Hard Template-Assisted Trans-Crystallization Synthesis of Hierarchically Porous Cu-SSZ-13 with Enhanced NH3-SCR Performance
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural and Morphological Characterizations of SSZ-13-x Zeolites
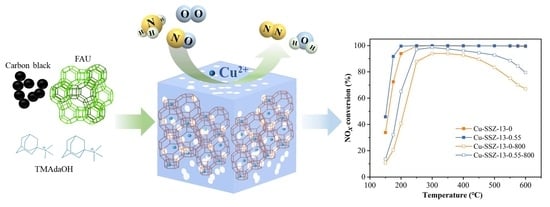
2.2. SCR Performance of Cu-SSZ-13-x Catalysts
2.3. Characterizations of Cu-SSZ-13-0.55 and Cu-SSZ-13-0 Catalysts
2.4. Redox Property and Acidity of Cu-SSZ-13-x Catalysts
2.5. Reaction Kinetics of Cu-SSZ-13-x Catalysts
3. Materials and Methods
3.1. Preparation
3.2. Characterizations
3.3. Catalyst Activity and Reaction Kinetics Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Zhao, H.; Haller, G.; Li, Y. Recent advances in the selective catalytic reduction of NOx with NH3 on Cu-Chabazite catalysts. Appl. Catal. B Environ. 2017, 202, 346–354. [Google Scholar] [CrossRef]
- Wu, T.; Cui, Y.; Lian, A.; Tian, Y.; Li, R.; Liu, X.; Yan, J.; Xue, Y.; Liu, H.; Wu, B. Vehicle emissions of primary air pollutants from 2009 to 2019 and projection for the 14th Five-Year Plan period in Beijing, China. J. Environ. Sci. 2023, 124, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Shan, W.; Yu, Y.; Shan, Y.; Wu, X.; Wu, Y.; Zhang, S.; He, L.; Shuai, S.; Pang, H.; et al. Advances in emission control of diesel vehicles in China. J. Environ. Sci. 2023, 123, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Xu, H.; Liang, X.; Wu, S.; Liu, Z.; Tie, Y.; Li, M.; Yang, D. Research progress on selective catalytic reduction of NOx by NH3 over copper zeolite catalysts at low temperature: Reaction mechanism and catalyst deactivation. Res. Chem. Intermed. 2022, 49, 2321–2357. [Google Scholar] [CrossRef]
- Xin, Y.; Li, Q.; Zhang, Z. Zeolitic materials for deNOx selective catalytic reduction. ChemCatChem 2018, 10, 29–41. [Google Scholar] [CrossRef]
- Kwak, J.; Tonkyn, R.; Kim, D.; Szanyi, J.; Peden, C. Excellent activity and selectivity of Cu-SSZ-13 in the selective catalytic reduction of NOx with NH3. J. Catal. 2010, 275, 187–190. [Google Scholar] [CrossRef]
- Qi, X.; Wang, Y.; Liu, C.; Liu, Q. The challenges and comprehensive evolution of Cu-based zeolite catalysts for SCR systems in diesel vehicles: A review. Catal. Surv. Asia 2022. [Google Scholar] [CrossRef]
- Xie, L.; Liu, F.; Ren, L.; Shi, X.; Xiao, F.; He, H. Excellent performance of one-pot synthesized Cu-SSZ-13 catalyst for the selective catalytic reduction of NOx with NH3. Environ. Sci. Technol. 2014, 48, 566–572. [Google Scholar] [CrossRef]
- Zhao, Z.; Yu, R.; Zhao, R.; Shi, C.; Gies, H.; Xiao, F.; DeVos, D.; Yokoi, T.; Bao, X.; Kolb, U.; et al. Cu-exchanged Al-rich SSZ-13 zeolite from organotemplate-free synthesis as NH3-SCR catalyst: Effects of Na+ ions on the activity and hydrothermal stabiliy. Appl. Catal. B Environ. 2017, 217, 421–428. [Google Scholar] [CrossRef]
- Li, P.; Xin, Y.; Zhang, H.; Yang, F.; Tang, A.; Han, D.; Jia, J.; Wang, J.; Li, Z.; Zhang, Z. Recent progress in performance optimization of Cu-SSZ-13 catalyst for selective catalytic reduction of NOx. Front. Chem. 2022, 10, 1033255. [Google Scholar] [CrossRef]
- Deka, U.; Lezcano-Gonzalez, I.; Weckhuysen, B.; Beale, A. Local environment and nature of Cu active sites in zeolite-based catalysts for the selective catalytic reduction of NOx. ACS Catal. 2013, 3, 413–427. [Google Scholar] [CrossRef]
- Liu, C.; Bi, Y.; Han, J.; Guo, M.; Liu, Q. A Perspective on the relationship between microstructure and performance of Cu-based zeolites for the selective catalytic reduction of NOx. Catal. Surv. Asia 2020, 24, 179–195. [Google Scholar] [CrossRef]
- Gao, F.; Walter, E.; Karp, E.; Luo, J.; Tonkyn, R.; Kwak, J.; Szanyi, J.; Peden, C. Structure-activity relationships in NH3-SCR over Cu-SSZ-13 as probed by reaction kinetics and EPR studies. J. Catal. 2013, 300, 20–29. [Google Scholar] [CrossRef]
- Kumar, M.; Luo, H.; Roman-Leshkov, Y.; Rimer, J. SSZ-13 crystallization by particle attachment and deterministic pathways to crystal size control. J. Am. Chem. Soc. 2015, 137, 13007–13017. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhao, X.; Mao, W.; Chen, H.; Han, L.; Zhu, K.; Zhou, X. Pickering emulsion mediated crystallization of hierarchical zeolite SSZ-13 with enhanced NH3 selective catalytic reduction performance. Microporous Mesoporous Mater. 2019, 285, 202–214. [Google Scholar] [CrossRef]
- Koohsaryan, E.; Anbia, M. Nanosized and hierarchical zeolites: A short review. Chin. J. Catal. 2016, 37, 447–467. [Google Scholar] [CrossRef]
- Chen, L.; Sun, M.; Wang, Z.; Yang, W.; Xie, Z.; Su, B. Hierarchically structured zeolites: From Design to application. Chem. Rev. 2020, 120, 11194–11294. [Google Scholar] [CrossRef]
- Liu, Z.; Hua, Y.; Wang, J.; Dong, X.; Tian, Q.; Han, Y. Recent progress in the direct synthesis of hierarchical zeolites: Synthetic strategies and characterization methods. Mater. Chem. Front. 2017, 1, 2195–2212. [Google Scholar] [CrossRef]
- Chen, L.; Li, X.; Rooke, J.C.; Zhang, Y.; Yang, X.; Tang, Y.; Xiao, F.; Su, B. Hierarchically structured zeolites: Synthesis, mass transport properties and applications. J. Mater. Chem. 2012, 22, 17381–17403. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X.; Pan, M.; Yang, X.; Liu, Y.; Sun, J.; Wan, Q.; Zheng, J.; Wang, Y.; Ma, J.; et al. Interfacial effects between carbon nanotube templates and precursors on fabricating a wall-crystallized hierarchical pore system in zeolite crystals. J. Mater. Sci. 2020, 55, 10412–10426. [Google Scholar] [CrossRef]
- Wang, H.; Du, G.; Jia, J.; Chen, S.; Su, Z.; Chen, R.; Chen, T. Hierarchically porous zeolites synthesized with carbon materials as templates. Front. Chem. Sci. Eng. 2021, 15, 1444–1461. [Google Scholar] [CrossRef]
- Wu, L.; Degirmenci, V.; Magusin, P.; Szyja, B.; Hensen, E. Dual template synthesis of a highly mesoporous SSZ-13 zeolite with improved stability in the methanol-to-olefins reaction. Chem. Commun. 2012, 48, 9492–9494. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Rohling, R.; Filonenko, G.; Mezari, B.; Hofmann, J.; Asahina, S.; Hensen, E. Synthesis of hierarchical zeolites using an inexpensive mono-quaternary ammonium surfactant as mesoporogen. Chem. Commun. 2014, 50, 14658–14661. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Z.; Qu, H.; Yuan, J.; Yu, M.; Xie, H.; Zhong, Q. Dual-template assembled hierarchical Cu-SSZ-13: Morphology evolution, crystal growth and stable high-temperature selective catalytic reduction performance. CrystEngComm 2020, 22, 7036–7045. [Google Scholar] [CrossRef]
- Peng, C.; Yan, R.; Mi, Y.; Li, G.; Zheng, Y.; Luo, Y.; Liang, J.; Liu, W.; Li, Z.; Wu, D.; et al. Toward rational design of a novel hierarchical porous Cu-SSZ-13 catalyst with boosted low-temperature NO reduction performance. J. Catal. 2021, 401, 309–320. [Google Scholar] [CrossRef]
- Serrano, D.; Escola, J.; Pizarro, P. Synthesis strategies in the search for hierarchical zeolites. Chem. Soc. Rev. 2013, 42, 4004–4035. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Song, Y.; Li, Y. Creating hierarchical pores in zeolite catalysts. Trends Chem. 2019, 1, 601–611. [Google Scholar] [CrossRef]
- Liang, J.; Tao, J.; Mi, Y.; Liu, W.; Wang, Z.; Li, Z.; Wu, D.; Wu, P.; Peng, H. Unraveling the boosting low-temperature performance of ordered mesoporous Cu-SSZ-13 catalyst for NOx reduction. Chem. Eng. J. 2021, 409, 128238. [Google Scholar] [CrossRef]
- Li, D.; Chen, Y.; Hu, J.; Deng, B.; Cheng, X.; Zhang, Y. Synthesis of hierarchical chabazite zeolite via interzeolite transformation of coke-containing spent MFI. Appl. Catal. B Environ. 2020, 270, 118881. [Google Scholar] [CrossRef]
- Itakura, M.; Goto, I.; Takahashi, A.; Fujitani, T.; Ide, Y.; Sadakane, M.; Sano, T. Synthesis of high-silica CHA type zeolite by interzeolite conversion of FAU type zeolite in the presence of seed crystals. Microporous Mesoporous Mater. 2011, 144, 91–96. [Google Scholar] [CrossRef]
- Liang, J.; Mi, Y.; Song, G.; Peng, H.; Li, Y.; Yan, R.; Liu, W.; Wang, Z.; Wu, P.; Liu, F. Environmental benign synthesis of nano-SSZ-13 via FAU trans-crystallization: Enhanced NH3-SCR performance on Cu-SSZ-13 with nano-size effect. J. Hazard. Mater. 2020, 398, 122986. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chang, H.; You, Y.; Shi, C.; Li, J. Excellent activity and selectivity of one-pot synthesized Cu-SSZ-13 catalyst in the selective catalytic oxidation of ammonia to nitrogen. Environ. Sci. Technol. 2018, 52, 4802–4808. [Google Scholar] [CrossRef]
- Zhang, N.; Xin, Y.; Li, Q.; Ma, X.; Qi, Y.; Zheng, L.; Zhang, Z. Ion exchange of one-pot synthesized Cu-SAPO-44 with NH4NO3 to promote Cu dispersion and activity for selective catalytic reduction of NOx with NH3. Catalysts 2019, 9, 882. [Google Scholar] [CrossRef]
- Zhang, T.; Qiu, F.; Li, J. Design and synthesis of core-shell structured meso-Cu-SSZ-13@mesoporous aluminosilicate catalyst for SCR of NO with NH3: Enhancement of activity, hydrothermal stability and propene poisoning resistance. Appl. Catal. B Environ. 2016, 195, 48–58. [Google Scholar] [CrossRef]
- Sunil Kumar, S.; Alphin, M.S.; Senthil Kumar, P.; Raja, S. A review on zeolite catalyst for deNOx performance in ammonia-selective catalytic reduction. Fuel 2023, 334, 126828. [Google Scholar] [CrossRef]
- Song, J.; Wang, Y.; Walter, E.; Washton, N.; Mei, D.; Kovarik, L.; Engelhard, M.; Prodinger, S.; Wang, Y.; Peden, C.; et al. Toward rational design of Cu/SSZ-13 selective catalytic reduction catalysts: Implications from atomic-level understanding of hydrothermal stability. ACS Catal. 2017, 7, 8214–8227. [Google Scholar] [CrossRef]
- Xiong, W.; Liu, L.; Guo, A.; Chen, D.; Shan, Y.; Fu, M.; Wu, J.; Ye, D.; Chen, P. Economical and sustainable synthesis of small-pore chabazite catalysts for NOx abatement by recycling organic structure-directing agents. Environ. Sci. Technol. 2023, 57, 655–665. [Google Scholar] [CrossRef]
- Dědeček, J.; Sklenak, S.; Li, C.; Gao, F.; Brus, J.; Zhu, Q.; Tatsumi, T. Effect of Al/Si substitutions and silanol nests on the local geometry of Si and Al framework sites in silicone-rich zeolites: A combined high resolution 27Al and 29Si NMR and density functional theory/molecular mechanics study. J. Phys. Chem. C 2009, 113, 14454–14466. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, X.; Shan, Y.; Du, J.; Liu, K.; He, H. Hydrothermal stability enhancement of Al-rich Cu-SSZ-13 for NH3 selective catalytic reduction reaction by ion exchange with cerium and samarium. Ind. Eng. Chem. Res. 2020, 59, 6416–6423. [Google Scholar] [CrossRef]
- Prodinger, S.; Derewinski, M.; Wang, Y.; Washton, N.; Walter, E.; Szanyi, J.; Gao, F.; Wang, Y.; Peden, C. Sub-micron Cu/SSZ-13: Synthesis and application as selective catalytic reduction (SCR) catalysts. Appl. Catal. B Environ. 2017, 201, 461–469. [Google Scholar] [CrossRef]
- Di Iorio, J.; Li, S.; Jones, C.; Nimlos, C.; Wang, Y.; Kunkes, E.; Vattipalli, V.; Prasad, S.; Moini, A.; Schneider, W.; et al. Cooperative and competitive occlusion of organic and inorganic structure-directing agents within chabazite zeolites influences their aluminum arrangement. J. Am. Chem. Soc. 2020, 142, 4807–4819. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, J.; Xue, W.; Wang, S.; Han, J.; Wei, Y.; Mei, D.; Li, Y.; Yu, J. Unveiling secondary-ion-promoted catalytic properties of Cu-SSZ-13 zeolites for selective catalytic reduction of NOx. J. Am. Chem. Soc. 2022, 144, 12816–12824. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Peng, Z.; Chen, Y.; Bao, W.; Chang, L.; Feng, G. In-situ hydrothermal synthesis of Cu-SSZ-13/cordierite for the catalytic removal of NOx from diesel vehicles by NH3. Chem. Eng. J. 2015, 263, 9–19. [Google Scholar] [CrossRef]
- Wan, J.; Chen, J.; Zhao, R. One-pot synthesis of Fe/Cu-SSZ-13 catalyst and its highly efficient performance for the selective catalytic reduction of nitrogen oxide with ammonia. J. Environ. Sci. 2021, 100, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhao, W.; Wei, Y.; Han, J.; Li, J.; Sun, C.; Mei, D.; Yu, J. La ions-enhanced NH3-SCR performance over Cu-SSZ-13 catalysts. Nano Res. 2023. [Google Scholar] [CrossRef]
- Xu, R.; Wang, Z.; Liu, N.; Dai, C.; Zhang, J.; Chen, B. Understanding Zn functions on hydrothermal stability in a one-pot-synthesized Cu&Zn-SSZ-13 catalyst for NH3 selective catalytic reduction. ACS Catal. 2020, 10, 6197–6212. [Google Scholar]
- Kim, Y.; Lee, J.; Min, K.; Hong, S.; Nam, I.; Cho, B. Hydrothermal stability of CuSSZ13 for reducing NOx by NH3. J. Catal. 2014, 311, 447–457. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, R.; Zhou, R. A new insight into active Cu2+ species properties in one-pot synthesized Cu-SSZ-13 catalysts for NOx reduction by NH3. ChemCatChem 2018, 10, 5182–5189. [Google Scholar] [CrossRef]
- Gao, F.; Szanyi, J. On the hydrothermal stability of Cu/SSZ-13 SCR catalysts. Appl. Catal. A Gen. 2018, 560, 185–194. [Google Scholar] [CrossRef]
- Du, J.; Shan, Y.; Sun, Y.; Gao, M.; Liu, Z.; Shi, Y.; Yu, Y.; He, H. Unexpected increase in low-temperature NH3-SCR catalytic activity over Cu-SSZ-39 after hydrothermal aging. Appl. Catal. B Environ. 2021, 294, 120237. [Google Scholar] [CrossRef]
- Tan, W.; Wang, C.; Yu, S.; Li, Y.; Xie, S.; Gao, F.; Dong, L.; Liu, F. Revealing the effect of paired redox-acid sites on metal oxide catalysts for efficient NOx removal by NH3-SCR. J. Hazard. Mater. 2021, 416, 125826. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Cheng, Y.; Cavataio, G.; McCabe, R.; Fu, L. In situ DRIFTS and temperature-programmed technology study on NH3-SCR of NO over Cu-SSZ-13 and Cu-SAPO-34 catalysts. Appl. Catal. B Environ. 2014, 156–157, 428–437. [Google Scholar] [CrossRef]
- Wang, D.; Gao, F.; Peden, C.; Li, J.; Kamasamudram, K.; Epling, W. Selective catalytic reduction of NOx with NH3 over a Cu-SSZ-13 catalyst prepared by a solid-state ion-exchange method. ChemCatChem 2014, 6, 1579–1583. [Google Scholar] [CrossRef]
- Gao, F.; Wang, Y.; Washton, N.; Kollár, M.; Szanyi, J.; Peden, C. Effects of alkali and alkaline earth cocations on the activity and hydrothermal stability of Cu/SSZ-13 NH3-SCR Catalysts. ACS Catal. 2015, 5, 6780–6791. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, X.; Cheng, S.; Cao, L.; Liu, L.; Xu, Y.; Liu, J.; Ran, R.; Si, Z.; Weng, D. Relationships between copper speciation and Brønsted acidity evolution over Cu-SSZ-13 during hydrothermal aging. Appl. Catal. A Gen. 2020, 602, 117650. [Google Scholar] [CrossRef]
- Jiang, H.; Guan, B.; Lin, H.; Huang, Z. Cu/SSZ-13 zeolites prepared by in situ hydrothermal synthesis method as NH3-SCR catalysts: Influence of the Si/Al ratio on the activity and hydrothermal properties. Fuel 2019, 255, 115587. [Google Scholar] [CrossRef]
- Wang, J.; Fan, D.; Yu, T.; Wang, J.; Hao, T.; Hu, X.; Shen, M.; Li, W. Improvement of low-temperature hydrothermal stability of Cu/SAPO-34 catalysts by Cu2+ species. J. Catal. 2015, 322, 84–90. [Google Scholar] [CrossRef]
- Han, S.; Ye, Q.; Cheng, S.; Kang, T.; Dai, H. Effect of the hydrothermal aging temperature and Cu/Al ratio on the hydrothermal stability of CuSSZ-13 catalysts for NH3-SCR. Catal. Sci. Technol. 2017, 7, 703–717. [Google Scholar] [CrossRef]
- Deng, D.; Deng, S.; He, D.; Wang, Z.; Chen, Z.; Ji, Y.; Yan, G.; Hou, G.; Li, L.; He, H. A comparative study of hydrothermal aging effect on cerium and lanthanum doped Cu/SSZ-13 catalysts for NH3-SCR. J. Rare Earths 2021, 39, 969–978. [Google Scholar] [CrossRef]
- Gao, Q.; Ye, Q.; Han, S.; Cheng, S.; Kang, T.; Dai, H. Effect of Ce doping on hydrothermal stability of Cu-SAPO-18 in the selective catalytic reduction of NO with NH3. Catal. Surv. Asia 2020, 24, 134–142. [Google Scholar] [CrossRef]
- Jiang, H.; Guan, B.; Peng, X.; Zhan, R.; Lin, H.; Huang, Z. Influence of synthesis method on catalytic properties and hydrothermal stability of Cu/SSZ-13 for NH3-SCR reaction. Chem. Eng. J. 2020, 379, 122358. [Google Scholar] [CrossRef]
- Wang, J.; Shao, L.; Wang, C.; Wang, J.; Shen, M.; Li, W. Controllable preparation of various crystal size and nature of intra-crystalline diffusion in Cu/SSZ-13 NH3-SCR catalysts. J. Catal. 2018, 367, 221–228. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, Y.; Li, J.; Groden, K.; McEwen, J.; Walter, E.; Chen, Y.; Wang, Y.; Gao, F. Probing active-site relocation in Cu/SSZ-13 SCR catalysts during hydrothermal aging by in situ EPR spectroscopy, kinetics studies, and DFT calculations. ACS Catal. 2020, 10, 9410–9419. [Google Scholar] [CrossRef]
- Fu, Y.; He, G.; Shan, Y.; Du, J. Promotion of the selective catalytic reduction of NOx with NH3 over microporous Cu-SSZ-13 by H2O and OH groups at low temperatures: A density functional theory study. Catal. Sci. Technol. 2022, 12, 5524–5532. [Google Scholar] [CrossRef]
- Xin, Y.; Wang, X.; Li, Q.; Ma, X.; Qi, Y.; Zheng, L.; Anderson, J.; Zhang, Z. The potential of Cu-SAPO-44 in the selective catalytic reduction of NOx with NH3. ChemCatChem 2016, 8, 3740–3745. [Google Scholar] [CrossRef]












| Sample | Surface Areas (m2∙g−1) | Pore Volume (cm3∙g−1) | Si/Al Ratio a | Average Grain Size a (nm) |
|---|---|---|---|---|
| SSZ-13-0 | 590.0 | 0.23 | 5.6 | 910 |
| SSZ-13-0.33 | 561.5 | 0.30 | 6.0 | 520 |
| SSZ-13-0.55 | 608.9 | 0.37 | 6.1 | 470 |
| SSZ-13-0.77 | 575.2 | 0.35 | 6.4 | 419 |
| Sample | Surface Areas (m2∙g−1) | Pore Volume (cm3∙g−1) | Si/Al Ratio a | Cu Loading a (wt.%) | Average Grain Size a (nm) |
|---|---|---|---|---|---|
| Cu-SSZ-13-0 | 586.3 | 0.26 | 5.3 | 3.8 | 961 |
| Cu-SSZ-13-0.55 | 607.2 | 0.29 | 6.1 | 4.1 | 449 |
| Cu-SSZ-13-0-800 | 559.4 | 0.26 | 5.7 | 3.9 | 591 |
| Cu-SSZ-13-0.55-800 | 564.4 | 0.27 | 6.0 | 4.2 | 470 |
| Sample | Si/Al Ratio a | Proportion of Q4(nAl) (%) | ||
|---|---|---|---|---|
| Q4(0Al) | Q4(1Al) | Q4(2Al) | ||
| Cu-SSZ-13-0 | 5.9 | 38 | 56 | 6 |
| Cu-SSZ-13-0.55 | 6.5 | 77 | 19 | 4 |
| Cu-SSZ-13-0-800 | 15.2 | 44 | 50 | 6 |
| Cu-SSZ-13-0.55-800 | 16.5 | 78 | 20 | 2 |
| Sample | Tera-Coordinated Al (%) | Penta-Coordinated Al (%) | Hexa-Coordinated Al (%) |
|---|---|---|---|
| Cu-SSZ-13-0 | 88 | 0 | 12 |
| Cu-SSZ-13-0.55 | 90 | 0 | 10 |
| Cu-SSZ-13-0-800 | 71 | 18 | 11 |
| Cu-SSZ-13-0.55-800 | 83 | 9 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Xin, Y.; Zhu, X.; Tang, A.; Yu, L.; Han, D.; Jia, J.; Lu, Y.; Zhang, Z. Hard Template-Assisted Trans-Crystallization Synthesis of Hierarchically Porous Cu-SSZ-13 with Enhanced NH3-SCR Performance. Catalysts 2023, 13, 1217. https://doi.org/10.3390/catal13081217
Yang F, Xin Y, Zhu X, Tang A, Yu L, Han D, Jia J, Lu Y, Zhang Z. Hard Template-Assisted Trans-Crystallization Synthesis of Hierarchically Porous Cu-SSZ-13 with Enhanced NH3-SCR Performance. Catalysts. 2023; 13(8):1217. https://doi.org/10.3390/catal13081217
Chicago/Turabian StyleYang, Fuzhen, Ying Xin, Xiaoli Zhu, Ahui Tang, Long Yu, Dongxu Han, Junxiu Jia, Yaning Lu, and Zhaoliang Zhang. 2023. "Hard Template-Assisted Trans-Crystallization Synthesis of Hierarchically Porous Cu-SSZ-13 with Enhanced NH3-SCR Performance" Catalysts 13, no. 8: 1217. https://doi.org/10.3390/catal13081217