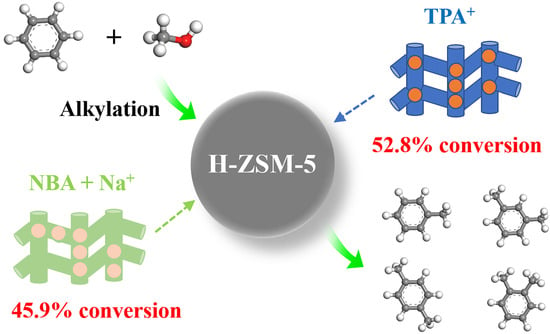
Selective Alkylation of Benzene with Methanol to Toluene and Xylene over H-ZSM-5 Zeolites: Impact of Framework Al Spatial Distribution
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural and Textural Properties
2.2. Acidic Properties
2.3. 29Si and 27Al MAS NMR, Constraint Index
2.4. Catalytic Performance
2.5. Periodic Density Functional Study
3. Materials and Methods
3.1. Synthesis of H-ZSM-5 Zeolites
3.2. Characterization of H-ZSM-5 Zeolites
3.3. Computational Method
3.4. Catalytic Performace Evaluation
3.4.1. Estimation of Constraint Index
3.4.2. Benzene Alkylation with Methanol
3.4.3. Cumene Cracking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahn, J.H.; Kolvenbach, R.; Al-Khattaf, S.S.; Jentys, A.; Lercher, J.A. Methanol usage in toluene methylation with medium and large pore zeolites. ACS Catal. 2013, 3, 817–825. [Google Scholar] [CrossRef]
- Gao, K.; Li, S.; Wang, L.; Wang, W. Study on the alkylation of benzene with methanol to selective formation of toluene and xylene over Co3O4-La2O3/ZSM-5. RSC Adv. 2015, 5, 45098–45105. [Google Scholar] [CrossRef]
- Liu, C.; Su, J.; Liu, S.; Zhou, H.; Yuan, X.; Ye, Y.; Wang, Y.; Jiao, W.; Zhang, L.; Lu, Y.; et al. Insights into the key factor of zeolite morphology on the selective conversion of syngas to light aromatics over a Cr2O3/ZSM-5 catalyst. ACS Catal. 2020, 10, 15227–15237. [Google Scholar] [CrossRef]
- Qian, J.; Xiong, G.; Liu, J.; Liu, C.; Guo, H. A preliminary study on the role of the internal and external surfaces of nano-ZSM-5 zeolite in the alkylation of benzene with methanol. Ind. Eng. Chem. Res. 2019, 58, 9006–9016. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Qi, G.; Li, B.; Wang, C.; Deng, F. Alkylation of benzene with methane over ZnZSM-5 zeolites studied with solid-state NMR spectroscopy. J. Phys. Chem. C 2013, 117, 4018–4023. [Google Scholar] [CrossRef]
- Rakoczy, J.; Romotowski, T. Alkylation of benzene with methanol on zeolites: Infrared spectroscopy studies. Zeolites 1993, 13, 256–260. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, Q.; Xie, Z.; Yang, W.; Li, C. The roles of acidity and structure of zeolite for catalyzing toluene alkylation with methanol to xylene. Micropor. Mesopor. Mater. 2006, 88, 16–21. [Google Scholar] [CrossRef]
- Hu, H.; Lyu, J.; Rui, J.; Cen, J.; Zhang, Q.; Wang, Q.; Han, W.; Li, X. The effect of Si/Al ratio on the catalytic performance of hierarchical porous ZSM-5 for catalyzing benzene alkylation with methanol. Catal. Sci. Technol. 2016, 6, 2647–2652. [Google Scholar] [CrossRef]
- Hu, H.; Lyu, J.; Cen, J.; Zhang, Q.; Wang, Q.; Han, W.; Rui, J.; Li, X. Promoting effects of MgO and Pd modification on the catalytic performance of hierarchical porous ZSM-5 for catalyzing benzene alkylation with methanol. RSC Adv. 2015, 5, 63044–63049. [Google Scholar] [CrossRef]
- Niziolek, A.M.; Onel, O.; Guzman, Y.A.; Floudas, C.A. Biomass-based production of benzene, toluene, and xylenes via methanol: Process synthesis and deterministic global optimization. Energy Fuels 2016, 30, 4970–4998. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, A.; Gawande, K.; Li, G.; Shang, S.; Dai, C.; Fan, W.; Han, Y.; Song, C.; Ren, L.; et al. b-Axis-oriented ZSM-5 nanosheets for efficient alkylation of benzene with methanol: Synergy of acid sites and diffusion. ACS Catal. 2023, 13, 3794–3805. [Google Scholar] [CrossRef]
- Ren, S.; Tian, C.; Yue, Y.; Zou, W.; Hua, W.; Gao, Z. Selective alkylation of benzene with methanol to toluene and xylene over sheet-like ZSM-5 with controllable b-oriented length. Catal. Lett. 2023. [Google Scholar] [CrossRef]
- Anderson, J.R.; Mole, T.; Christov, V. Mechanism of some conversions over ZSM-5 catalyst. J. Catal. 1980, 61, 477–484. [Google Scholar] [CrossRef]
- Svelle, S.; Visur, M.; Olsbye, U.; Saepurahman; Bjørgen, M. Mechanistic aspects of the zeolite catalyzed methylation of alkenes and aromatics with methanol: A review. Top. Catal. 2011, 54, 897–906. [Google Scholar] [CrossRef]
- Ivanova, I.I.; Corma, A. Surface species formed and their reactivity during the alkylation of toluene by methanol and dimethyl ether on zeolites as determined by in situ 13C MAS NMR. J. Phys. Chem. B 1997, 101, 547–551. [Google Scholar] [CrossRef]
- Vos, A.M.; Nulens, K.H.L.; De Proft, F.; Schoonheydt, R.A.; Geerlings, P. Reactivity descriptors and rate constants for electrophilic aromatic substitution: Acid zeolite catalyzed methylation of benzene and toluene. J. Phys. Chem. B 2002, 106, 2026–2034. [Google Scholar] [CrossRef]
- Wen, Z.; Zhu, H.; Zhu, X. Density functional theory study of the zeolite-catalyzed methylation of benzene with methanol. Catal. Lett. 2019, 150, 21–30. [Google Scholar] [CrossRef]
- Wen, Z.; Yang, D.; He, X.; Li, Y.; Zhu, X. Methylation of benzene with methanol over HZSM-11 and HZSM-5: A density functional theory study. J. Mol. Catal. A 2016, 424, 351–357. [Google Scholar] [CrossRef]
- Maihom, T.; Boekfa, B.; Sirijaraensre, J.; Nanok, T.; Probst, M.; Limtrakul, J. Reaction mechanisms of the methylation of ethene with methanol and dimethyl ether over H-ZSM-5: An ONIOM study. J. Phys. Chem. C 2009, 113, 6654–6662. [Google Scholar] [CrossRef]
- Van der Mynsbrugge, J.; Visur, M.; Olsbye, U.; Beato, P.; Bjørgen, M.; Van Speybroeck, V.; Svelle, S. Methylation of benzene by methanol: Single-site kinetics over H-ZSM-5 and H-beta zeolite catalysts. J. Catal. 2012, 292, 201–212. [Google Scholar] [CrossRef]
- Kaeding, W.W. Conversion of methanol to hydrocarbons III. Methylation, ethylation, and propylation of benzene with methanol. J. Catal. 1988, 114, 271–276. [Google Scholar] [CrossRef]
- Hu, H.; Lyu, J.; Wang, Q.; Zhang, Q.; Cen, J.; Li, X. Alkylation of benzene with methanol over hierarchical porous ZSM-5: Synergy effects of hydrogen atmosphere and zinc modification. RSC Adv. 2015, 5, 32679–32684. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, Q.; Cen, J.; Li, X. Catalytic activity of Pt modified hierarchical ZSM-5 catalysts in benzene alkylation with methanol. Catal. Lett. 2015, 145, 715–722. [Google Scholar] [CrossRef]
- Khare, R.; Bhan, A. Mechanistic studies of methanol-to-hydrocarbons conversion on diffusion-free MFI samples. J. Catal. 2015, 329, 218–228. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, H.; Chen, H.; Chen, Z.; Zhang, L.; Ren, J.; Wen, X.; Yang, Y.; Li, Y. Fabrication of a core–shell MFI@TON material and its enhanced catalytic performance for toluene alkylation. Catal. Sci. Technol. 2020, 10, 1281–1291. [Google Scholar] [CrossRef]
- Jalil, A.A.; Zolkifli, A.S.; Triwahyono, S.; Rahman, A.F.A.; Ghani, N.N.M.; Hamid, M.Y.S.; Mustapha, F.H.; Izan, S.M.; Nabgan, B.; Ripin, A. Altering dendrimer structure of fibrous-silica-HZSM5 for enhanced product selectivity of benzene methylation. Ind. Eng. Chem. Res. 2019, 58, 553–562. [Google Scholar] [CrossRef]
- Magusin, P.C.M.M.; Zorin, V.E.; Aerts, A.; Houssin, C.J.Y.; Yakovlev, A.L.; Martens, J.A.; van Santen, R.A. Template-aluminosilicate structures at the early stages of zeolite ZSM-5 formation. A combined preparative, solid-state NMR, and computational study. J. Phys. Chem. B 2005, 109, 22767–22774. [Google Scholar] [CrossRef]
- Yokoi, T.; Mochizuki, H.; Namba, S.; Kondo, J.N.; Tatsumi, T. Control of the Al distribution in the framework of ZSM-5 zeolite and its evaluation by solid-state NMR technique and catalytic properties. J. Phys. Chem. C 2015, 119, 15303–15315. [Google Scholar] [CrossRef]
- Pashkova, V.; Sklenak, S.; Klein, P.; Urbanova, M.; Dedecek, J. Location of framework Al atoms in the channels of ZSM-5: Effect of the (hydrothermal) synthesis. Chem. Eur. J. 2016, 22, 3937–3941. [Google Scholar] [CrossRef]
- Dedecek, J.; Balgová, V.; Pashkova, V.; Klein, P.; Wichterlová, B. Synthesis of ZSM-5 zeolites with defined distribution of Al atoms in the framework and multinuclear MAS NMR analysis of the control of Al distribution. Chem. Mater. 2012, 24, 3231–3239. [Google Scholar] [CrossRef]
- Gábová, V.; Dědecěk, J.; Čejka, J. Control of Al distribution in ZSM-5 by conditions of zeolite synthesis. Chem. Commun. 2003, 39, 1196–1197. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Chen, J.; Qin, Z.; Li, J.; Wang, P.; Wang, S.; Wang, G.; Dong, M.; Fan, W.; Wang, J. Conversion of methanol to olefins over H-ZSM-5 zeolite: Reaction pathway is related to the framework aluminum siting. ACS Catal. 2016, 6, 7311–7325. [Google Scholar] [CrossRef]
- Hur, Y.G.; Kester, P.M.; Nimlos, C.T.; Cho, Y.; Miller, J.T.; Gounder, R. Influence of tetrapropylammonium and ethylenediamine structure-directing agents on the framework Al distribution in B-Al-MFI zeolites. Ind. Eng. Chem. Res. 2019, 58, 11849–11860. [Google Scholar] [CrossRef]
- Biligetu, T.; Wang, Y.; Nishitoba, T.; Otomo, R.; Park, S.; Mochizuki, H.; Kondo, J.N.; Tatsumi, T.; Yokoi, T. Al distribution and catalytic performance of ZSM-5 zeolites synthesized with various alcohols. J. Catal. 2017, 353, 1–10. [Google Scholar] [CrossRef]
- Kim, S.; Park, G.; Woo, M.H.; Kwak, G.; Kim, S.K. Control of hierarchical structure and framework-Al distribution of ZSM-5 via adjusting crystallization temperature and their effects on methanol conversion. ACS Catal. 2019, 9, 2880–2892. [Google Scholar] [CrossRef]
- Al-Nahari, S.; Dib, E.; Cammarano, C.; Saint-Germes, E.; Massiot, D.; Sarou-Kanian, V.; Alonso, B. Impact of mineralizing agents on aluminum distribution and acidity of ZSM-5 zeolites. Angew. Chem. Int. Ed. 2023, 62, e202217992. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, H.; Xing, A.; Cheng, J. Effect of Al distribution in MFI framework channels on the catalytic performance of ethane and ethylene aromatization. J. Phys. Chem. C 2019, 123, 15637–15647. [Google Scholar] [CrossRef]
- Wang, S.; Wang, P.; Qin, Z.; Chen, Y.; Dong, M.; Li, J.; Zhang, K.; Liu, P.; Wang, J.; Fan, W. Relation of catalytic performance to the aluminum siting of acidic zeolites in the conversion of methanol to olefins, viewed via a comparison between ZSM-5 and ZSM-11. ACS Catal. 2018, 8, 5485–5505. [Google Scholar] [CrossRef]
- Park, S.; Biligetu, T.; Wang, Y.; Nishitoba, T.; Kondo, J.N.; Yokoi, T. Acidic and catalytic properties of ZSM-5 zeolites with different Al distributions. Catal. Today 2018, 303, 64–70. [Google Scholar] [CrossRef]
- Wang, Y.; He, X.; Yang, F.; Su, Z.; Zhu, X. Control of framework aluminum distribution in MFI channels on the catalytic performance in alkylation of benzene with methanol. Ind. Eng. Chem. Res. 2020, 59, 13420–13427. [Google Scholar] [CrossRef]
- Yue, Y.; Gu, L.; Zhou, Y.; Liu, H.; Yuan, P.; Zhu, H.; Bai, Z.; Bao, X. Template-free synthesis and catalytic applications of microporous and hierarchical ZSM-5 zeolites from natural aluminosilicate minerals. Ind. Eng. Chem. Res. 2017, 56, 10069–10077. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, T.; Cao, S.; Wang, J.; Meng, X.; Gong, Y.; Zhang, Z.; Ma, A.; Liu, P. Defective sites in ZSM-5 zeolite synthesized by n-butylamine template facilitating uniform meso-microporosity by alkali-treatment. Micropor. Mesopor. Mater. 2021, 326, 11360–11368. [Google Scholar] [CrossRef]
- Yang, J.; Gong, K.; Miao, D.; Jiao, F.; Pan, X.; Meng, X.; Xiao, F.; Bao, X. Enhanced aromatic selectivity by the sheet-like ZSM-5 in syngas conversion. J. Energy Chem. 2019, 35, 44–48. [Google Scholar] [CrossRef]
- Wu, D.; Yu, X.; Chen, X.; Yu, G.; Zhang, K.; Qiu, M.; Xue, W.; Yang, C.; Liu, Z.; Sun, Y. Morphology-controlled synthesis of H-type MFI zeolites with unique stacked structures through a one-pot solvent-free strategy. ChemSusChem 2019, 12, 3871–3877. [Google Scholar] [CrossRef]
- Soghrati, E.; Ong, T.K.C.; Poh, C.K.; Kawi, S.; Borgna, A. Zeolite-supported nickel phyllosilicate catalyst for C-O hydrogenolysis of cyclic ethers and polyols. Appl. Catal. B 2018, 235, 130–142. [Google Scholar] [CrossRef]
- Shang, S.; Li, W.; Zhou, A.; Zhang, J.; Yang, H.; Zhang, A.; Guo, X. Fe-Substituted Pt/HZSM-48 for superior selectivity of i-C12 in n-dodecane hydroisomerization. Ind. Eng. Chem. Res. 2022, 61, 1056–1065. [Google Scholar] [CrossRef]
- Parry, E.P. An infrared study of pyridine adsorbed on acidic solids. Characterization of surface acidity. J. Catal. 1963, 2, 371–379. [Google Scholar] [CrossRef]
- Shishido, T.; Hattori, H. Hydrogen effects on cumene cracking over zirconium oxide promoted by sulfate ion and platinum. J. Catal. 1996, 161, 194–197. [Google Scholar] [CrossRef]
- Nie, Y.; Shang, S.; Xu, X.; Hua, W.; Yue, Y.; Gao, Z. In2O3-doped Pt/WO3/ZrO2 as a novel efficient catalyst for hydroisomerization of n-heptane. Appl. Catal. A 2012, 433–434, 69–74. [Google Scholar] [CrossRef]
- Meng, L.; Zhu, X.; Mezari, B.; Pestman, R.; Wannapakdee, W.; Hensen, E.J.M. On the role of acidity in bulk and nanosheet [T]MFI (T = Al3+, Ga3+, Fe3+, B3+) zeolites in the methanol-to-hydrocarbons reaction. ChemCatChem 2017, 9, 3942–3954. [Google Scholar] [CrossRef] [PubMed]
- Fyfe, C.A.; Gobbi, G.C.; Kennedy, G.J. Investigation of the conversion (dealumination) of ZSM-5 into silicalite by high-resolution solid-state silicon-29 and aluminum-27 MAS NMR spectroscopy. J. Phys. Chem. 1984, 88, 3248–3253. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, X.; Zhu, L.; Ding, L.; Gao, P.; Wang, X.; Pan, S.; Bian, C.; Meng, X.; Xu, J.; et al. Solvent-free synthesis of zeolites from anhydrous starting raw solids. J. Am. Chem. Soc. 2015, 137, 1052–1055. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, L.; Magusin, P.; Mezari, B.; Hensen, E. On the synthesis of highly acidic nanolayered ZSM-5. J. Catal. 2015, 327, 10–21. [Google Scholar] [CrossRef]
- Petushkov, A.; Yoon, S.; Larsen, S.C. Synthesis of hierarchical nanocrystalline ZSM-5 with controlled particle size and mesoporosity. Micropor. Mesopor. Mater. 2011, 137, 92–100. [Google Scholar] [CrossRef]
- Nagy, J.B.; Gabelica, Z.; Debras, G.; Derouane, E.G.; Gilson, J.P.; Jacobs, P.A. 27Al-n.m.r. characterization of natural and synthetic zeolites. Zeolites 1984, 4, 133–139. [Google Scholar] [CrossRef]
- Deng, F.; Du, Y.; Ye, C.; Wang, J.; Ding, T.; Li, H. Acid sites and hydration behavior of dealuminated zeolite HZSM-5: A high-resolution solid state NMR study. J. Phys. Chem. 1995, 99, 15208–15214. [Google Scholar] [CrossRef]
- Blasco, T.; Corma, A.; Martínez-Triguero, J. Hydrothermal stabilization of ZSM-5 catalytic cracking additives by phosphorus addition. J. Catal. 2006, 237, 267–277. [Google Scholar] [CrossRef]
- Saenluang, K.; Imyen, T.; Wannapakdee, W.; Suttipat, D.; Dugkhuntod, P.; Ketkaew, M.; Thivasasith, A.; Wattanakit, C. Hierarchical nanospherical ZSM-5 nanosheets with uniform Al distribution for alkylation of benzene with ethanol. ACS Appl. Nano Mater. 2020, 3, 3252–3263. [Google Scholar] [CrossRef]
- Li, R.; Chawla, A.; Linares, N.; Sutjianto, J.G.; Chapman, K.W.; Martínez, J.G.; Rimer, J.D. Diverse physical states of amorphous precursors in zeolite synthesis. Ind. Eng. Chem. Res. 2018, 57, 8460–8471. [Google Scholar] [CrossRef]
- Jiang, Y.; Hunger, M.; Wang, W. On the reactivity of surface methoxy species in acidic zeolites. J. Am. Chem. Soc. 2006, 128, 11679–11692. [Google Scholar] [CrossRef]
- Svelle, S.; Rønning, P.O.; Kolboe, S. Kinetic studies of zeolite-catalyzed methylation reactions: 1. Coreaction of [12C]ethene and [13C]methanol. J. Catal. 2004, 224, 115–123. [Google Scholar] [CrossRef]
- Svelle, S.; Kolboe, S.; Swang, O.; Olsbye, U. Methylation of alkenes and methylbenzenes by dimethyl ether or methanol on acidic zeolites. J. Phys. Chem. B 2005, 109, 12874–12878. [Google Scholar] [CrossRef] [PubMed]
- Mehio, N.; Dai, S.; Jiang, D.E. Quantum mechanical basis for kinetic diameters of small gaseous molecules. J. Phys. Chem. A 2014, 118, 1150–1154. [Google Scholar] [CrossRef] [PubMed]
- Emeis, C.A. Determination of integrated molar extinction coefficients for infrared absorption bands of pyridine adsorbed on solid acid catalysts. J. Catal. 1993, 141, 347–354. [Google Scholar] [CrossRef]
- Yang, F.; Zhong, J.; Liu, X.; Zhu, X. A novel catalytic alkylation process of syngas with benzene over the cerium modified platinum supported on HZSM-5 zeolite. Appl. Energy 2018, 226, 22–30. [Google Scholar] [CrossRef]
- Frillette, V.J.; Haag, W.O.; Lago, R.M. Catalysis by crystalline aluminosilicates: Characterization of intermediate pore-size zeolites by the “Constraint Index”. J. Catal. 1981, 67, 218–222. [Google Scholar] [CrossRef]









| Sample | Si/Al | Crystallinity | Surface Area (m2/g) | Pore Volume (cm3/g) | ||
|---|---|---|---|---|---|---|
| Ratio a | (%) | Total | Micro b | Micro b | Meso c | |
| Z5-TPA | 151 | 105 | 398 | 361 | 0.17 | 0.10 |
| Z5-NBA | 149 | 100 | 393 | 358 | 0.17 | 0.10 |
| Sample | Acidity by NH3-TPD (μmol/g) | Acidity by Py-IR (μmol/g) | BAS Distribution (%) a | Conv. of Cumene Cracking (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Weak | Strong | Total | Brønsted | Lewis | Total | Weak | Medium | Strong | ||
| Z5-TPA | 59 | 70 | 129 | 78 | 17 | 95 | 22 | 31 | 47 | 33.9 |
| Z5-NBA | 60 | 70 | 130 | 78 | 17 | 95 | 22 | 31 | 47 | 33.6 |
| Sample | Proportion of Various Peaks (%) | |||
|---|---|---|---|---|
| Q3(0Al), −103 ppm | Q4(1Al), −107 ppm | Q4(0Al), −113 ppm | Q4(0Al), −116 ppm | |
| Z5-TPA | 4.7 | 14.4 | 60.1 | 20.8 |
| Z5-NBA | 4.6 | 14.5 | 60.4 | 20.5 |
| Sample | Aluminum Distribution (%) | CI a | ||
|---|---|---|---|---|
| Straight | Sinusoidal | Intersection | ||
| Z5-TPA | 3.7 | 9.9 | 86.4 | 3.4 |
| Z5-NBA | 5.3 | 25.7 | 69.0 | 5.0 |
| Catalyst | Conversion | Selectivity (%) a | ST+X b | YT+X c | Rate d | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| (%) | T | PX | MX | OX | EB | C9+ | (%) | (%) | (mmol/(g h)) | |
| Z5-TPA | 52.8 | 52.4 | 8.7 | 16.5 | 6.8 | 10.5 | 5.1 | 84.4 | 44.6 | 2445 |
| Z5-NBA | 45.9 | 48.5 | 9.2 | 18.1 | 8.2 | 7.3 | 8.7 | 84.0 | 38.6 | 1510 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, S.; Yang, F.; Tian, C.; Yue, Y.; Zou, W.; Hua, W.; Gao, Z. Selective Alkylation of Benzene with Methanol to Toluene and Xylene over H-ZSM-5 Zeolites: Impact of Framework Al Spatial Distribution. Catalysts 2023, 13, 1295. https://doi.org/10.3390/catal13091295
Ren S, Yang F, Tian C, Yue Y, Zou W, Hua W, Gao Z. Selective Alkylation of Benzene with Methanol to Toluene and Xylene over H-ZSM-5 Zeolites: Impact of Framework Al Spatial Distribution. Catalysts. 2023; 13(9):1295. https://doi.org/10.3390/catal13091295
Chicago/Turabian StyleRen, Shu, Fan Yang, Chao Tian, Yinghong Yue, Wei Zou, Weiming Hua, and Zi Gao. 2023. "Selective Alkylation of Benzene with Methanol to Toluene and Xylene over H-ZSM-5 Zeolites: Impact of Framework Al Spatial Distribution" Catalysts 13, no. 9: 1295. https://doi.org/10.3390/catal13091295