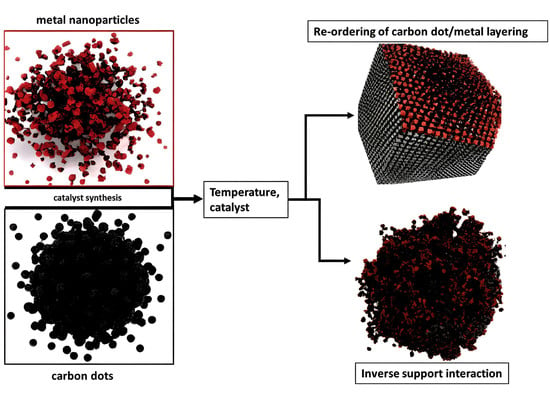
The Behavior of Carbon Dots in Catalytic Reactions
Abstract
:1. Introduction
2. The Structure of Carbon Dots (CDs)
2.1. Types of CDs
2.2. Surface Properties of CDs
2.3. Synthesis of Carbon Dots and Their Application as Reducing Agents
3. Carbon Dots in Catalysis
3.1. Applications of Carbon Dots-Based Catalysts
- -
- A limitation of using carbon dots in catalysis
3.2. Metals Doped into CDs and Metals Supported on CDs as Catalysts
3.2.1. High-Temperature Reactions of Metals Supported on CDs
3.2.2. Low-Temperature Reactions of Metals Supported on/in CDs
- -
- Examples where the CD structure is retained
- -
- Examples where the CD structure is lost
3.2.3. Post Reduction of CDs as Metal Supports
4. Techniques to Evaluate CD and CD/Metal Transformations
- -
- Summary of CD conversion reactions
- (i)
- CDs can react with each other, through physical or chemical bonds, to create CD assemblies. These assemblies could then be used as carbocatalysts.
- (ii)
- CDs can react at temperatures of ca. 200 °C to convert to carbon sheet-like structures and the graphicity of the sheets has been shown to increase with temperature.
- (iii)
- CDs can lose many of their surface functional groups at temperatures between 200 and 450 °C, and this will impact on their chemical properties.
- (iv)
- In the presence of easily reducible metal oxide catalysts, CDs can be converted to carbon sheet-like materials at temperatures < 100 °C. The ability to achieve this will be dependent on the metal reducibility, with directions given by Ellingham diagrams.
- (v)
- Ellingham diagrams have been determined for bulk metals, but metal nanoparticles are expected to have similar temperature versus free energy values.
- (vi)
- The chemical process by which a CD is converted to a sheet-like carbon is not known.
5. Future Directions
- (i)
- Is the CD conversion dependent on the type of CD?
- (ii)
- Is the conversion dependent on the type of functional group?
- (iii)
- How does the conversion from a CD to other dimensional carbon structures take place?
- (iv)
- The morphology conversion reaction is temperature dependent. What are the temperature ranges in which the CD/metal acts as a catalyst with/without a morphology change?
- (v)
- Is there a carbon layer thickness in which a carbon will only be oxidized by a metal but not undergo a change in morphology? For example, in the presence of Co it is clear that CSs (d = 400 nm) only undergo carbon removal while CDs (d < 10 nm) undergo a morphology change.
- (vi)
- In situ studies could provide valuable information on the CD conversion process.
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Coville, N.J.; Mhlanga, S.D.; Nxumalo, E.N.; Shaikjee, A. A review of shaped carbon nanomaterials. S. Afr. J. Sci. 2011, 107, 44–58. [Google Scholar] [CrossRef]
- Nasir, S.; Hussein, M.Z.; Zainal, Z.; Yusof, N.A. Carbon-Based Nanomaterials/Allotropes: A Glimpse of Their Synthesis, Properties and Some Applications. Materials 2018, 11, 295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karthik, P.S.; Himaja, A.L.; Singh, S.P. Carbon-allotropes: Synthesis methods, applications and future perspectives. Carbon Lett. 2014, 15, 219–237. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Kumar, V.; Huczko, A.; Oraon, R.; De Adhikari, A.; Nayak, G.C. Magical Allotropes of Carbon: Prospects and Applications. Crit. Rev. Solid State Mater. Sci. 2016, 41, 257–317. [Google Scholar] [CrossRef]
- Rodriguez-reinoso, F. The role of carbon materials in heterogeneous catalysis. Carbon 1998, 36, 159–175. [Google Scholar] [CrossRef]
- Berseth, P.A.; Harter, A.G.; Zidan, R.; Blomqvist, A.; Araújo, C.M.; Scheicher, R.H.; Ahuja, R.; Jena, P. Carbon nanomaterials as catalysts for hydrogen uptake and release in NaAIH4. Nano Lett 2009, 9, 1501–1505. [Google Scholar] [CrossRef] [Green Version]
- Védrine, J.C. Heterogeneous catalysis on metal oxides. Catalysts 2017, 7, 341. [Google Scholar] [CrossRef] [Green Version]
- Tauster, S.J.; Fung, S.C.; Baker, R.T.K.; Horsley, J.A. Strong Interactions in Supported-Metal Catalysts. Science 1981, 211, 1121–1125. [Google Scholar] [CrossRef] [Green Version]
- Iglesia, E. Design, synthesis, and use of cobalt-based Fischer-Tropsch synthesis catalysts. Appl. Catal. A Gen. 1997, 161, 59–78. [Google Scholar] [CrossRef]
- Macheli, L.; Carleschi, E.; Doyle, B.P.; Leteba, G.; van Steen, E. Tuning catalytic performance in Fischer-Tropsch synthesis by metal-support interactions. J. Catal. 2021, 395, 70–79. [Google Scholar] [CrossRef]
- Tsakoumis, N.E.; Rønning, M.; Borg, Ø.; Rytter, E.; Holmen, A. Deactivation of cobalt based Fischer—Tropsch catalysts: A review. Catal. Today 2010, 154, 162–182. [Google Scholar] [CrossRef]
- Xiong, H.; Jewell, L.L.; Coville, N.J. Shaped Carbons As Supports for the Catalytic Conversion of Syngas to Clean Fuels. ACS Catal. 2015, 5, 2640–2658. [Google Scholar] [CrossRef]
- Macheli, L.; Roy, A.; Carleschi, E.; Doyle, B.P.; van Steen, E. Surface modification of Co3O4 nanocubes with TEOS for an improved performance in the Fischer-Tropsch synthesis. Catal. Today 2020, 343, 176–182. [Google Scholar] [CrossRef]
- Nguyen, B.H.; Nguyen, V.H.; Vu, D.L. Photocatalytic composites based on titania nanoparticles and carbon nanomaterials. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015, 6, 033001. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Hu, L.; Li, J.; Wei, Z. Enhanced stability of Pt nanoparticle electrocatalysts for fuel cells. Nano Res. 2015, 8, 418–440. [Google Scholar] [CrossRef]
- Baker, S.N.; Baker, G.A. Luminescent Carbon Nanodots: Emergent Nanolights. Angew. Chem. Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef]
- Kang, Z.; Lee, S.T. Carbon dots: Advances in nanocarbon applications. Nanoscale 2019, 11, 19214–19224. [Google Scholar] [CrossRef]
- Kurian, M.; Paul, A. Recent trends in the use of green sources for carbon dot synthesis—A short review. Carbon Trends 2021, 3, 100032. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Li, Y.; Feng, Y.; Feng, W. Frontiers in carbon dots: Design, properties and applications. Mater. Chem. Front. 2019, 3, 2571–2601. [Google Scholar] [CrossRef]
- Sharma, A.; Das, J. Small molecules derived carbon dots: Synthesis and applications in sensing, catalysis, imaging, and biomedicine. J. Nanobiotechnol. 2019, 17, 92. [Google Scholar] [CrossRef] [Green Version]
- Yuan, F.; Yuan, T.; Sui, L.; Wang, Z.; Xi, Z.; Li, Y.; Li, X.; Fan, L.; Chen, A.; Jin, M. Engineering triangular carbon quantum dots with unprecedented narrow bandwidth emission for multicolored LEDs. Nat. Commun. 2018, 9, 2249. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.B.; Liu, M.L.; Huang, C.Z. Carbon dot-based composites for catalytic applications. Green Chem. 2020, 22, 4034–4054. [Google Scholar] [CrossRef]
- Manjupriya, R.; Roopan, S.M. Carbon dots-based catalyst for various organic transformations. J. Mater. Sci. 2021, 56, 17369–17410. [Google Scholar] [CrossRef]
- Rosso, C.; Filippini, G.; Prato, M. Carbon Dots as Nano-Organocatalysts for Synthetic Applications. ACS Catal. 2020, 10, 8090–8105. [Google Scholar] [CrossRef]
- Dey, D.; Bhattacharya, T.; Majumdar, B.; Mandani, S.; Sharma, B.; Sarma, T.K. Carbon dot reduced palladium nanoparticles as active catalysts for carbon–carbon bond formation. Dalton Trans. 2013, 42, 13821. [Google Scholar] [CrossRef]
- Jana, J.; Chung, J.S.; Hur, S.H. Carbon dot supported bimetallic nanocomposite for the hydrogen evolution reaction. J. Alloys Compd. 2021, 859, 157895. [Google Scholar] [CrossRef]
- Mokoloko, L.L.; Forbes, R.P.; Coville, N.J. The Transformation of 0-D Carbon Dots into 1-, 2- and 3-D Carbon Allotropes: A Minireview. Nanomaterials 2022, 12, 2515. [Google Scholar] [CrossRef]
- Mokoloko, L.L.; Forbes, R.P.; Coville, N.J. Use of carbon dots-derived graphene-like sheets on the autoreduction of cobalt nanoparticles for Fischer-Tropsch synthesis: A limitation on the use of carbon supports. Presented at the Gauteng Catalysis Seminar, University of the Witwatersrand, Gauteng, South Africa, 2022. To be published.
- Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Evolution and Synthesis of Carbon Dots: From Carbon Dots to Carbonized Polymer Dots. Adv. Sci. 2019, 6, 1901316. [Google Scholar] [CrossRef]
- Sarkar, S.; Banerjee, D.; Ghorai, U.; Das, N.; Chattopadhyay, K. Size dependent photoluminescence property of hydrothermally synthesized crystalline carbon quantum dots. J. Lumin. 2016, 178, 314–323. [Google Scholar] [CrossRef]
- Li, L.; Dong, T. Photoluminescence tuning in carbon dots: Surface passivation or/and functionalization, heteroatom doping. J. Mater. Chem. C 2018, 6, 7944–7970. [Google Scholar] [CrossRef]
- Guo, R.; Li, L.; Wang, B.; Xiang, Y.; Zou, G.; Zhu, Y.; Hou, H.; Ji, X. Functionalized carbon dots for advanced batteries. Energy Storage Mater. 2021, 37, 8–39. [Google Scholar] [CrossRef]
- Ai, L.; Shi, R.; Yang, J.; Zhang, K.; Zhang, T.; Lu, S. Efficient Combination of G-C3N4 and CDs for Enhanced Photocatalytic Performance: A Review of Synthesis, Strategies, and Applications. Small 2021, 17, 2007523. [Google Scholar] [CrossRef] [PubMed]
- Mongwe, T.H.; Coville, N.J.; Maubane-Nkadimeng, M.S. Synthesis of onion-like carbon nanoparticles by flame pyrolysis. In Nanoscience; SPR-Nanoscience, Royal Society of Chemistry: London, UK, 2022; pp. 198–220. [Google Scholar]
- Li, X.; Fu, Y.; Zhao, S.; Xiao, J.; Lan, M.; Wang, B.; Zhang, K.; Song, X.; Zeng, L. Metal ions-doped carbon dots: Synthesis, properties, and applications. Chem. Eng. J. 2022, 430, 133101. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, W.; Leblanc, R.M. Structure-Property-Activity Relationships in Carbon Dots. J. Phys. Chem. B 2022, 126, 10777–10796. [Google Scholar] [CrossRef]
- Ding, H.; Li, X.H.; Chen, X.B.; Wei, J.S.; Li, X.B.; Xiong, H.M. Surface states of carbon dots and their influences on luminescence. J. Appl. Phys. 2020, 127, 231101. [Google Scholar] [CrossRef]
- Yan, F.; Jiang, Y.; Sun, X.; Bai, Z.; Zhang, Y.; Zhou, X. Surface modification and chemical functionalization of carbon dots: A review. Microchim. Acta 2018, 185, 424. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Lu, D.; Wang, L.-P.; Zhang, Q.; Hao, J.; Li, G.; Li, H. Hydrophobic Carbon Dots from Aliphatic Compounds with One Terminal Functional Group. J. Phys. Chem. C 2019, 123, 22447–22456. [Google Scholar] [CrossRef]
- Yin, K.; Lu, D.; Tian, W.; Zhang, R.; Yu, H.; Gorecka, E.; Pociecha, D.; Godbert, N.; Hao, J.; Li, H. Ordered Structures of Alkylated Carbon Dots and Their Applications in Nonlinear Optics. J. Mater. Chem. C 2020, 8, 8980–8991. [Google Scholar] [CrossRef]
- Xie, Z.; Sun, X.; Jiao, J.; Xin, X. Ionic liquid-functionalized carbon quantum dots as fluorescent probes for sensitive and selective detection of iron ion and ascorbic acid. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 38–44. [Google Scholar] [CrossRef]
- Sun, X.; Yin, K.; Liu, B.; Zhou, S.; Cao, J.; Zhang, G.; Li, H. Carbon quantum dots in ionic liquids: A new generation of environmentally benign photoluminescent inks. J. Mater. Chem. C Mater. 2017, 5, 4951–4958. [Google Scholar] [CrossRef]
- Yin, K.; Feng, N.; Godbert, N.; Xing, P.; Li, H. Self-Assembly of Cholesteryl Carbon Dots with Circularly Polarized Luminescence in Solution and Solvent-Free Phases. J. Phys. Chem. Lett. 2023, 14, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wu, S.; Hui, L.; Zhao, Y.; Ye, J.; Tan, Z.; Zeng, W.; Tao, Z.; Yang, L.; Zhu, Y. Assembling carbon quantum dots to a layered carbon for high-density supercapacitor electrodes. Sci. Rep. 2016, 6, 19028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Mintz, K.J.; Cheng, L.; Chen, J.; Ferreira, B.C.; Hettiarachchi, S.D.; Liyanage, P.Y.; Seven, E.S.; Miloserdov, N.; Pandey, R.R.; et al. Direct conjugation of distinct carbon dots as Lego-like building blocks for the assembly of versatile drug nanocarriers. J. Colloid Interface Sci. 2020, 576, 412–425. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, J.; Miloserdov, N.; Zhang, W.; Mintz, K.J.; Ferreira, B.C.L.B.; Micic, M.; Li, S.; Peng, Z.; Leblanc, R.M. Versatile drug nanocarrier assembly via conjugation of distinct carbon dots. Mor. J. Chem. 2020, 8, 994–1007. [Google Scholar]
- Sun, X.; Wang, H.; Qi, J.; Zhou, S.; Li, H. Supramolecular self-assemblies formed by co-assembly of carbon dots and tannic acid. Dyes Pigments 2021, 190, 109287. [Google Scholar] [CrossRef]
- Jana, P.; Dev, A. Carbon quantum dots: A promising nanocarrier for bioimaging and drug delivery in cancer. Mater. Today Commun. 2022, 32, 104068. [Google Scholar] [CrossRef]
- Roy, P.; Chen, P.; Periasamy, A.P.; Chen, Y.; Chang, H. Photoluminescent carbon nanodots: Synthesis, physicochemical properties and analytical applications. Mater. Today 2015, 18, 447–458. [Google Scholar] [CrossRef]
- Batool, M.; Junaid, H.M.; Tabassumb, S.; Kanwala, F.; Abid, K.; Fatima, Z.; Shah, A.T. Metal Ion Detection by Carbon Dots—A Review. Crit. Rev. Anal. Chem. 2022, 52, 756–767. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, X.; Long, Y.; Wang, X.; Zhang, H.; Zhu, R.; Liang, L.; Teng, P.; Zheng, H. Hollow luminescent carbon dots for drug delivery. Carbon 2013, 59, 192–199. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, F.; Guo, B.; Gao, N.; Zhang, X. Synthesis of N-doped Micropore carbon quantum dots with high quantum yield and dual-wavelength photoluminescence emission from biomass for cellular imaging. Nanomaterials 2019, 9, 495. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Zhang, L.; Zhang, J.; Su, Y. Recent Insight in Transition Metal Anchored on Nitrogen-Doped Carbon Catalysts: Preparation and Catalysis Application. Electrochem 2022, 3, 520–537. [Google Scholar] [CrossRef]
- Xiong, H.; Motchelaho, M.A.M.; Moyo, M.; Jewell, L.L.; Coville, N.J. Correlating the preparation and performance of cobalt catalysts supported on carbon nanotubes and carbon spheres in the Fischer-Tropsch synthesis. J. Catal. 2011, 278, 26–40. [Google Scholar] [CrossRef]
- Kumar, K.S.; Pittala, S.; Sanyadanam, S.; Paik, P. A new single/few-layered graphene oxide with a high dielectric constant of 106: Contribution of defects and functional groups. RSC Adv. 2015, 5, 14768–14779. [Google Scholar] [CrossRef]
- Moyo, M.; Motchelaho, M.A.M.; Xiong, H.; Jewell, L.L.; Coville, N.J. Promotion of Co/carbon sphere Fischer-Tropsch catalysts by residual K and Mn from carbon oxidation by KMnO4. Appl. Catal. A Gen. 2012, 413–414, 223–229. [Google Scholar] [CrossRef]
- Mansuriya, B.D.; Altintas, Z. Carbon Dots: Classification, Properties, Synthesis, Characterization, and Applications in Health Care-An Updated Review (2018–2021). Nanomaterials 2018, 11, 2525. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wu, M.; Feng, X.Z.; Yin, X.B.; He, X.W.; Zhang, Y.K. Reduced carbon dots versus oxidized carbon dots: Photo- and electrochemiluminescence investigations for selected applications. Chem. Eur. J. 2013, 19, 6282–6288. [Google Scholar] [CrossRef]
- De, B.; Karak, N. A green and facile approach for the synthesis of water soluble fluorescent carbon dots from banana juice. RSC Adv. 2013, 3, 8286–8290. [Google Scholar] [CrossRef]
- Chu, K.W.; Lee, S.L.; Chang, C.J.; Liu, L. Recent progress of carbon dot precursors and photocatalysis applications. Polymers 2019, 11, 689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Q.; Deng, J.; Hou, Y.; Wang, H.; Li, H.; Zhang, Y.; Yao, S. Hydroxyl-rich C-dots synthesized by a one-pot method and their application in the preparation of noble metal nanoparticles. Chem. Commun. 2015, 51, 7164–7167. [Google Scholar] [CrossRef]
- Zhuo, Y.; Zhong, D.; Miao, H.; Yang, X. Reduced carbon dots employed for synthesizing metal nanoclusters and nanoparticles. RSC Adv. 2015, 5, 32669–32674. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Q.; Long, Y.; Zhang, H.; Huang, X.; Zhu, R. Enhancing the luminescence of carbon dots with a reduction pathway. Chem. Commun. 2011, 47, 10650–10652. [Google Scholar] [CrossRef] [PubMed]
- Dandia, A.; Saini, P.; Sethi, M.; Kumar, K.; Saini, S.; Meena, S.; Meena, S.; Parewa, V. Nanocarbons in quantum regime: An emerging sustainable catalytic platform for organic synthesis. Catal. Rev. Sci. Eng. 2021, 65, 874–928. [Google Scholar] [CrossRef]
- Corti, V.; Bartolomei, B.; Mamone, M.; Gentile, G.; Prato, M.; Filippini, G. Amine-Rich Carbon Dots as Novel Nano-Aminocatalytic Platforms in Organic Synthesis. Eur. J. Org. Chem. 2022, 2022, e202200879. [Google Scholar] [CrossRef] [PubMed]
- Su, D.S.; Perathoner, S.; Centi, G. Nanocarbons for the development of advanced catalysts. Chem. Rev. 2013, 113, 5782–5816. [Google Scholar] [CrossRef]
- Cheng, H.; Zhao, Y.; Fan, Y.; Xie, X.; Qu, L.; Shi, G. Graphene-quantum-dot assembled nanotubes: A new platform for efficient Raman enhancement. ACS Nano 2012, 6, 2237–2244. [Google Scholar] [CrossRef]
- Hou, H.; Banks, C.E.; Jing, M.; Zhang, Y.; Ji, X. Carbon Quantum Dots and Their Derivative 3D Porous Carbon Frameworks for Sodium-Ion Batteries with Ultralong Cycle Life. Adv. Mater. 2015, 27, 7861–7866. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Y.; Hao, J.; Liu, Y.; Li, W.; Li, J. N and P co-functionalized three-dimensional porous carbon networks as efficient metal-free electrocatalysts for oxygen reduction reaction. Carbon 2017, 122, 64–73. [Google Scholar] [CrossRef]
- Hou, H.; Shao, L.; Zhang, Y.; Zou, G.; Chen, J.; Ji, X. Large-Area Carbon Nanosheets Doped with Phosphorus: A High-Performance Anode Material for Sodium-Ion Batteries. Adv. Sci. 2016, 4, 1600243. [Google Scholar] [CrossRef] [Green Version]
- Mokoloko, L.L.; Matsoso, B.J.; Forbes, R.P.; Barrett, D.H.; Moreno, B.D.; Coville, N.J. Evolution of large-area reduced graphene oxide nanosheets from carbon dots via thermal treatment. Carbon Trends 2021, 4, 100074. [Google Scholar] [CrossRef]
- Luo, H.; Lari, L.; Kim, H.; Hérou, S.; Tanase, L.C.; Lazarov, V.K.; Titirici, M.-M. Structural evolution of carbon dots during low temperature pyrolysis. Nanoscale 2022, 14, 910–918. [Google Scholar] [CrossRef]
- Wu, X.; Yu, F.; Han, Y.; Jiang, L.; Li, Z.; Zhu, J.; Xu, Q.; Tedesco, A.C.; Zhang, J.; Bi, H. Enhanced chemodynamic and photoluminescence efficiencies of Fe-O4 coordinated carbon dots via the core-shell synergistic effect. Nanoscale 2022, 15, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhao, S.; Guo, S.; Wang, H.; Sun, Y.; Yao, B.; Liu, Y.; Haung, H.; Kang, Z. Carbon quantum dot-covered porous Ag with enhanced activity for selective electroreduction of CO2 to CO. Inorg. Chem. Front. 2019, 6, 1453–1460. [Google Scholar] [CrossRef]
- Atkins, P.W.; Overton, T.L.; Rourke, J.P.; Weller, M.T.; Armstrong, F.A. Shriver and Atkins’ Inorganic Chemistry, 5th ed.; Oxford University Press: Oxford, UK, 2010; p. 171. ISBN 978-1-42-921820-7. [Google Scholar]
- Xiong, H.; Moyo, M.; Rayner, M.K.; Jewell, L.L.; Billing, D.G.; Coville, N.J. Autoreduction and Catalytic Performance of a Cobalt Fischer-Tropsch Synthesis Catalyst Supported on Nitrogen-Doped Carbon Spheres. ChemCatChem 2010, 2, 514–518. [Google Scholar] [CrossRef]
- Petersen, A.P.; Claeys, M.; Kooyman, P.J.; van Steen, E. Cobalt-Based Fischer—Tropsch Synthesis: A Kinetic Inverse Model System. Catalysts 2019, 9, 794. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Medlin, J.W. Catalyst design using an inverse strategy: From mechanistic studies on inverted model catalysts to applications of oxide-coated metal nanoparticles. Surf. Sci. Rep. 2018, 73, 117–152. [Google Scholar] [CrossRef]
- Nabaho, D.; Niemantsverdriet, J.W.; Claeys, M.; van Steen, E. Hydrogen spillover in the Fischer-Tropsch synthesis: An analysis of platinum as a promoter for cobalt-alumina catalysts. Catal. Today 2016, 261, 17–27. [Google Scholar] [CrossRef]
- Ma, C.; Zhou, Y.; Yan, W.; He, W.; Liu, Q.; Li, Z.; Wang, H.; Li, G.; Yang, Y.; Han, W.; et al. Predominant Catalytic Performance of Nickel Nanoparticles Embedded into Nitrogen-Doped Carbon Quantum Dot-Based Nanosheets for the Nitroreduction of Halogenated Nitrobenzene. ACS Sustain. Chem. Eng. 2022, 10, 8162–8171. [Google Scholar] [CrossRef]
- Zhang, Y.; Foster, C.W.; Banks, C.E.; Shao, L.; Hou, H.; Zou, G.; Chen, J.; Huang, Z.; Ji, X. Graphene-Rich Wrapped Petal-Like Rutile TiO2 tuned by Carbon Dots for High-Performance Sodium Storage. Adv. Mater. 2016, 28, 9391–9399. [Google Scholar] [CrossRef]
- Li, X.; Ding, S.; Lyu, Z.; Tieu, P.; Wang, M.; Feng, Z.; Pan, X.; Zhou, Y.; Niu, X.; Du, D.; et al. Single-Atomic Iron Doped Carbon Dots with Both Photoluminescence and Oxidase-Like Activity. Small 2022, 18, 2203001. [Google Scholar] [CrossRef]
- Zeng, J.; Zhao, B.; Luo, X.; Wu, F. Glucose-sensitive colorimetric sensor based on peroxidase mimics activity of carbon dots-functionalized Fe3O4 nanocomposites. Diam. Relat. Mater. 2023, 136, 109914. [Google Scholar] [CrossRef]
- Zhang, B.T.; Wang, Q.; Zhang, Y.; Teng, Y.; Fan, M. Degradation of ibuprofen in the carbon dots/Fe3O4@carbon sphere pomegranate-like composites activated persulfate system. Sep. Purif. Technol. 2020, 242, 116820. [Google Scholar] [CrossRef]
- Juang, R.S.; Ju, Y.-C.; Liao, C.-S.; Lin, K.-S.; Lu, H.-C.; Wang, S.-F.; Sun, A.-C. Synthesis of Carbon Dots on Fe3O4 Nanoparticles as Recyclable Visible-Light Photocatalysts. IEEE Trans. Magn. 2017, 53, 2710541. [Google Scholar] [CrossRef]
- Abbas, M.W.; Soomro, R.A.; Kalwar, N.H.; Zahoor, M.; Avci, A.; Pehlivan, E.; Hallam, K.R.; Willander, M. Carbon quantum dot coated Fe3O4 hybrid composites for sensitive electrochemical detection of uric acid. Microchem. J. 2019, 146, 517–524. [Google Scholar] [CrossRef]
- Zhang, P.; Song, T.; Wang, T.; Zeng, H. In-situ synthesis of Cu nanoparticles hybridized with carbon quantum dots as a broad spectrum photocatalyst for improvement of photocatalytic H2 evolution. Appl. Catal. B 2017, 206, 328–335. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, F.; Lang, Z.; Nie, H.; Wang, Z.; Shao, M.; Liu, Y.; Tan, H.; Li, Y.; Kang, Z. Carbon dots/PtW6O24 composite as efficient and stable electrocatalyst for hydrogen oxidation reaction in PEMFCs. Chem. Eng. J. 2021, 426, 130709. [Google Scholar] [CrossRef]
- Yang, L.; Liu, X.; Lu, Q.; Huang, N.; Liu, M.; Zhang, Y.; Yao, S. Catalytic and peroxidase-like activity of carbon based-AuPd bimetallic nanocomposite produced using carbon dots as the reductant. Anal. Chim. Acta 2016, 930, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.C.; Xu, X.Q.; Dong, P.; Lai, L.; Lan, J.Y.; Jiang, F.L.; Liu, Y. One-step synthesis of silver nanoparticles using carbon dots as reducing and stabilizing agents and their antibacterial mechanisms. Carbon N. Y. 2015, 94, 129–141. [Google Scholar] [CrossRef]
- Liu, T.; Dong, J.X.; Liu, S.G.; Li, N.; Lin, S.M.; Fan, Y.Z.; Lei, J.L.; Luo, H.Q.; Li, N.B. Carbon quantum dots prepared with polyethyleneimine as both reducing agent and stabilizer for synthesis of Ag/CQDs composite for Hg2+ ions detection. J. Hazard. Mater. 2017, 322, 430–436. [Google Scholar] [CrossRef]
- Shen, L.; Chen, M.; Hu, L.; Chen, X.; Wang, J. Growth and stabilization of silver nanoparticles on carbon dots and sensing application. Langmuir 2013, 29, 16135–16140. [Google Scholar] [CrossRef]
- Lu, C.; Zhu, Q.; Zhang, X.; Ji, H.; Zhou, Y.; Wang, H.; Liu, Q.; Nie, J.; Han, W.; Li, X. Decoration of Pd Nanoparticles with N and S Doped Carbon Quantum Dots as a Robust Catalyst for the Chemoselective Hydrogenation Reaction. ACS Sustain. Chem. Eng. 2019, 7, 8542–8553. [Google Scholar] [CrossRef]
- Liu, R.; Huang, H.; Li, H.; Liu, Y.; Zhong, J.; Li, Y.; Zhang, S.; Kang, Z. Metal nanoparticle/carbon quantum dot composite as a photocatalyst for high-efficiency cyclohexane oxidation. ACS Catal. 2014, 4, 328–336. [Google Scholar] [CrossRef]
- Jin, J.; Zhu, S.; Song, Y.; Zhao, H.; Zhang, Z.; Guo, Y.; Li, J.; Song, W.; Yang, B.; Zhao, B. Precisely Controllable Core-Shell Ag@Carbon Dots Nanoparticles: Application to in Situ Super-Sensitive Monitoring of Catalytic Reactions. ACS Appl. Mater. Interfaces 2016, 8, 27965. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Tan, J.; Sang, H.; Zhang, L.; Yue, D. The synthesis of rhodium/carbon dots nanoparticles and its hydrogenation application. Appl. Surf. Sci. 2017, 396, 1138–1145. [Google Scholar] [CrossRef]
- Bharathi, G.; Nataraj, D.; Premkumar, S.; Sowmiya, M.; Senthilkumar, K.; Thangadurai, T.D.; Khyzhun, O.Y.; Gupta, M.; Phase, D.; Patra, N.; et al. Graphene Quantum Dot Solid Sheets: Strong blue-light-emitting & photocurrent-producing band-gap-opened nanostructures. Sci. Rep. 2017, 7, 1085. [Google Scholar]
- Wang, X.; Long, Y.; Wang, Q.; Zhang, H.; Huang, X.; Zhu, R.; Teng, P.; Liang, L.; Zheng, H. Reduced state carbon dots as both reductant and stabilizer for the synthesis of gold nanoparticles. Carbon 2013, 64, 499–506. [Google Scholar] [CrossRef]







 ; cobalt metal =
; cobalt metal =  ).
).
 ; cobalt metal =
; cobalt metal =  ).
).













Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokoloko, L.L.; Forbes, R.P.; Coville, N.J. The Behavior of Carbon Dots in Catalytic Reactions. Catalysts 2023, 13, 1201. https://doi.org/10.3390/catal13081201
Mokoloko LL, Forbes RP, Coville NJ. The Behavior of Carbon Dots in Catalytic Reactions. Catalysts. 2023; 13(8):1201. https://doi.org/10.3390/catal13081201
Chicago/Turabian StyleMokoloko, Lerato L., Roy P. Forbes, and Neil J. Coville. 2023. "The Behavior of Carbon Dots in Catalytic Reactions" Catalysts 13, no. 8: 1201. https://doi.org/10.3390/catal13081201