The Sonocatalytic Activation of Persulfates on Iron Nanoparticle Decorated Zeolite for the Degradation of 1,4-Dioxane in Aquatic Environments
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Catalyst
2.2. Effect of Persulfate and Catalyst on Dioxane Degradation
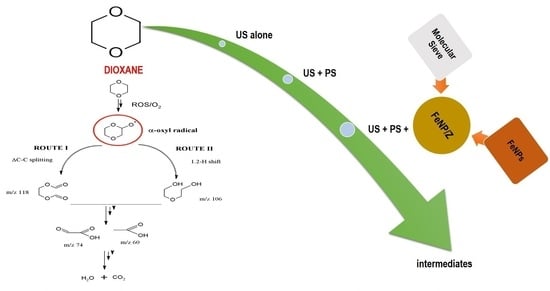
2.3. Identification of Reactive Species, Degradation Routes, and Intermediates
3. Materials and Methods
3.1. Materials
3.2. Experimental
3.2.1. Sonochemical Impregnation of Iron Nanoparticles on Molecular Sieves
3.2.2. Degradation Studies of Dioxane
3.3. Process Control and Intermediates Identification by Gas Chromatography
4. Cost Estimation
persulfate (Technical Grade) is INR 130/kg (Antares Chem Pvt Ltd., Mumbai, MS, India)] = 2055 INR/m3
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geissen, V.; Mol, H.; Klumpp, E.; Umlauf, G.; Nadal, M.; van der Ploeg, M.; van de Zee, S.E.A.T.M.; Ritsema, C.J. Emerging pollutants in the environment: A challenge for water resource management. Int. Soil Water Conserv. Res. 2015, 3, 57–65. [Google Scholar] [CrossRef]
- Ghumra, D.P.; Agarkoti, C.; Gogate, P.R. Improvements in effluent treatment technologies in Common Effluent Treatment Plants (CETPs): Review and recent advances. Process Saf. Environ. Prot. 2021, 147, 1018–1051. [Google Scholar] [CrossRef]
- Malkapuram, S.T.; Sharma, V.; Gumfekar, S.P.; Sonawane, S.; Sonawane, S.; Boczkaj, G.; Seepana, M.M. A review on recent advances in the application of biosurfactants in wastewater treatment. Sustain. Energy Technol. Assess. 2021, 48, 101576. [Google Scholar] [CrossRef]
- Kumari, S.; Debnath, M.; Sonawane, S.H.; Malkapuram, S.T.; Seepana, M.M. Dye Decolorization by Rhodococcus ruber Strain TES III Isolated from Textile Effluent Wastewater Contaminated Soil. ChemistrySelect 2022, 7, e202200421. [Google Scholar] [CrossRef]
- Salimi, M.; Esrafili, A.; Gholami, M.; Jafari, A.J.; Kalantary, R.R.; Farzadkia, M.; Kermani, M.; Sobhi, H.R. Contaminants of emerging concern: A review of new approach in AOP technologies. Environ. Monit. Assess. 2017, 189. [Google Scholar] [CrossRef]
- Kim, S.; Chu, K.H.; Al-Hamadani, Y.A.J.; Park, C.M.; Jang, M.; Kim, D.H.; Yu, M.; Heo, J.; Yoon, Y. Removal of contaminants of emerging concern by membranes in water and wastewater: A review. Chem. Eng. J. 2018, 335, 896–914. [Google Scholar] [CrossRef]
- Joseph, L.; Jun, B.M.; Jang, M.; Park, C.M.; Muñoz-Senmache, J.C.; Hernández-Maldonado, A.J.; Heyden, A.; Yu, M.; Yoon, Y. Removal of contaminants of emerging concern by metal-organic framework nanoadsorbents: A review. Chem. Eng. J. 2019, 369, 928–946. [Google Scholar] [CrossRef]
- Zhu, J.; Li, B. Degradation Kinetic and Remediation Effectiveness of 1,4-Dioxane-Contaminated Groundwater by a Sono-Activated Persulfate Process. J. Environ. Eng. 2018, 144, 1–8. [Google Scholar] [CrossRef]
- Klečka, G.M.; Gonsior, S.J. Removal of 1,4-dioxane from wastewater. J. Hazard. Mater. 1986, 13, 161–168. [Google Scholar] [CrossRef]
- Stepien, D.K.; Diehl, P.; Helm, J.; Thoms, A.; Püttmann, W. Fate of,4-dioxane in the aquatic environment: From sewage to drinking water. Water Res. 2014, 48, 406–419. [Google Scholar] [CrossRef]
- Doherty, A.-C.; Lee, C.-S.; Meng, Q.; Sakano, Y.; Noble, A.E.; Grant, K.A.; Esposito, A.; Gobler, C.J.; Venkatesan, A.K. Contribution of household and personal care products to 1,4-dioxane contamination of drinking water. Curr. Opin. Environ. Sci. Health 2023, 31, 100414. [Google Scholar] [CrossRef]
- Vatankhah, H.; Szczuka, A.; Mitch, W.A.; Almaraz, N.; Brannum, J.; Bellona, C. Evaluation of Enhanced Ozone-Biologically Active Filtration Treatment for the Removal of 1,4-Dioxane and Disinfection Byproduct Precursors from Wastewater Effluent. Environ. Sci. Technol. 2019, 53, 2720–2730. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, T.; Sengupta, S. Contaminants of Emerging Concern: Occurrence, Fate, and Remediation. In Advances in Water Purification Techniques; Elsevier: Amsterdam, The Netherlands, 2019; pp. 67–114. [Google Scholar] [CrossRef]
- Ghatak, H.R. Advanced Oxidation Processes for the Treatment of Biorecalcitrant Organics in Wastewater. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1167–1219. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Xu, X.; Zhong, Y.; Shao, Z. Double Perovskites in Catalysis, Electrocatalysis, and Photo(electro)catalysis. Trends Chem. 2019, 1, 410–424. [Google Scholar] [CrossRef]
- Krishnan, S.; Rawindran, H.; Sinnathambi, C.M.; Lim, J.W. Comparison of various advanced oxidation processes used in remediation of industrial wastewater laden with recalcitrant pollutants. IOP Conf. Ser. Mater. Sci. Eng. 2017, 206. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef]
- Trellu, C.; Chaplin, B.P.; Coetsier, C.; Esmilaire, R.; Cerneaux, S.; Causserand, C.; Cretin, M. Electro-oxidation of organic pollutants by reactive electrochemical membranes. Chemosphere 2018, 208, 159–175. [Google Scholar] [CrossRef] [Green Version]
- Barisci, S.; Suri, R. Degradation of,4-dioxane from water and plating industry wastewater using electrochemical batch and plug flow reactors. J. Appl. Electrochem. 2022, 53, 1169–1181. [Google Scholar] [CrossRef]
- Byrne, C.; Dervin, S.; Hermosilla, D.; Merayo, N.; Blanco, Á.; Hinder, S.; Harb, M.; Dionysiou, D.D.; Pillai, S.C. Solar light assisted photocatalytic degradation of 1,4-dioxane using high temperature stable anatase W-TiO2 nanocomposites. Catal. Today 2021, 380, 199–208. [Google Scholar] [CrossRef]
- Vikas, S.S.S.S.; Ghodke, S.; Teja, S.; Dilipkumar, P.; Sonawane, S.H.; Gaikwad, R. Ultrasonication based wastewater treatment. In Novel Approaches towards Wastewater Treatment and Resource Recovery; Elsevier: Amsterdam, The Netherlands, 2022; pp. 221–240. [Google Scholar] [CrossRef]
- Gogate, P.R.; Pandit, A.B. A review of imperative technologies for wastewater treatment II: Hybrid methods. Adv. Environ. Res. 2004, 8, 553–597. [Google Scholar] [CrossRef]
- Taylor, P.; Wang, J.L.; Xu, L.J. Advanced Oxidation Processes for Wastewater Treatment: Formation of Hydroxyl Radical and Application. Crit. Rev. Environ. Sci. Technol. 2011, 42, 37–41. [Google Scholar] [CrossRef]
- Xu, Z.; Mochida, K.; Naito, T.; Yasuda, K. Effects of operational conditions on 1,4-Dioxane degradation by combined use of ultrasound and ozone microbubbles. Jpn. J. Appl. Phys. 2012, 51, 07GD08. [Google Scholar] [CrossRef]
- Yang, L.; Xue, J.; He, L.; Wu, L.; Ma, Y.; Chen, H.; Li, H.; Peng, P.; Zhang, Z. Review on ultrasound assisted persulfate degradation of organic contaminants in wastewater: Influences, mechanisms and prospective. Chem. Eng. J. 2019, 378, 122146. [Google Scholar] [CrossRef]
- Sonawane, S.; Rayaroth, M.P.; Landge, V.K.; Fedorov, K.; Boczkaj, G. Thermally activated persulfate-based Advanced Oxidation Processes—Recent progress and challenges in mineralization of persistent organic chemicals: A review. Curr. Opin. Chem. Eng. 2022, 37, 100839. [Google Scholar] [CrossRef]
- Panda, D.; Saharan, V.K.; Manickam, S. Controlled hydrodynamic cavitation: A review of recent advances and perspectives for greener processing. Processes 2020, 8, 220. [Google Scholar] [CrossRef] [Green Version]
- Wacławek, S.; Lutze, H.V.; Grübel, K.; Padil, V.V.T.; Černík, M.; Dionysiou, D.D. Chemistry of persulfates in water and wastewater treatment: A review. Chem. Eng. J. 2017, 330, 44–62. [Google Scholar] [CrossRef]
- Fagan, W.P.; Zhao, J.; Villamena, F.A.; Zweier, J.L.; Weavers, L.K. Synergistic, aqueous PAH degradation by ultrasonically-activated persulfate depends on bulk temperature and physicochemical parameters. Ultrason. Sonochem. 2020, 67, 105172. [Google Scholar] [CrossRef]
- Yang, L.; Jiao, Y.; Xu, X.; Pan, Y.; Su, C.; Duan, X.; Sun, H.; Liu, S.; Wang, S.; Shao, Z. Superstructures with Atomic-Level Arranged Perovskite and Oxide Layers for Advanced Oxidation with an Enhanced Non-Free Radical Pathway. ACS Sustain. Chem. Eng. 2022, 10, 1899–1909. [Google Scholar] [CrossRef]
- Deng, J.; Feng, S.F.; Zhang, K.; Li, J.; Wang, H.; Zhang, T.; Ma, X. Heterogeneous activation of peroxymonosulfate using ordered mesoporous Co3O4 for the degradation of chloramphenicol at neutral pH. Chem. Eng. J. 2017, 308, 505–515. [Google Scholar] [CrossRef]
- Hu, Y.; He, Y.; Wang, X.; Wei, C. Efficient adsorption of phenanthrene by simply synthesized hydrophobic MCM-41 molecular sieves. Appl. Surf. Sci. 2014, 311, 825–830. [Google Scholar] [CrossRef]
- Karataev, O.R.; Novikov, V.F.; Karataeva, E.S.; Kartashova, A.A. Use of modified molecular sieves in mechanical filters. IOP Conf. Ser. Mater. Sci. Eng. 2019, 570, 11–14. [Google Scholar] [CrossRef]
- Wu, M.; Deng, H.; Shi, J.; Wang, Z. Transition element doped octahedral manganese molecular sieves (Me-OMS-2) as diclofenac adsorbents. Chemosphere 2020, 258, 127120. [Google Scholar] [CrossRef]
- Leal, T.W.; Lourenço, L.A.; de L. Brandão, H.; da Silva, A.; de Souza, S.M.A.G.U.; de Souza, A.A.U. Low-cost iron-doped catalyst for phenol degradation by heterogeneous Fenton. J. Hazard. Mater. 2018, 359, 96–103. [Google Scholar] [CrossRef]
- Ruíz-Baltazar, A.; Esparza, R.; Gonzalez, M.; Rosas, G.; Pérez, R. Preparation and characterization of natural zeolite modified with iron nanoparticles. J. Nanomater. 2015, 2015, 364763. [Google Scholar] [CrossRef] [Green Version]
- Vasconcellos, C.M.; Gonçalves, M.L.A.; Pereira, M.M.; Carvalho, N.M.F. Iron doped manganese oxide octahedral molecular sieve as potential catalyst for SOx removal at FCC. Appl. Catal. A Gen. 2015, 498, 69–75. [Google Scholar] [CrossRef]
- Guaya, D.; Jiménez, R.; Sarango, J.; Valderrama, C.; Cortina, J.L. Iron-doped natural clays: Low-cost inorganic adsorbents for phosphate recovering from simulated urban treated wastewater. J. Water Process Eng. 2021, 43. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Meng, F.; Wang, G.; Zhang, D. Preparation and evaluation of iron nanoparticles embedded CNTs grown on ZSM-5 as catalysts for NO decomposition. Chem. Eng. J. 2020, 392, 123798. [Google Scholar] [CrossRef]
- Chen, C.; Yang, X.-D.; Zhou, Z.-Y.; Lai, Y.-J.; Rauf, M.; Wang, Y.; Pan, J.; Zhuang, L.; Wang, Q.; Wang, Y.-C.; et al. Aminothiazole-derived N,S,Fe-doped graphene nanosheets as high performance electrocatalysts for oxygen reduction. Chem. Commun. 2015, 51, 17092–17095. [Google Scholar] [CrossRef]
- Xiao, S.; Cheng, M.; Zhong, H.; Liu, Z.; Liu, Y.; Yang, X.; Liang, Q. Iron-mediated activation of persulfate and peroxymonosulfate in both homogeneous and heterogeneous ways: A review. Chem. Eng. J. 2020, 384, 123265. [Google Scholar] [CrossRef]
- Ramakrishna, C.; Krishna, R.; Gopi, T.; Swetha, G.; Saini, B.; Shekar, S.C.; Srivastava, A. Complete oxidation of 1,4-dioxane over zeolite-13X-supported Fe catalysts in the presence of air. Cuihua Xuebao/Chin. J. Catal. 2016, 37, 240–249. [Google Scholar] [CrossRef]
- Sonawane, S.; Fedorov, K.; Rayaroth, M.P.; Boczkaj, G. Degradation of, 4-dioxane by sono-activated persulfates for water and wastewater treatment applications. Water Resour. Ind. 2022, 28, 100183. [Google Scholar] [CrossRef]
- Entezari, M.H.; Heshmati, A.; Sarafraz-Yazdi, A. A combination of ultrasound and inorganic catalyst: Removal of 2-chlorophenol from aqueous solution. Ultrason. Sonochem. 2005, 12, 137–141. [Google Scholar] [CrossRef]
- Chen, N.; Lee, D.; Kang, H.; Cha, D.; Lee, J.; Lee, C. Catalytic persulfate activation for oxidation of organic pollutants: A critical review on mechanisms and controversies. J. Environ. Chem. Eng. 2022, 10, 107654. [Google Scholar] [CrossRef]
- Zhao, L.; Hou, H.; Fujii, A.; Hosomi, M.; Li, F. Degradation of,4-dioxane in water with heat- and Fe2+-activated persulfate oxidation. Environ. Sci. Pollut. Res. 2014, 21, 7457–7465. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-Y.; Wang, L.; Liu, Y.-L.; Zhao, Q.; Ma, J. Unraveling the interaction of hydroxylamine and Fe(III) in Fe(II)/Persulfate system: A kinetic and simulating study. Water Res. 2020, 168, 115093. [Google Scholar] [CrossRef]
- Tawfik, A. Degradation pathways of 1,4-dioxane in biological and advanced oxidation processes. Desalin. Water Treat. 2020, 178, 360–386. [Google Scholar] [CrossRef]
- Beckett, M.A.; Hua, I. Elucidation of the 1,4-dioxane decomposition pathway at discrete ultrasonic frequencies. Environ. Sci. Technol. 2000, 34, 3944–3953. [Google Scholar] [CrossRef]
- Beckett, M.A.; Hua, I. Enhanced sonochemical decomposition of 1,4-dioxane by ferrous iron. Water Res. 2003, 37, 2372–2376. [Google Scholar] [CrossRef]
- Boczkaj, G.; Makoś, P.; Przyjazny, A. Application of dispersive liquid-liquid microextraction and gas chromatography with mass spectrometry for the determination of oxygenated volatile organic compounds in effluents from the production of petroleum bitumen. J. Sep. Sci. 2016, 39, 2604–2615. [Google Scholar] [CrossRef]
- Makoś, P.; Fernandes, A.; Boczkaj, G. Method for the simultaneous determination of monoaromatic and polycyclic aromatic hydrocarbons in industrial effluents using dispersive liquid-liquid microextraction with gas chromatography-mass spectrometry. J. Sep. Sci. 2018, 41, 2360–2367. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, K.; Plata-Gryl, M.; Khan, J.A.; Boczkaj, G. Ultrasound-assisted heterogeneous activation of persulfate and peroxymonosulfate by asphaltenes for the degradation of BTEX in water. J. Hazard. Mater. 2020, 397, 122804. [Google Scholar] [CrossRef] [PubMed]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malkapuram, S.T.; Sonawane, S.H.; Rayaroth, M.P.; Seepana, M.M.; Manickam, S.; Karczewski, J.; Boczkaj, G. The Sonocatalytic Activation of Persulfates on Iron Nanoparticle Decorated Zeolite for the Degradation of 1,4-Dioxane in Aquatic Environments. Catalysts 2023, 13, 1065. https://doi.org/10.3390/catal13071065
Malkapuram ST, Sonawane SH, Rayaroth MP, Seepana MM, Manickam S, Karczewski J, Boczkaj G. The Sonocatalytic Activation of Persulfates on Iron Nanoparticle Decorated Zeolite for the Degradation of 1,4-Dioxane in Aquatic Environments. Catalysts. 2023; 13(7):1065. https://doi.org/10.3390/catal13071065
Chicago/Turabian StyleMalkapuram, Surya Teja, Shirish Hari Sonawane, Manoj P. Rayaroth, Murali Mohan Seepana, Sivakumar Manickam, Jakub Karczewski, and Grzegorz Boczkaj. 2023. "The Sonocatalytic Activation of Persulfates on Iron Nanoparticle Decorated Zeolite for the Degradation of 1,4-Dioxane in Aquatic Environments" Catalysts 13, no. 7: 1065. https://doi.org/10.3390/catal13071065