Efficient and Stable Degradation of Triazophos Pesticide by TiO2/WO3 Nanocomposites with S-Scheme Heterojunctions and Oxygen Defects
Abstract
:1. Introduction
2. Results
2.1. Phase and Microscopic Morphology Analysis
2.2. XPS and Elemental Analysis
2.3. Specific Surface Area
2.4. Optical Property and Band Gap Analysis
2.5. Photocatalytic Performance of Triazophos and Electrochemical Analysis
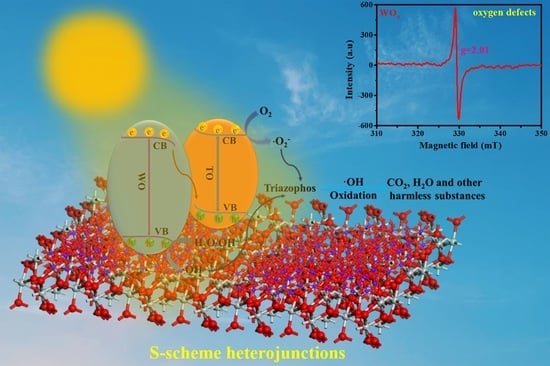
2.6. The Exploration Process of Photocatalytic Mechanism
2.7. Photocatalytic Mechanism
3. Experimental
3.1. Material
3.2. Synthesis of WO Nanosheets
3.3. Synthesis of TO/WO Nanocomposites
3.4. Characterization
3.5. Evaluation of Photocatalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bose, S.; Kumar, P.S.; Vo, D.-V.N. A review on the microbial degradation of chlorpyrifos and its metabolite TCP. Chemosphere 2021, 283, 131447. [Google Scholar] [CrossRef] [PubMed]
- Dash, D.M.; Osborne, W.J. A systematic review on the implementation of advanced and evolutionary biotechnological tools for efficient bioremediation of organophosphorus pesticides. Chemosphere 2023, 313, 137506. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Tan, P.; Wang, R.; Li, S.; Liu, H.; Yang, Y.; Wu, Z. Advances in organophosphorus pesticides pollution: Current status and challenges in ecotoxicological, sustainable agriculture, and degradation strategies. J. Hazard. Mater. 2022, 424, 127494. [Google Scholar] [CrossRef]
- Gong, C.; Fan, Y.; Zhao, H. Recent advances and perspectives of enzyme-based optical biosensing for organophosphorus pesticides detection. Talanta 2022, 240, 123145. [Google Scholar] [CrossRef] [PubMed]
- Issaka, E.; Wariboko, M.A.; Johnson, N.A.N.; Nyame-do Aniagyei, O. Advanced visual sensing techniques for on-site detection of pesticide residue in water environments. Heliyon 2023, 9, e13986. [Google Scholar] [CrossRef]
- Jain, U.; Saxena, K.; Hooda, V.; Balayan, S.; Singh, A.P.; Tikadar, M.; Chauhan, N. Emerging vistas on pesticides detection based on electrochemical biosensors—An update. Food Chem. 2022, 371, 131126. [Google Scholar] [CrossRef]
- Kou, J.; Li, X.; Zhang, M.; Wang, L.; Hu, L.; Liu, X.; Mei, S.; Xu, G. Accumulative levels, temporal and spatial distribution of common chemical pollutants in the blood of Chinese adults. Environ. Pollut. 2022, 311, 119980. [Google Scholar] [CrossRef]
- Li, H.; Jiao, Y.; Li, L.; Jiao, X. Research progress and trend of effects of organophosphorus pesticides on aquatic organisms in the past decade. Comp. Biochem. Physiol. C 2023, 271, 109673. [Google Scholar] [CrossRef]
- Mali, H.; Shah, C.; Patel, D.H.; Trivedi, U.; Subramanian, R.B. Bio-catalytic system of metallohydrolases for remediation of neurotoxin organophosphates and applications with a future vision. J. Inorg. Biochem. 2022, 231, 111771. [Google Scholar] [CrossRef] [PubMed]
- Matula, M.; Kucera, T.; Soukup, O.; Pejchal, J. Enzymatic Degradation of Organophosphorus Pesticides and Nerve Agents by EC: 3.1.8.2. Catalysts 2020, 10, 1365. [Google Scholar] [CrossRef]
- Mdeni, N.L.; Adeniji, A.O.; Okoh, A.I.; Okoh, O.O. Analytical Evaluation of Carbamate and Organophosphate Pesticides in Human and Environmental Matrices: A Review. Molecules 2022, 27, 618. [Google Scholar] [CrossRef]
- Nandhini, A.R.; Harshiny, M.; Gummadi, S.N. Chlorpyrifos in environment and food: A critical review of detection methods and degradation pathways. Environ. Sci. Process. Impacts 2021, 23, 1255–1277. [Google Scholar] [CrossRef]
- Paidi, M.K.; Satapute, P.; Haider, M.S.; Udikeri, S.S.; Ramachandra, Y.L.; Vo, D.-V.N.; Govarthanan, M.; Jogaiah, S. Mitigation of organophosphorus insecticides from environment: Residual detoxification by bioweapon catalytic scavengers. Environ. Res. 2021, 200, 111368. [Google Scholar] [CrossRef] [PubMed]
- Raj, A.; Kumar, A. Recent advances in assessment methods and mechanism of microbe-mediated chlorpyrifos remediation. Environ. Res. 2022, 214, 114011. [Google Scholar] [CrossRef]
- Veloo, K.V.; Ibrahim, N.A.S. Analytical Extraction Methods and Sorbents’ Development for Simultaneous Determination of Organophosphorus Pesticides’ Residues in Food and Water Samples: A Review. Molecules 2021, 26, 5495. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.L.; Rani, V. Biosensors for toxic metals, polychlorinated biphenyls, biological oxygen demand, endocrine disruptors, hormones, dioxin, phenolic and organophosphorus compounds: A review. Environ. Chem. Lett. 2021, 19, 1657–1666. [Google Scholar] [CrossRef]
- Vyas, T.; Singh, V.; Kodgire, P.; Joshi, A. Insights in detection and analysis of organophosphates using organophosphorus acid anhydrolases (OPAA) enzyme-based biosensors. Crit. Rev. Biotechnol. 2023, 43, 521–539. [Google Scholar] [CrossRef]
- Xu, W.; Zhao, S.; Zhang, W.; Wu, H.; Guang, C.; Mu, W. Recent advances and future prospective of organophosphorus-degrading enzymes: Identification, modification, and application. Crit. Rev. Biotechnol. 2021, 41, 1096–1113. [Google Scholar] [CrossRef]
- Zammataro, A.; Santonocito, R.; Pappalardo, A.; Trusso Sfrazzetto, G. Catalytic Degradation of Nerve Agents. Catalysts 2020, 10, 881. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Li, J.; Pang, S.; Mishra, S.; Bhatt, P.; Zeng, D.; Chen, S. Emerging Technologies for Degradation of Dichlorvos: A Review. Int. J. Env. Res. Public Health 2021, 18, 5789. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, B.; Chen, H.; Yuan, R. Heterogeneous photocatalytic oxidation for the removal of organophosphorus pollutants from aqueous solutions: A review. Sci. Total Environ. 2023, 856, 159048. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Xu, W.; Zhang, W.; Wu, H.; Guang, C.; Mu, W. Overview of a bioremediation tool: Organophosphorus hydrolase and its significant application in the food, environmental, and therapy fields. Appl. Microbiol. Biotechnol. 2021, 105, 8241–8253. [Google Scholar] [CrossRef]
- Ma, J.; Long, R.; Liu, D.; Low, J.; Xiong, Y. Defect Engineering in Photocatalytic Methane Conversion. Small Struct. 2022, 3, 2100147. [Google Scholar] [CrossRef]
- Sultana, S.; Mansingh, S.; Parida, K.M. Crystal facet and surface defect engineered low dimensional CeO2 (0D, 1D, 2D) based photocatalytic materials towards energy generation and pollution abatement. Mater. Adv. 2021, 2, 6942–6983. [Google Scholar] [CrossRef]
- Zhao, Y.; Mao, Q.; Zhai, X.; Zhang, G. Structural Defects Regulation of Bismuth Molybdate Photocatalyst. Prog. Chem. 2021, 33, 1331–1343. [Google Scholar]
- Cheng, C.; Zhang, J.; Zhu, B.; Liang, G.; Zhang, L.; Yu, J. Verifying the Charge-Transfer Mechanism in S-Scheme Heterojunctions Using Femtosecond Transient Absorption Spectroscopy. Angew. Chem. 2023, 62, e202218688. [Google Scholar] [CrossRef]
- Xiao, Y.; Tian, X.; Chen, Y.; Xiao, X.; Chen, T.; Wang, Y. Recent Advances in Carbon Nitride-Based S-scheme Photocatalysts for Solar Energy Conversion. Materials 2023, 16, 3745. [Google Scholar] [CrossRef]
- Xu, Q.; Wageh, S.; Al-Ghamdi, A.A.; Li, X. Design principle of S-scheme heterojunction photocatalyst. J. Mater. Sci. Technol. 2022, 124, 171–173. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, Z.-C.; Yang, Y.-P.; Zhang, Z.; Chen, W.; Yan, R.-Q.; Jin, Y.; Zhang, J. Defective WO3 nanoplates controllably decorated with MIL-101(Fe) nanoparticles to efficiently remove tetracycline hydrochloride by S-scheme mechanism. Sep. Purif. Technol. 2022, 300, 121846. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Wang, W.; Yu, J. In Situ Irradiated X-ray Photoelectron Spectroscopy Investigation on Electron Transfer Mechanism in S-Scheme Photocatalyst. J. Phys. Chem. Lett. 2022, 13, 8462–8469. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, Y.; Cheng, X.; Ma, J.; Li, L.; Fan, X.; Ding, Y.; Han, Y.; Tao, R. K+-Doped ZnO/g-C3N4 Heterojunction: Controllable Preparation, Efficient Charge Separation, and Excellent Photocatalytic VOC Degradation Performance. Ind. Eng. Chem. Res. 2022, 61, 187–197. [Google Scholar] [CrossRef]
- Wang, K.; Li, X.; Wang, N.; Shen, Q.; Liu, M.; Zhou, J.; Li, N. Z-Scheme Core-Shell meso-TiO2@ZnIn2S4/Ti3C2 MXene Enhances Visible Light-Driven CO2-to-CH4 Selectivity. Ind. Eng. Chem. Res. 2021, 60, 8720–8732. [Google Scholar] [CrossRef]
- Mirmasoomi, S.R.; Ghazi, M.M.; Galedari, M. Photocatalytic degradation of diazinon under visible light using TiO2/Fe2O3 nanocomposite synthesized by ultrasonic-assisted impregnation method. Sep. Purif. Technol. 2017, 175, 418–427. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, W.; Xia, Z.; Xian, M.; Bu, F.; Liang, F.; Feng, D. One-pot synthesis of S-scheme WO3/BiOBr heterojunction nanoflowers enriched with oxygen vacancies for enhanced tetracycline photodegradation. Sep. Purif. Technol. 2022, 290, 12087. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Yu, H.; Yu, J. Emerging S-Scheme Photocatalyst. Adv. Mater. 2022, 34, 2107668. [Google Scholar] [CrossRef]
- Xu, X.T.; Huang, Y.; Dai, K.; Wang, Z.L.; Zhang, J.F. Non-noble-metal CuSe promotes charge separation and photocatalytic CO2 reduction on porous g-C3N4 nanosheets. Sep. Purif. Technol. 2023, 317, 123887. [Google Scholar] [CrossRef]
- Li, Z.; Jin, D.; Wang, Z. WO3(H2O)0.333/CdSe-diethylenetriamine nanocomposite as a step-scheme photocatalyst for hydrogen production. Surf. Interfaces 2022, 29, 101702. [Google Scholar] [CrossRef]
- Brown, J.J.; Ke, Z.; Ma, T.; Page, A.J. Defect Engineering for Photocatalysis: From Ternary to Perovskite Oxynitrides. ChemNanoMat 2020, 6, 708–719. [Google Scholar] [CrossRef]
- Kumar, A.; Raizada, P.; Khan, A.A.P.; Nguyen, V.-H.; Van Le, Q.; Singh, A.; Saini, V.; Selvasembian, R.; Huynh, T.-T.; Singh, P. Phenolic compounds degradation: Insight into the role and evidence of oxygen vacancy defects engineering on nanomaterials. Sci. Total Environ. 2021, 800, 149410. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, S.; He, H.; Xie, C.; Tang, Y.; He, C.; Shao, M.; Wang, H. Oxygen Vacancy Engineering in Titanium Dioxide for Sodium Storage. Chem. Asian J. 2021, 16, 3–19. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, R.; Huo, Y.; Li, H.; Wang, L. Formation, Detection, and Function of Oxygen Vacancy in Metal Oxides for Solar Energy Conversion. Adv. Funct. Mater. 2022, 32, 2109503. [Google Scholar] [CrossRef]
- Ye, K.; Li, K.; Lu, Y.; Guo, Z.; Ni, N.; Liu, H.; Huang, Y.; Ji, H.; Wang, P. An overview of advanced methods for the characterization of oxygen vacancies in materials. TrAC Trends Anal. Chem. 2019, 116, 102–108. [Google Scholar] [CrossRef]
- Salarian, A.-A.; Hami, Z.; Mirzaei, N.; Mohseni, S.M.; Asadi, A.; Bahrami, H.; Vosoughi, M.; Alinejad, A.; Zare, M.-R. N-doped TiO2 nanosheets for photocatalytic degradation and mineralization of diazinon under simulated solar irradiation: Optimization and modeling using a response surface methodology. J. Mol. Liq. 2016, 220, 183–191. [Google Scholar] [CrossRef]
- Pathania, D.; Sharma, A.; Kumar, S.; Srivastava, A.K.; Kumar, A.; Singh, L. Bio-synthesized Cu-ZnO hetro-nanostructure for catalytic degradation of organophosphate chlorpyrifos under solar illumination. Chemosphere 2021, 277, 130315. [Google Scholar] [CrossRef] [PubMed]
- Vigneshwaran, S.; Preethi, J.; Meenakshi, S. Removal of chlorpyrifos, an insecticide using metal free heterogeneous graphitic carbon nitride (g-C3N4) incorporated chitosan as catalyst: Photocatalytic and adsorption studies. Int. J. Biol. Macromol. 2019, 132, 289–299. [Google Scholar] [CrossRef]
- Hossaini, H.; Moussavi, G.; Farrokhi, M. Oxidation of diazinon in cns-ZnO/LED photocatalytic process: Catalyst preparation, photocatalytic examination, and toxicity bioassay of oxidation by-products. Sep. Purif. Technol. 2017, 174, 320–330. [Google Scholar] [CrossRef]
- Isari, A.A.; Moradi, S.; Rezaei, S.S.; Ghanbari, F.; Dehghanifard, E.; Kakavandi, B. Peroxymonosulfate catalyzed by core/shell magnetic ZnO photocatalyst towards malathion degradation: Enhancing synergy, catalytic performance and mechanism. Sep. Purif. Technol. 2021, 275, 119163. [Google Scholar] [CrossRef]
- Antonopoulou, M.; Giannakas, A.; Bairamis, F.; Papadaki, M.; Konstantinou, I. Degradation of organophosphorus flame retardant tris (1-chloro-2-propyl) phosphate (TCPP) by visible light N,S-codoped TiO2 photocatalysts. Chem. Eng. J. 2017, 318, 231–239. [Google Scholar] [CrossRef]
- Tang, Q.-Y.; Chen, W.-F.; Lv, Y.-R.; Yang, S.-Y.; Xu, Y.-H. Z-scheme hierarchical Cu2S/Bi2WO6 composites for improved photocatalytic activity of glyphosate degradation under visible light irradiation. Sep. Purif. Technol. 2020, 236, 116243. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Chen, C.; Yang, R.; Cheng, S.; Sang, X.; Zhang, M.; Zhang, J.; Wang, Z.; Li, Z. Efficient and Stable Degradation of Triazophos Pesticide by TiO2/WO3 Nanocomposites with S-Scheme Heterojunctions and Oxygen Defects. Catalysts 2023, 13, 1136. https://doi.org/10.3390/catal13071136
Li W, Chen C, Yang R, Cheng S, Sang X, Zhang M, Zhang J, Wang Z, Li Z. Efficient and Stable Degradation of Triazophos Pesticide by TiO2/WO3 Nanocomposites with S-Scheme Heterojunctions and Oxygen Defects. Catalysts. 2023; 13(7):1136. https://doi.org/10.3390/catal13071136
Chicago/Turabian StyleLi, Wen, Chunxu Chen, Renqiang Yang, Shuangli Cheng, Xiaoyu Sang, Meiwen Zhang, Jinfeng Zhang, Zhenghua Wang, and Zhen Li. 2023. "Efficient and Stable Degradation of Triazophos Pesticide by TiO2/WO3 Nanocomposites with S-Scheme Heterojunctions and Oxygen Defects" Catalysts 13, no. 7: 1136. https://doi.org/10.3390/catal13071136