Sulfadiazine Elimination from Wastewater Effluents under Ozone-Based Catalysis Processes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Degradation Performance of Different Oxidation Processes
2.2. Influence of Reaction Parameters
2.2.1. Effects of Initial SFD Concentrations and Oxidant Dosages
2.2.2. Effect of Initial pH
2.3. Influence of Water Matrix
2.4. Influence of Anions on SFD Degradation
2.5. TOC Abatement in SFD Degradation
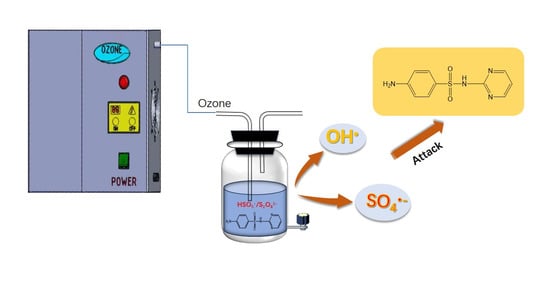
2.6. Contributions of Different Reactive Species
2.7. Proposed Degradation Products and Pathways
3. Methodology
3.1. Chemicals and Reagents
3.2. Experimental Procedures
3.3. Analytic Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Deniere, E.; Van Langenhove, H.; Van Hulle, S.W.H.; Demeestere, K. Improving the ozone-activated peroxymonosulfate process for removal of trace organic contaminants in real waters through implementation of an optimized sequential ozone dosing strategy. Sci. Total Environ. 2023, 856, 158764. [Google Scholar] [CrossRef]
- Deniere, E.; Van Langenhove, H.; Van Hulle, S.; Demeestere, K. The ozone-activated peroxymonosulfate process for the removal of a mixture of TrOCs with different ozone reactivity at environmentally relevant conditions: Technical performance, radical exposure and online monitoring by spectral surrogate parameters. Chem. Eng. J. 2023, 454, 140128. [Google Scholar] [CrossRef]
- Deniere, E.; Alagappan, R.P.; Langenhove, H.V.; Hulle, S.V.; Demeestere, K. The ozone-activated peroxymonosulfate process (O3/PMS) for removal of trace organic contaminants in natural and wastewater: Effect of the (in)organic matrix composition. Chem. Eng. J. 2022, 430, 133000. [Google Scholar] [CrossRef]
- Tian, Y.; Yao, S.; Zhou, L.; Hu, Y.; Lei, J.; Wang, L.; Zhang, J.; Liu, Y.; Cui, C. Efficient removal of antibiotic-resistant bacteria and intracellular antibiotic resistance genes by heterogeneous activation of peroxymonosulfate on hierarchical macro-mesoporous Co3O4-SiO2 with enhanced photogenerated charges. J. Hazard. Mater. 2022, 430, 127414. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, Z.; Yang, W.; Chen, C.; Li, J.; He, R.; Liu, S. Insights into the radical and nonradical oxidation degradation of ciprofloxacin in peroxodisulfate activation by ultraviolet light. J. Water Process Eng. 2022, 49, 103184. [Google Scholar] [CrossRef]
- Zhou, C.S.; Wu, J.W.; Dong, L.L.; Liu, B.F.; Xing, D.F.; Yang, S.S.; Wu, X.K.; Wang, Q.; Fan, J.N.; Feng, L.P.; et al. Removal of antibiotic resistant bacteria and antibiotic resistance genes in wastewater effluent by UV-activated persulfate. J. Hazard. Mater. 2020, 388, 122070. [Google Scholar] [CrossRef]
- Giannakis, S.; Le, T.M.; Entenza, J.M.; Pulgarin, C. Solar photo-Fenton disinfection of 11 antibiotic-resistant bacteria (ARB) and elimination of representative AR genes. Evidence that antibiotic resistance does not imply resistance to oxidative treatment. Water Res. 2018, 143, 334–345. [Google Scholar] [CrossRef]
- Modak, S.M.; Sampath, L.; Fox, C.L., Jr. Combined topical use of silver sulfadiazine and antibiotics as a possible solution to bacterial resistance in burn wounds. J. Burn Care Rehabil. 1988, 9, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Schauss, K.; Focks, A.; Heuer, H.; Kotzerke, A.; Schmitt, H.; Thiele-Bruhn, S.; Smalla, K.; Wilke, B.-M.; Matthies, M.; Amelung, W.; et al. Analysis, fate and effects of the antibiotic sulfadiazine in soil ecosystems. TrAC Trends Anal. Chem. 2009, 28, 612–618. [Google Scholar] [CrossRef]
- Schauss, K.; Focks, A.; Leininger, S.; Kotzerke, A.; Heuer, H.; Thiele-Bruhn, S.; Sharma, S.; Wilke, B.M.; Matthies, M.; Smalla, K.; et al. Dynamics and functional relevance of ammonia-oxidizing archaea in two agricultural soils. Environ. Microbiol. 2009, 11, 446–456. [Google Scholar] [CrossRef]
- Miao, X.-S.; Bishay, F.; Chen, M.; Metcalfe, C.D. Occurrence of Antimicrobials in the Final Effluents of Wastewater Treatment Plants in Canada. Environ. Sci. Technol. 2004, 38, 3533–3541. [Google Scholar] [CrossRef]
- Qin, L.T.; Pang, X.R.; Zeng, H.H.; Liang, Y.P.; Mo, L.Y.; Wang, D.Q.; Dai, J.F. Ecological and human health risk of sulfonamides in surface water and groundwater of Huixian karst wetland in Guilin, China. Sci. Total Environ. 2020, 708, 134552. [Google Scholar] [CrossRef]
- de Orte, M.R.; Carballeira, C.; Viana, I.G.; Carballeira, A. Assessing the toxicity of chemical compounds associated with marine land-based fish farms: The use of mini-scale microalgal toxicity tests. Chem. Ecol. 2013, 29, 554–563. [Google Scholar] [CrossRef]
- Lin, T.; Chen, Y.; Chen, W. Impact of toxicological properties of sulfonamides on the growth of zebrafish embryos in the water. Environ. Toxicol. Pharmacol. 2013, 36, 1068–1076. [Google Scholar] [CrossRef]
- Wollenberger, L.; Halling-Sørensen, B.; Kusk, K.O. Acute and chronic toxicity of veterinary antibiotics to Daphnia magna. Chemosphere 2000, 40, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Qian, K.; Xie, X.; Chen, J.; Tang, W.; Tao, F.; Wang, Y. In situ degradation of fluoroquinolone antibiotics in groundwater by CoFe2O4 nanoparticle-activated peroxymonosulfate: Performance, activation mechanism, degradation pathway. Appl. Geochem. 2023, 152, 105605. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; Yu, X.; Sanganyado, E.; Wang, L.; Li, P.; Liu, W. Effects of norfloxacin, copper, and their interactions on microbial communities in estuarine sediment. Environ. Res. 2022, 212, 113506. [Google Scholar] [CrossRef]
- Castrignano, E.; Yang, Z.; Feil, E.J.; Bade, R.; Castiglioni, S.; Causanilles, A.; Gracia-Lor, E.; Hernandez, F.; Plosz, B.G.; Ramin, P.; et al. Enantiomeric profiling of quinolones and quinolones resistance gene qnrS in European wastewaters. Water Res. 2020, 175, 115653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Su, H.; Gao, P.; Li, B.; Feng, L.; Liu, Y.; Du, Z.; Zhang, L. Effects and mechanisms of aged polystyrene microplastics on the photodegradation of sulfamethoxazole in water under simulated sunlight. J. Hazard. Mater. 2022, 433, 128813. [Google Scholar] [CrossRef] [PubMed]
- Gulkowska, A.; Leung, H.W.; So, M.K.; Taniyasu, S.; Yamashita, N.; Yeung, L.W.; Richardson, B.J.; Lei, A.P.; Giesy, J.P.; Lam, P.K. Removal of antibiotics from wastewater by sewage treatment facilities in Hong Kong and Shenzhen, China. Water Res. 2008, 42, 395–403. [Google Scholar] [CrossRef]
- Li, J.; Ren, S.; Qiu, X.; Zhao, S.; Wang, R.; Wang, Y. Electroactive ultrafiltration membrane for simultaneous removal of antibiotic, antibiotic resistant bacteria, and antibiotic resistance genes from wastewater effluent. Environ. Sci. Technol. 2022, 56, 15120–15129. [Google Scholar] [CrossRef] [PubMed]
- Avramiotis, E.; Frontistis, Z.; Manariotis, I.D.; Vakros, J.; Mantzavinos, D. On the Performance of a Sustainable Rice Husk Biochar for the Activation of Persulfate and the Degradation of Antibiotics. Catalysts 2021, 11, 1303. [Google Scholar] [CrossRef]
- Huang, H.; Jiang, L.; Yang, J.; Zhou, S.; Yuan, X.; Liang, J.; Wang, H.; Wang, H.; Bu, Y.; Li, H. Synthesis and modification of ultrathin g-C3N4 for photocatalytic energy and environmental applications. Renew. Sust. Energy Rev. 2023, 173, 113110. [Google Scholar] [CrossRef]
- Jiang, L.; Zhou, S.; Yang, J.; Wang, H.; Yu, H.; Chen, H.; Zhao, Y.; Yuan, X.; Chu, W.; Li, H. Near-Infrared Light Responsive TiO2 for Efficient Solar Energy Utilization. Adv. Funct. Mater. 2021, 32, 2108977. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z.; Duan, J.; Li, N.; Li, B.; Song, T.; Sardar, M.F.; Lv, X.; Zhu, C. Electrochemical disinfection of secondary effluent from a wastewater treatment plant: Removal efficiency of ARGs and variation of antibiotic resistance in surviving bacteria. Chem. Eng. J. 2020, 392, 123674. [Google Scholar] [CrossRef]
- Cui, C.; Jin, L.; Jiang, L.; Han, Q.; Lin, K.; Lu, S.; Zhang, D.; Cao, G. Removal of trace level amounts of twelve sulfonamides from drinking water by UV-activated peroxymonosulfate. Sci. Total Environ. 2016, 572, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Travis, B.R.; Borhan, B. Direct Oxidative Cleavage of α- and β-Dicarbonyls and α-Hydroxyketones to Diesters with KHSO5. J. Org. Chem. 2004, 69, 9299–9302. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, Z.; Dong, Q.; Nie, C.; Wang, S.; Zhang, B.; Han, P.; Tong, M. Catalyst-free periodate activation by solar irradiation for bacterial disinfection: Performance and mechanisms. Environ. Sci. Technol. 2022, 56, 4413–4424. [Google Scholar] [CrossRef] [PubMed]
- Rivas, F.J.; Gimeno, O.; Borallho, T. Aqueous pharmaceutical compounds removal by potassium monopersulfate. Uncatalyzed and catalyzed semicontinuous experiments. Chem. Eng. J. 2012, 192, 326–333. [Google Scholar] [CrossRef]
- Chu, L.; Zhuan, R.; Chen, D.; Wang, J.; Shen, Y. Degradation of macrolide antibiotic erythromycin and reduction of antimicrobial activity using persulfate activated by gamma radiation in different water matrices. Chem. Eng. J. 2019, 361, 156–166. [Google Scholar] [CrossRef]
- How, Z.T.; Fang, Z.; Chelme-Ayala, P.; Ganiyu, S.O.; Zhang, X.; Xu, B.; Chen, C.; Gamal El-Din, M. Ozone-activated peroxymonosulfate (O3/PMS) process for the removal of model naphthenic acids compounds: Kinetics, reactivity, and contribution of oxidative species. J. Environ. Chem. Eng. 2023, 11, 109935. [Google Scholar] [CrossRef]
- Zawadzki, P.; Deska, M. Degradation Efficiency and Kinetics Analysis of an Advanced Oxidation Process Utilizing Ozone, Hydrogen Peroxide and Persulfate to Degrade the Dye Rhodamine B. Catalysts 2021, 11, 974. [Google Scholar] [CrossRef]
- Yang, S.; Wang, P.; Yang, X.; Shan, L.; Zhang, W.; Shao, X.; Niu, R. Degradation efficiencies of azo dye Acid Orange 7 by the interaction of heat, UV and anions with common oxidants: Persulfate, peroxymonosulfate and hydrogen peroxide. J. Hazard. Mater. 2010, 179, 552–558. [Google Scholar] [CrossRef]
- Gao, P.; Tian, X.; Nie, Y.; Yang, C.; Zhou, Z.; Wang, Y. Promoted peroxymonosulfate activation into singlet oxygen over perovskite for ofloxacin degradation by controlling the oxygen defect concentration. Chem. Eng. J. 2019, 359, 828–839. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.-G.; Yang, D.; Zhao, Y. Insight into the removal of tetracycline-resistant bacteria and resistance genes from mariculture wastewater by ultraviolet/persulfate advanced oxidation process. J. Hazard. Mater. Adv. 2022, 7, 100129. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, J.; Wang, Y.; Duan, W.; Liu, J.; Zhang, Y.; Zhang, H.; Zhao, M. The removal of antibiotic resistant bacteria and genes and inhibition of the horizontal gene transfer by contrastive research on sulfidated nanoscale zerovalent iron activating peroxymonosulfate or peroxydisulfate. J. Hazard. Mater. 2022, 423, 126866. [Google Scholar] [CrossRef]
- Yu, Z.; Rabiee, H.; Guo, J. Synergistic effect of sulfidated nano zerovalent iron and persulfate on inactivating antibiotic resistant bacteria and antibiotic resistance genes. Water Res. 2021, 198, 117141. [Google Scholar] [CrossRef]
- Feng, M.; Cizmas, L.; Wang, Z.; Sharma, V.K. Synergistic effect of aqueous removal of fluoroquinolones by a combined use of peroxymonosulfate and ferrate(VI). Chemosphere 2017, 177, 144–148. [Google Scholar] [CrossRef]
- Lange, F.; Cornelissen, S.; Kubac, D.; Sein, M.M.; von Sonntag, J.; Hannich, C.B.; Golloch, A.; Heipieper, H.J.; Moder, M.; von Sonntag, C. Degradation of macrolide antibiotics by ozone: A mechanistic case study with clarithromycin. Chemosphere 2006, 65, 17–23. [Google Scholar] [CrossRef]
- Qiao, J.; Luo, S.; Yang, P.; Jiao, W.; Liu, Y. Degradation of Nitrobenzene-containing wastewater by ozone/persulfate oxidation process in a rotating packed bed. J. Taiwan Inst. Chem. Eng. 2019, 99, 1–8. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Yang, Z.; Shih, K.; Ying, G.-G.; Feng, Y. Activation of ozone by peroxymonosulfate for selective degradation of 1,4-dioxane: Limited water matrices effects. J. Hazard. Mater. 2022, 436, 129223. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Qiu, W.; Zhao, Y.; Li, J.; Jiang, J.; Yang, Y.; Pang, S.-Y.; Liu, G. The degradation of chloramphenicol by O3/PMS and the impact of O3-based AOPs pre-oxidation on dichloroacetamide generation in post-chlorination. Chem. Eng. J. 2020, 401, 126146. [Google Scholar] [CrossRef]
- Adil, S.; Maryam, B.; Kim, E.J.; Dulova, N. Individual and simultaneous degradation of sulfamethoxazole and trimethoprim by ozone, ozone/hydrogen peroxide and ozone/persulfate processes: A comparative study. Environ. Res. 2020, 189, 109889. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Li, H.; Li, X.; He, H.; Yang, C. Performances and mechanisms of efficient degradation of atrazine using peroxymonosulfate and ferrate as oxidants. Chem. Eng. J. 2018, 353, 533–541. [Google Scholar] [CrossRef]
- Zhai, G.; Liu, S.; Si, S.; Liu, Y.; Zhang, H.; Mao, Y.; Zhang, M.; Wang, Z.; Cheng, H.; Wang, P.; et al. Oxygen vacancies enhanced ozonation toward phenol derivatives removal over Ov-Bi2O3. ACS EST Water 2022, 2, 1725–1733. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, Z.; Chen, Y.; Feng, H.; Yu, J.; Tang, J.; Ren, X.; Tang, J.; Wang, J.; Tang, L. Single-atom Ru loaded on layered double hydroxide catalyzes peroxymonosulfate for effective E. coli inactivation via a non-radical pathway: Efficiency and mechanism. J. Hazard. Mater. 2022, 440, 129720. [Google Scholar] [CrossRef]
- Jiang, Z.-R.; Li, Y.; Zhou, Y.-X.; Liu, X.; Wang, C.; Lan, Y.; Li, Y. Co3O4-MnO2 nanoparticles moored on biochar as a catalyst for activation of peroxymonosulfate to efficiently degrade sulfonamide antibiotics. Sep. Purif. Technol. 2022, 281, 119935. [Google Scholar] [CrossRef]
- Zou, Y.; Hu, J.; Li, B.; Lin, L.; Li, Y.; Liu, F.; Li, X.-Y. Tailoring the coordination environment of cobalt in a single-atom catalyst through phosphorus doping for enhanced activation of peroxymonosulfate and thus efficient degradation of sulfadiazine. Appl. Catal. B Environ. 2022, 312, 121408. [Google Scholar] [CrossRef]
- Wang, T.; Lu, J.; Lei, J.; Zhou, Y.; Zhao, H.; Chen, X.; Faysal Hossain, M.; Zhou, Y. Highly efficient activation of peroxymonosulfate for rapid sulfadiazine degradation by Fe3O4@Co3S4. Sep. Purif. Technol. 2023, 307, 122755. [Google Scholar] [CrossRef]
- Ling, C.; Wu, S.; Dong, T.; Dong, H.; Wang, Z.; Pan, Y.; Han, J. Sulfadiazine removal by peroxymonosulfate activation with sulfide-modified microscale zero-valent iron: Major radicals, the role of sulfur species, and particle size effect. J. Hazard. Mater. 2022, 423, 127082. [Google Scholar] [CrossRef]
- Dong, F.-X.; Yan, L.; Huang, S.-T.; Liang, J.-Y.; Zhang, W.-X.; Yao, X.-W.; Chen, X.; Qian, W.; Guo, P.-R.; Kong, L.-J.; et al. Removal of antibiotics sulfadiazine by a biochar based material activated persulfate oxidation system: Performance, products and mechanism. Process Saf. Environ. Prot. 2022, 157, 411–419. [Google Scholar] [CrossRef]
- Cui, H.; Tian, Y.; Zhang, J.; Ma, S.; Li, L.; Zuo, W.; Zhang, L.; Wang, T. Enhanced oxidation of sulfadiazine by two-stage ultrasound assisted zero-valent iron catalyzed persulfate process: Factors and pathways. Chem. Eng. J. 2021, 417, 128152. [Google Scholar] [CrossRef]
- Chen, J.; Dai, C.; Zhu, Y.; Gao, Y.; Chu, W.; Gao, N.; Wang, Q. Degradation of sulfadiazine by UV/Oxone: Roles of reactive oxidative species and the formation of disinfection byproducts. Environ. Sci. Pollut. Res. Int. 2022, 29, 54407–54420. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Chu, W.; Huang, Y.; Xu, L.; Chen, M.; Yan, M. Solar photocatalytic degradation of ibuprofen with a magnetic catalyst: Effects of parameters, efficiency in effluent, mechanism and toxicity evolution. Environ. Pollut. 2021, 276, 116691. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Guo, H.; Zhang, Y.; Wu, X.; Liu, Y. Non-photochemical production of singlet oxygen via activation of persulfate by carbon nanotubes. Water Res. 2017, 113, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Lin, Q.; Fu, H.; Luo, H.; Zhong, Q.; Li, J.; Chen, Y. Role of sulfide-modified nanoscale zero-valent iron on carbon nanotubes in nonradical activation of peroxydisulfate. J. Hazard. Mater. 2022, 422, 126949. [Google Scholar] [CrossRef] [PubMed]
- Deniere, E.; Van Hulle, S.; Van Langenhove, H.; Demeestere, K. Advanced oxidation of pharmaceuticals by the ozone-activated peroxymonosulfate process: The role of different oxidative species. J. Hazard. Mater. 2018, 360, 204–213. [Google Scholar] [CrossRef]
- Chen, Y.; Bai, X.; Ji, Y.; Chen, D. Enhanced activation of peroxymonosulfate using ternary MOFs-derived MnCoFeO for sulfamethoxazole degradation: Role of oxygen vacancies. J. Hazard. Mater. 2023, 441, 129912. [Google Scholar] [CrossRef]
- Mao, Y.; Dong, H.; Liu, S.; Zhang, L.; Qiang, Z. Accelerated oxidation of iopamidol by ozone/peroxymonosulfate (O3/PMS) process: Kinetics, mechanism, and simultaneous reduction of iodinated disinfection by-product formation potential. Water Res. 2020, 173, 115615. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, J.; Lu, X.; Ma, J.; Liu, Y. Production of sulfate radical and hydroxyl radical by reaction of ozone with peroxymonosulfate: A novel advanced oxidation process. Environ. Sci. Technol. 2015, 49, 7330–7339. [Google Scholar] [CrossRef]
- Wang, X.-X.; Lin, Y.-L.; Zhang, T.-Y.; Dong, Z.-Y.; Luo, Z.-N.; Hu, C.-Y.; Tang, Y.-L.; Xu, B. Feasibility of UVC laser-activated persulfate with concentrated beam for micropollutant degradation in water. Sep. Purif. Technol. 2022, 299, 121598. [Google Scholar] [CrossRef]
- Furman, O.S.; Teel, A.L.; Watts, R.J. Mechanism of base activation of persulfate. Environ. Sci. Technol. 2010, 44, 6423–6428. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Hou, H.; Fujii, A.; Hosomi, M.; Li, F. Degradation of 1,4-dioxane in water with heat- and Fe2+-activated persulfate oxidation. Environ. Sci. Pollut. Res. Int. 2014, 21, 7457–7465. [Google Scholar] [CrossRef]
- Hayat, W.; Zhang, Y.; Huang, S.; Hussain, I.; Huang, R. Insight into the degradation of methomyl in water by peroxymonosulfate. J. Environ. Chem. Eng. 2021, 9, 105358. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Feng, M.; Tu, W.; Xiao, T.; Xiong, T.; Ang, H.; Yuan, X.; Chew, J.W. Visible-light-driven removal of tetracycline antibiotics and reclamation of hydrogen energy from natural water matrices and wastewater by polymeric carbon nitride foam. Water Res. 2018, 144, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Polo, M.; Lopez-Penalver, J.; Prados-Joya, G.; Ferro-Garcia, M.A.; Rivera-Utrilla, J. Gamma irradiation of pharmaceutical compounds, nitroimidazoles, as a new alternative for water treatment. Water Res. 2009, 43, 4028–4036. [Google Scholar] [CrossRef]
- Wang, F.; Wang, W.; Yuan, S.; Wang, W.; Hu, Z.-H. Comparison of UV/H2O2 and UV/PS processes for the degradation of thiamphenicol in aqueous solution. J. Photochem. Photobiol. A 2017, 348, 79–88. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, X.; Li, H.; Wang, C.; Zhang, T. Combination of peroxymonosulfate and Fe(VI) for enhanced degradation of sulfamethoxazole: The overlooked roles of high-valent iron species. Chem. Eng. J. 2023, 453, 139742. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Pan, Z.; Zhu, Y.; Shao, Y.; Wang, Y.; Yu, K. Degradation of sulfamethoxazole by UV/persulfate in different water samples: Influential factors, transformation products and toxicity. Chem. Eng. J. 2020, 379, 122354. [Google Scholar] [CrossRef]
- Ghanbari, F.; Khatebasreh, M.; Mahdavianpour, M.; Lin, K.A. Oxidative removal of benzotriazole using peroxymonosulfate/ozone/ultrasound: Synergy, optimization, degradation intermediates and utilizing for real wastewater. Chemosphere 2020, 244, 125326. [Google Scholar] [CrossRef]
- Jaafarzadeh, N.; Ghanbari, F.; Ahmadi, M. Efficient degradation of 2,4-dichlorophenoxyacetic acid by peroxymonosulfate/magnetic copper ferrite nanoparticles/ozone: A novel combination of advanced oxidation processes. Chem. Eng. J. 2017, 320, 436–447. [Google Scholar] [CrossRef]
- Jorfi, S.; Kakavandi, B.; Motlagh, H.R.; Ahmadi, M.; Jaafarzadeh, N. A novel combination of oxidative degradation for benzotriazole removal using TiO2 loaded on FeIIFe2IIIO4@C as an efficient activator of peroxymonosulfate. Appl. Catal. B Environ. 2017, 219, 216–230. [Google Scholar] [CrossRef]
- Klu, P.K.; Zhang, H.; Nasir Khan, M.A.; Wang, C.; Qi, J.; Sun, X.; Li, J. TiO2/C coated Co3O4 nanocages for peroxymonosulfate activation towards efficient degradation of organic pollutants. Chemosphere 2022, 308, 136255. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, D.; Zhao, X. Heterogeneous degradation of refractory pollutants by peroxymonosulfate activated by CoOx-doped ordered mesoporous carbon. Chem. Eng. J. 2017, 328, 1112–1121. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, J.; Gao, Y.; Pang, S.Y.; Ma, J.; Duan, J.; Guo, Q.; Li, J.; Yang, Y. Oxidation of steroid estrogens by peroxymonosulfate (PMS) and effect of bromide and chloride ions: Kinetics, products, and modeling. Water Res. 2018, 138, 56–66. [Google Scholar] [CrossRef]
- Gábor, L.; József, K.; Zsuzsa, B.; Alíz, K.; Ildikó, K.; Dávid, B.; Marcell, T.; Lilla, V.; Fábián, I. One-versus two-electron oxidation with peroxomonosulfate ion reactions with iron(II), vanadium(IV), halide ions, and photoreaction with cerium(III). Inorg. Chem. 2009, 48, 1763–1773. [Google Scholar]
- Li, Y.; Feng, Y.; Yang, B.; Yang, Z.; Shih, K. Activation of peroxymonosulfate by molybdenum disulfide-mediated traces of Fe(III) for sulfadiazine degradation. Chemosphere 2021, 283, 131212. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; He, D.; Zhu, D.; Lu, Y.; Li, C.; Li, X.; Dong, S.; Lyu, C. Electron-rich ketone-based covalent organic frameworks supported nickel oxyhydroxide for highly efficient peroxymonosulfate activation and sulfadiazine removal: Performance and multi-path reaction mechanisms. Sep. Purif. Technol. 2022, 296, 121350. [Google Scholar] [CrossRef]
- Yao, J.; Dong, Z.; Ye, X.; Yang, J.; Jia, Y.; Zhang, Y.; Liu, H. Electrochemically activated peroxymonosulfate with mixed metal oxide electrodes for sulfadiazine degradation: Mechanism, DFT study and toxicity evaluation. Chemosphere 2022, 309, 136695. [Google Scholar] [CrossRef]
- Lastre-Acosta, A.M.; Palharim, P.H.; Barbosa, I.M.; Mierzwa, J.C.; Silva Costa Teixeira, A.C. Removal of sulfadiazine from simulated industrial wastewater by a membrane bioreactor and ozonation. J. Environ. Manag. 2020, 271, 111040. [Google Scholar] [CrossRef]











| Processes | Reaction Conditions | SFD | Matrix | Removal Rate | k (min−1) | Refs. |
|---|---|---|---|---|---|---|
| Co3O4-MnO2/BC/PMS | [catalyst] = 0.1 g/L [PMS] = 1 mM pH = 7.0 | 25 mg/L | Ultrapure water | 100% (10 min) | 0.482 | [47] |
| ZIF-CoN3P-C/PMS | [catalyst] = 0.05 g/L [PMS] = 1 mM pH = 3.35 | 10 mg/L | Ultrapure water | 98.4% (5 min) | 1.074 | [48] |
| Fe3O4@Co3S4-3/PMS | [catalyst] = 0.2 g/L [PMS] = 1 mM pH = 6.9 | 20 mg/L | Ultrapure water | 95% (2 min) | 1.1609 | [49] |
| S-Fe0/PMS | [catalyst] = 0.05 g/L [PMS] = 0.5 mM pH = 5.4 | 0.08 mM | Ultrapure water | 99.4% (60 min) | 0.296 | [50] |
| MBC/PS | [catalyst] = 1.0 mg/L [PS] = 1.5 mM pH = 5.16 | 40 mg/L | Ultrapure water | 91.79% (60 min) | 0.0309 | [51] |
| Two-stage US-ZVI/PS | [US power] = 90 W [catalyst] = 0.6 mM [PS] = 1.4 mM pH = 6.5 | 20 mg/L | Ultrapure water | 97.4% (15 min) | 0.279 | [52] |
| UV/Oxone | [UV intensity] = 0.272 mW/cm2 [Oxone] = 80 μM pH = 7.0 | 20 μM | Ultrapure water | 98.9% (10 min) | 0.4518 | [53] |
| Ozone/PS | [Ozone flow rate] = 2.14 g/min [PS] = 3.0 mM pH = 8.0 | 0.03 mM | Ultrapure water | 96.5% (20 min) | 0.163 | This work |
| Effluent | 91.8% (20 min) | 0.1315 | ||||
| Ozone/PMS | [Ozone flow rate] = 2.14 g/min [PMS] = 3.0 mM pH = 8.0 | 0.03 mM | Ultrapure water | 99.3% (20 min) | 0.2267 | This work |
| Effluent | 97.5% (1 min) | 3.579 |
| Name | CAS No. | Molecular Weight | Formula | Purity | Manufacturer |
|---|---|---|---|---|---|
| Target compounds | |||||
| Sulfadiazine (SFD) | 68-35-9 | 250.28 | C10H10N4O2S | 99.0% | Sigma Aldrich Inc. |
| Compounds used in HPLC and LC/MS analyses | |||||
| Acetonitrile | 75-05-8 | 41.05 | C2H3N | ≥99.9% | Sigma Aldrich Inc. |
| Potassium phosphate | 7778-77-0 | 136.09 | KH2PO4 | ≥99.5% | Sigma Aldrich Inc. |
| Formic acid | 64-18-6 | 46.03 | CH2O2 | ≥98.0% | Sigma Aldrich Inc. |
| Compounds used for degradation experiments | |||||
| Hydrochloric acid | 7647-01-0 | 36.46 | HCl | ≥96.0% | Sigma Aldrich Inc. |
| Sodium hydroxide | 1310-73-2 | 40.00 | NaOH | ≥97.0% | Sigma Aldrich Inc. |
| Peroxymonosulfate | 37222-66-5 | 152.17 | KHSO5·0. 5KHSO4·0.5 K2SO4 | >4.0% (active oxygen) | Sigma Aldrich Inc. |
| Persulfate | 7727-21-1 | 270.02 | K2S2O8 | ≥99.0% (RT) | Sigma Aldrich Inc. |
| Sodium thiosulfate pentahydrate | 10102-17-7 | 248.18 | Na2S2O3·5H2O | ≥99.5% | Sigma Aldrich Inc. |
| Sodium nitrite | 7632-00-0 | 69.00 | NaNO2 | ≥97.0% | Sigma Aldrich Inc. |
| Sodium nitrate | 7631-99-4 | 84.99 | NaNO3 | ≥99.0% | Sigma Aldrich Inc. |
| Sodium chloride | 7647-14-5 | 58.44 | NaCl | ≥99.5% | Sigma Aldrich Inc. |
| Sodium bromide | 7647-15-6 | 102.89 | NaBr | ≥99.0% | Sigma Aldrich Inc. |
| Sodium sulfate | 7757-82-6 | 142.04 | Na2SO4 | ≥99.0% | Sigma Aldrich Inc. |
| Sodium phosphate | 7601-54-9 | 163.94 | Na3PO4 | 96% | Sigma Aldrich Inc. |
| Methanol | 67-56-1 | 32.04 | CH4O | ≥99.8% | Sigma Aldrich Inc. |
| Tert-Butanol | 75-65-0 | 74.12 | C4H10O | ≥99.0% | Sigma Aldrich Inc. |
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| pH | 6.55 | [Cu2+] (mg/L) | 0.019 |
| [PO43−] (mg/L) | 2 | [Zn2+] (mg/L) | 0.05 |
| [SO42−] (mg/L) | 567.9 | [Mg2+] (mg/L) | >149.171 |
| [Br−] (mg/L) | 13.3 | [Sr2+] (mg/L) | 0.976 |
| [Cl−] (mg/L) | 3750 | [Ca2+] (mg/L) | 66.986 |
| [NO3−] (mg/L) | 0.127 | [Se2+] (mg/L) | 0.01 |
| [NO2−] (mg/L) | 1.1 | [K+] (mg/L) | >96.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Zhang, Y.; Lu, F.; Li, F.; Xu, L.; Gan, L.; Cui, C.; Li, X.; Jin, Q.; Chu, W.; et al. Sulfadiazine Elimination from Wastewater Effluents under Ozone-Based Catalysis Processes. Catalysts 2023, 13, 1076. https://doi.org/10.3390/catal13071076
Li R, Zhang Y, Lu F, Li F, Xu L, Gan L, Cui C, Li X, Jin Q, Chu W, et al. Sulfadiazine Elimination from Wastewater Effluents under Ozone-Based Catalysis Processes. Catalysts. 2023; 13(7):1076. https://doi.org/10.3390/catal13071076
Chicago/Turabian StyleLi, Ruixue, Yanqiong Zhang, Fengru Lu, Feng Li, Lijie Xu, Lu Gan, Chao Cui, Xuesong Li, Qiutong Jin, Wei Chu, and et al. 2023. "Sulfadiazine Elimination from Wastewater Effluents under Ozone-Based Catalysis Processes" Catalysts 13, no. 7: 1076. https://doi.org/10.3390/catal13071076