZrO2 Catalysts for Transformation of Cellulose to Glucose and 5-Hydroxymethylfurfural in Pure Hot Water. Catal. Lett. 2017, 147, 1485–1495. [Google Scholar] [CrossRef]
- Gromov, N.V.; Yakovleva, I.S.; Isupova, L.A.; Parmon, V.N.; Taran, O.P. Solid acid catalysts based on niobium and zirconium oxides for hydrolysis-dehydration of cellulose into glucose and 5-hydroxymethylfurfural. Impact of preparation technique on catalytic properties. J. Sib. Fed. Univ. Chem. 2020, 13, 283–296. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Li, X.; Liu, X.; Xia, Y.; Hu, B.; Lu, G.; Wang, Y. Direct conversion of biomass-derived carbohydrates to 5-hydroxymethylfurural over water-tolerant niobium-based catalysts. Fuel 2015, 139, 301–307. [Google Scholar] [CrossRef]
- Ordomsky, V.V.; Sushkevich, V.L.; Schouten, J.C.; van der Schaaf, J.; Nijhuis, T.A. Glucose dehydration to 5-hydroxymethylfurfural over phosphate catalysts. J. Catal. 2013, 300, 37–46. [Google Scholar] [CrossRef]
- Carlini, C.; Giuttari, M.; Maria Raspolli Galletti, A.; Sbrana, G.; Armaroli, T.; Busca, G. Selective saccharides dehydration to 5-hydroxymethyl-2-furaldehyde by heterogeneous niobium catalysts. Appl. Catal. A Gen. 1999, 183, 295–302. [Google Scholar] [CrossRef]
- Marzo, M.; Gervasini, A.; Carniti, P. Hydrolysis of disaccharides over solid acid catalysts under green conditions. Carbohydr. Res. 2012, 347, 23–31. [Google Scholar] [CrossRef]
- Carniti, P.; Gervasini, A.; Biella, S.; Auroux, A. Niobic acid and niobium phosphate as highly acidic viable catalysts in aqueous medium: Fructose dehydration reaction. Catal. Today 2006, 118, 373–378. [Google Scholar] [CrossRef]
- Carniti, P.; Gervasini, A.; Biella, S.; Auroux, A. Intrinsic and Effective Acidity Study of Niobic Acid and Niobium Phosphate by a Multitechnique Approach. Chem. Mater. 2005, 17, 6128–6136. [Google Scholar] [CrossRef]
- Ding, S.; Zhao, J.; Yu, Q. Effect of Zirconia Polymorph on Vapor-Phase Ketonization of Propionic Acid. Catalysts 2019, 9, 768. [Google Scholar] [CrossRef]
- Medvedeva, T.B.; Ogorodnikova, O.L.; Yakovleva, I.S.; Isupova, L.A.; Taran, O.P.; Gromov, N.V.; Parmon, V.N. Impact of Design on the Activity of ZrO2 Catalysts in Cellulose Hydrolysis-Dehydration to Glucose and 5-Hydroxymethylfurfural. Catalysts 2021, 11, 1359. [Google Scholar] [CrossRef]
- Armaroli, T.; Busca, G.; Carlini, C.; Giuttari, M.; Raspolli Galletti, A.M.; Sbrana, G. Acid sites characterization of niobium phosphate catalysts and their activity in fructose dehydration to 5-hydroxymethyl-2-furaldehyde. J. Mol. Catal. A Chem. 2000, 151, 233–243. [Google Scholar] [CrossRef]
- İlknur, Ç. Preparation and Characterization of Niobia-Containing Solid Acid. Ph.D. Thesis, Bilkent University, Ankara, Turkey, 2010. [Google Scholar]
- Onfroy, T.; Clet, G.; Houalla, M. Correlations between Acidity, Surface Structure, and Catalytic Activity of Niobium Oxide Supported on Zirconia. J. Phys. Chem. B 2005, 109, 14588–14594. [Google Scholar] [CrossRef]
- Gao, X.; Wachs, I.E.; Wong, M.S.; Ying, J.Y. Structural and Reactivity Properties of Nb-MCM-41: Comparison with That of Highly Dispersed Nb2O5/SiO2 Catalysts. J. Catal. 2001, 203, 18–24. [Google Scholar] [CrossRef]
- Shao, R.; Zeng, X.; Cao, Z.; Dong, H.; Wang, L.; Wang, F.; Liu, J.; Li, Z.; Liang, Q. A novel Ag3PO4/Nb2O5 fiber composite with enhanced photocatalytic performance and stability. RSC Adv. 2015, 5, 102101–102107. [Google Scholar] [CrossRef]
- Watanabe, M.; Aizawa, Y.; Iida, T.; Nishimura, R.; Inomata, H. Catalytic glucose and fructose conversions with TiO2 and ZrO2 in water at 473K: Relationship between reactivity and acid–base property determined by TPD measurement. Appl. Catal. A Gen. 2005, 295, 150–156. [Google Scholar] [CrossRef]
- Chareonlimkun, A.; Champreda, V.; Shotipruk, A.; Laosiripojana, N. Catalytic conversion of sugarcane bagasse, rice husk and corncob in the presence of TiO2, ZrO2 and mixed-oxide TiO2–ZrO2 under hot compressed water (HCW) condition. Bioresour. Technol. 2010, 101, 4179–4186. [Google Scholar] [CrossRef]
- Hassan, N.S.; Jalil, A.A. Cellulose conversion into renewable chemicals and fuels over supported metal catalysts. IOP Conf. Ser. Mater. Sci. Eng. 2020, 808, 012002. [Google Scholar] [CrossRef]
- Sasaki, M.; Kabyemela, B.; Malaluan, R.; Hirose, S.; Takeda, N.; Adschiri, T.; Arai, K. Cellulose hydrolysis in subcritical and supercritical water. J. Supercrit. Fluids 1998, 13, 261–268. [Google Scholar] [CrossRef]
- Rogalinski, T.; Ingram, T.; Brunner, G. Hydrolysis of lignocellulosic biomass in water under elevated temperatures and pressures. J. Supercrit. Fluids 2008, 47, 54–63. [Google Scholar] [CrossRef]
- Rogalinski, T.; Liu, K.; Albrecht, T.; Brunner, G. Hydrolysis kinetics of biopolymers in subcritical water. J. Supercrit. Fluids 2008, 46, 335–341. [Google Scholar] [CrossRef]
- Dhepe, P.L.; Fukuoka, A. Cellulose Conversion under Heterogeneous Catalysis. ChemSusChem 2008, 1, 969–975. [Google Scholar] [CrossRef]
- Onda, A.; Ochi, T.; Yanagisawa, K. Selective hydrolysis of cellulose into glucose over solid acid catalysts. Green Chem. 2008, 10, 1033–1037. [Google Scholar] [CrossRef]
- Yang, F.; Li, G.; Gao, P.; Lv, X.-N.; Sun, X.; Liu, Z.-H.; Fan, H. Mild Hydrothermal Degradation of Cotton Cellulose by using a Mixed-Metal-Oxide ZnO–ZrO2 Catalyst. Energy Technol. 2013, 1, 581–586. [Google Scholar] [CrossRef]
- Gavilà, L.; Güell, E.J.; Maru, B.T.; Medina, F.; Constantí, M. Combining catalytical and biological processes to transform cellulose into high value-added products. Phys. Sci. Rev. 2017, 2, 26. [Google Scholar] [CrossRef]
- Lai, D.-m.; Deng, L.; Li, J.; Liao, B.; Guo, Q.-x.; Fu, Y. Hydrolysis of Cellulose into Glucose by Magnetic Solid Acid. ChemSusChem 2011, 4, 55–58. [Google Scholar] [CrossRef]
- Yatsenko, D.A.; Medvedeva, T.B. Estimating Crystality Index of Microcrystalline Cellulose Using Diffraction Methods. J. Struct. Chem. 2019, 60, 1430–1436. [Google Scholar] [CrossRef]
- Gromov, N.V.; Medvedeva, T.B.; Taran, O.P.; Bukhtiyarov, A.V.; Aymonier, C.; Prosvirin, I.P.; Parmon, V.N. Hydrothermal Solubilization–Hydrolysis–Dehydration of Cellulose to Glucose and 5-Hydroxymethylfurfural Over Solid Acid Carbon Catalysts. Top. Catal. 2018, 61, 1912–1927. [Google Scholar] [CrossRef]
- Gromov, N.V.; Taran, O.P.; Aymonier, C.; Parmon, V.N. Kinetic modeling of the multistep hydrolysis-dehydration of cellulose to platform molecules over a solid carbon acid catalyst in pure water. React. Kinet. Mech. Catal. 2020, 130, 669–684. [Google Scholar] [CrossRef]






| № | Catalyst | Nb Content (wt.%) | Textural Properties | Phase Composition 1 | ||||
|---|---|---|---|---|---|---|---|---|
| SBET (m2·g−1) | VΣ (cm3·g−1) | Vμ (cm3·g−1) | Dpore (nm) | ZrO2 | NbxOy | |||
| Niobium oxide supported on ZrO2 (NbZr-MAW) | ||||||||
| 1 | NbZr-MAW0 | 0 | 10 | 0.39 | 0.0009 | 86.6 | M + T | A |
| 2 | NbZr-MAW1 | 0.6 | 49 | 0.83 | 0.0010 | 61.3 | M + T | A |
| 3 | NbZr-MAW2 | 1.2 | 51 | 0.72 | 0.0014 | 48.9 | M + T | A |
| 4 | NbZr-MAW3 | 6 | 60 | 0.49 | 0.0013 | 25.0 | M + T | A |
| Mixed oxide of NbOx-ZrO2 (NbZr-W) | ||||||||
| 5 | NbZr-W0 | 0 | 134 | 0.37 | n.d. | 9.5 | A | A |
| 6 | NbZr-W1 | 2 | 221 | 0.49 | n.d. | 7.1 | A | A |
| 7 | NbZr-W2 | 4 | 194 | 0.40 | n.d. | 7.3 | A | A |
| 8 | NbZr-W3 | 17 | 154 | 0.72 | n.d. | 22.5 | A | A |
| No | Catalyst Sample | Nb Content (wt.%) | BAS 1 (µmol g−1) | LAS 2 (µmol g−1) | Ʃ(LAS + BAS) (µmol g−1) | LAS/BAS | ρAS 3 (µmol·m−2) |
|---|---|---|---|---|---|---|---|
| NbZr-MAW samples | |||||||
| 1 | NbZr-MAW0 | 0 | n.d. | 14 | 14 | n/d | 1.4 |
| 2 | NbZr-MAW1 | 0.6 | 11 | 13 | 23 | 0.28 | 0.28 |
| 3 | NbZr-MAW2 | 1.2 | 21 | 14 | 35 | 0.68 | 0.68 |
| 4 | NbZr-MAW3 | 6.0 | 23 | 28 | 51 | 1.20 | 0.85 |
| NbZr-W samples | |||||||
| 5 | NbZr-W0 | 0 | n/d | n/d | n/d | n/d | 0 |
| 6 | NbZr-W1 | 2 | 10 | 22 | 32 | 2.18 | 0.14 |
| 7 | NbZr-W2 | 4 | 6 | 13 | 19 | 2.16 | 0.10 |
| 8 | NbZr-W3 | 17 | 10 | 24 | 34 | 2.5 | 0.22 |
| 9 | Nb2O5 | - | 63 | 109 | 172 | 1.74 | n/d |
| No | Catalyst | Nb Content (wt.%) | pHsusp | Conversion of Cellulose (%) | Yield | R 2 (mmol·L−1·h−1) | ||
|---|---|---|---|---|---|---|---|---|
| Glucose | 5-HMF | Other 3 | ||||||
| NbZr-MAW samples | ||||||||
| 1 | NbZr-MAW0 | 0 | 5.8 | 36 | 7.3 | 9.4 | 19.3 | 1.6 |
| 2 | NbZr-MAW1 | 0.6 | 8.2 | 41 | 13.8 | 11.3 | 15.9 | 3.1 |
| 3 | NbZr-MAW2 | 1.2 | 7.8 | 40 | 14.0 | 11.7 | 14.3 | 3.1 |
| 4 | NbZr-MAW3 | 6.0 | 5.7 | 39 | 14.0 | 13.0 | 12.0 | 3.5 |
| NbZr-W samples | ||||||||
| 5 | NbZr-W0 | 0 | 2.2 | 50 | 17.5 | 12.5 | 20.0 | 7.5 |
| 6 | NbZr-W1 | 2 | 2.1 | 51 | 16.9 | 12.5 | 21.6 | 8.4 |
| 7 | NbZr-W2 | 4 | 2.2 | 37 | 14.7 | 11.5 | 10.8 | 8.4 |
| 8 | NbZr-W3 | 17 | 2.4 | 41 | 15.6 | 12.9 | 12.5 | 4.7 |
| Catalyst | Nb Content (wt.%) | Preparation Method |
|---|---|---|
| Niobium oxide supported on Nb/ZrO2 (NbZr-MAW) | ||
| NbZr-MAW0 | 0 | Step 1. Preparation of ZrO2 by thermal decomposition of ZrO(NO3)2·5H2O at 873 K for 4 h. Step 2. Mechanical activation of ZrO2 and Nb(HC2O4)5 for 2 min. Step 3. Microwave treatment for 30 min |
| NbZr-MAW1 | 0.6 | |
| NbZr-MAW2 | 1.2 | |
| NbZr-MAW3 | 6 | |
| Mixed oxide of Nb-ZrO2 (NbZr-W) | ||
| NbZr-W0 | 0 | Microwave treatment mixture of ZrO(NO3)2 and Nb(HC2O4)5) for 30 min |
| NbZr-W1 | 2 | |
| NbZr-W2 | 4 | |
| NbZr-W3 | 17 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gromov, N.V.; Ogorodnikova, O.L.; Medvedeva, T.B.; Panchenko, V.N.; Yakovleva, I.S.; Isupova, L.A.; Timofeeva, M.N.; Taran, O.P.; Aymonier, C.; Parmon, V.N. Hydrolysis–Dehydration of Cellulose: Efficiency of NbZr Catalysts under Batch and Flow Conditions. Catalysts 2023, 13, 1298. https://doi.org/10.3390/catal13091298
Gromov NV, Ogorodnikova OL, Medvedeva TB, Panchenko VN, Yakovleva IS, Isupova LA, Timofeeva MN, Taran OP, Aymonier C, Parmon VN. Hydrolysis–Dehydration of Cellulose: Efficiency of NbZr Catalysts under Batch and Flow Conditions. Catalysts. 2023; 13(9):1298. https://doi.org/10.3390/catal13091298
Chicago/Turabian StyleGromov, Nikolay V., Olga L. Ogorodnikova, Tatiana B. Medvedeva, Valentina N. Panchenko, Irina S. Yakovleva, Lyubov A. Isupova, Maria N. Timofeeva, Oxana P. Taran, Cyril Aymonier, and Valentin N. Parmon. 2023. "Hydrolysis–Dehydration of Cellulose: Efficiency of NbZr Catalysts under Batch and Flow Conditions" Catalysts 13, no. 9: 1298. https://doi.org/10.3390/catal13091298