Ultrafast and Facile Synthesis of (Ni/Fe/Mo)OOH on Ni Foam for Oxygen Evolution Reaction in Seawater Electrolysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Electrode Characterization
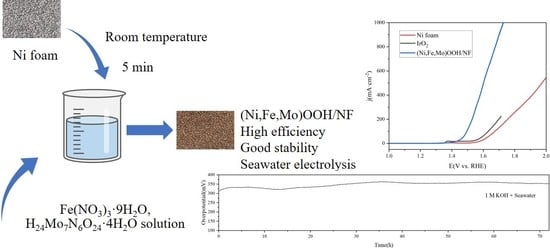
2.2. Electrocatalytic Activity for the OER
2.3. Durability Testing and Seawater Splitting Performance
3. Experimental Sections
3.1. Chemicals
3.2. Synthesis of (Ni/Fe/Mo)OOH and (Ni/Fe)OOH Catalysts on Nickel Foam
3.3. Synthesis of IrO2 Electrocatalyst on Nickel Foam
3.4. Materials Characterization
3.5. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, J.-Y. High safety, low cost, large capacity storage of high pressure gaseous hydrogen. Zhejiang Daxue Xuebao (Gongxue Ban)/J. Zhejiang Univ. (Eng. Sci.) 2020, 54, 1655–1657. [Google Scholar]
- Pei, Z.; Xu, L.; Xu, W. Hierarchical honeycomb-like Co3O4 pores coating on CoMoO4 nanosheets as bifunctional efficient electrocatalysts for overall water splitting. Appl. Surf. Sci. 2018, 433, 256–263. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, X.; Wen, H.; Pei, A. Hydrogen production from offshore wind power in South China. Int. J. Hydrogen Energy 2022, 47, 24558–24568. [Google Scholar] [CrossRef]
- Shao, Z.; Zhang, W.; An, D.; Zhang, G.; Wang, Y. Pyrolyzed egg yolk as an efficient bifunctional electrocatalyst for oxygen reduction and evolution reactions. RSC Adv. 2015, 5, 97508–97511. [Google Scholar] [CrossRef]
- Kunitski, M.; Eicke, N.; Huber, P.; Köhler, J.; Zeller, S.; Voigtsberger, J.; Schlott, N.; Henrichs, K.; Sann, H.; Trinter, F.; et al. Double-slit photoelectron interference in strong-field ionization of the neon dimer. Nat. Commun. 2019, 10, 1. [Google Scholar] [CrossRef]
- Shi, Q.; Zhu, C.; Du, D.; Lin, Y. Robust noble metal-based electrocatalysts for oxygen evolution reaction. Chem. Soc. Rev. 2019, 48, 3181–3192. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, H.; Lin, D.; Liu, C.; Hsu, P.-C.; Liu, W.; Chen, W.; Cui, Y. Electrochemical tuning of olivine-type lithium transition-metal phosphates as efficient water oxidation catalysts. Energy Environ. Sci. 2015, 8, 1719–1724. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Ferrer, I.M.; Chatman, S.M.; Peters, J.C.; Jaramillo, T.F. Benchmarking Hydrogen Evolving Reaction and Oxygen Evolving Reaction Electrocatalysts for Solar Water Splitting Devices. J. Am. Chem. Soc. 2015, 137, 4347–4357. [Google Scholar] [CrossRef]
- Xu, X.; Song, F.; Hu, X. A nickel iron diselenide-derived efficient oxygen-evolution catalyst. Nat. Commun. 2016, 7, 12324. [Google Scholar] [CrossRef] [PubMed]
- Song, J. Are Metal Chalcogenides, Nitrides, and Phosphides Oxygen Evolution Catalysts or Bifunctional Catalysts? ACS Energy Lett. 2017, 2, 1937–1938. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, H.; Liu, C.; Zhao, Y.; Liu, S.; Wang, D.; Fritz, M.; Ispas, A.; Bund, A.; Schaaf, P.; et al. Efficient preparation of Ni-M (M=Fe, Co, Mo) bimetallic oxides layer on Ni nanorod arrays for electrocatalytic oxygen evolution. Appl. Mater. Today 2021, 25, 101185. [Google Scholar] [CrossRef]
- Xu, L.; Yuan, B.; Min, L.; Xu, W.; Zhang, W. Preparation of NiCo-LDH@NiCoV-LDH interconnected nanosheets as high-performance electrocatalysts for overall water splitting. Int. J. Hydrog. Energy 2022, 47, 15583–15592. [Google Scholar] [CrossRef]
- Xu, L.; Cao, L.; Xu, W.; Pei, Z. One-step electrosynthesis of NiFe-NF electrodes for highly efficient overall water splitting. Appl. Surf. Sci. 2020, 503, 144122. [Google Scholar] [CrossRef]
- Liu, Z.; Yuan, C.; Teng, F.; Tang, M.; Abideen, Z.U.; Teng, Y. First insight on Mo(II) as electrocatalytically active species for oxygen evolution reaction. Int. J. Hydrogen Energy 2019, 44, 1345–1351. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Huang, C.-L.; Chen, Y.-A.; Lu, S.-Y. NiFeMo alloy inverse-opals on Ni foam as outstanding bifunctional catalysts for electrolytic water splitting of ultra-low cell voltages at high current densities. Appl. Catal. B Environ. 2020, 267, 118376. [Google Scholar] [CrossRef]
- Qin, F.; Zhao, Z.; Alam, M.K.; Ni, Y.; Robles-Hernandez, F.; Yu, L.; Chen, S.; Ren, Z.; Wang, Z.; Bao, J. Trimetallic NiFeMo for Overall Electrochemical Water Splitting with a Low Cell Voltage. ACS Energy Lett. 2018, 3, 546–554. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, H.; Sun, J.; Qin, F.; Yu, F.; Bao, J.; Yu, Y.; Chen, S.; Ren, Z. Cu nanowires shelled with NiFe layered double hydroxide nanosheets as bifunctional electrocatalysts for overall water splitting. Energy Environ. Sci. 2017, 10, 1820–1827. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, S.; Liu, P.; Li, Q.; Xu, S.; Liu, Y.; Jiang, K.; He, P.; Chen, M.; Wang, P.; et al. Capturing Reversible Cation Migration in Layered Structure Materials for Na-Ion Batteries. Adv. Energy Mater. 2019, 9, 1900189. [Google Scholar] [CrossRef]
- Wang, T.; Nam, G.; Jin, Y.; Wang, X.; Ren, P.; Kim, M.G.; Liang, J.; Wen, X.; Jang, H.; Han, J.; et al. NiFe (Oxy) Hydroxides Derived from NiFe Disulfides as an Efficient Oxygen Evolution Catalyst for Rechargeable Zn–Air Batteries: The Effect of Surface S Residues. Adv. Mater. 2018, 30, 1800757. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wu, Y.; Zhang, Z.; Yang, L.; Zang, Q. Rapid electrodeposited of self-supporting Ni-Fe-Mo film on Ni foam as affordable electrocatalysts for oxygen evolution reaction. Electrochim. Acta 2021, 390, 138754. [Google Scholar] [CrossRef]
- Liang, C.; Zou, P.; Nairan, A.; Zhang, Y.; Liu, J.; Liu, K.; Hu, S.; Kang, F.; Fan, H.J.; Yang, C. Exceptional performance of hierarchical Ni–Fe oxyhydroxide@NiFe alloy nanowire array electrocatalysts for large current density water splitting. Energy Environ. Sci. 2020, 13, 86–95. [Google Scholar] [CrossRef]
- Wang, A.-L.; Xu, H.; Li, G.-R. NiCoFe Layered Triple Hydroxides with Porous Structures as High-Performance Electrocatalysts for Overall Water Splitting. ACS Energy Lett. 2016, 1, 445–453. [Google Scholar] [CrossRef]
- Zhu, M.; Yan, Y.; Yan, Q.; Yin, J.; Cheng, K.; Ye, K.; Zhu, K.; Yan, J.; Cao, D.; Wang, G. In situ growth of Ni0·85Se on graphene as a robust electrocatalyst for hydrogen evolution reaction. Int. J. Hydrogen Energy 2020, 45, 10486–10493. [Google Scholar] [CrossRef]
- Wang, Q.; Shang, L.; Shi, R.; Zhang, X.; Zhao, Y.; Waterhouse, G.I.N.; Wu, L.-Z.; Tung, C.-H.; Zhang, T. NiFe Layered Double Hydroxide Nanoparticles on Co,N-Codoped Carbon Nanoframes as Efficient Bifunctional Catalysts for Rechargeable Zinc–Air Batteries. Adv. Energy Mater. 2017, 7, 1700467. [Google Scholar] [CrossRef]
- Wu, Y.; He, H. Electrodeposited nickel–iron–carbon–molybdenum film as efficient bifunctional electrocatalyst for overall water splitting in alkaline solution. Int. J. Hydrog. Energy 2019, 44, 1336–1344. [Google Scholar] [CrossRef]
- Duan, Y.; Yu, Z.-Y.; Hu, S.-J.; Zheng, X.-S.; Zhang, C.-T.; Ding, H.-H.; Hu, B.-C.; Fu, Q.-Q.; Yu, Z.-L.; Zheng, X.; et al. Scaled-Up Synthesis of Amorphous NiFeMo Oxides and Their Rapid Surface Reconstruction for Superior Oxygen Evolution Catalysis. Angew. Chem. Int. Ed. 2019, 58, 15772–15777. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wu, L.B.; McElhenny, B.; Song, S.W.; Luo, D.; Zhang, F.H.; Yu, Y.; Chen, S.; Ren, Z.F. Ultrafast room-temperature synthesis of porous S-doped Ni/Fe (oxy)hydroxide electrodes for oxygen evolution catalysis in seawater splitting. Energy Environ. Sci. 2020, 13, 3439–3446. [Google Scholar] [CrossRef]
- Li, Y.-K.; Zhang, G.; Lu, W.-T.; Cao, F.-F. Amorphous Ni–Fe–Mo Suboxides Coupled with Ni Network as Porous Nanoplate Array on Nickel Foam: A Highly Efficient and Durable Bifunctional Electrode for Overall Water Splitting. Adv. Sci. 2020, 7, 1902034. [Google Scholar] [CrossRef]
- Li, T.; Ma, X.; Wu, J.; Chu, F.; Qiao, L.; Song, Y.; Wu, M.; Lin, J.; Peng, L.; Chen, Z. Ni(OH)2 microspheres in situ self-grown on ultra-thin layered g-C3N4 as a heterojunction electrocatalyst for oxygen evolution reaction. Electrochim. Acta 2021, 400, 139473. [Google Scholar] [CrossRef]
- Mukai, K.; Suzuki, T.M.; Uyama, T.; Nonaka, T.; Morikawa, T.; Yamada, I. High-pressure synthesis of ε-FeOOH from β-FeOOH and its application to the water oxidation catalyst. RSC Adv. 2020, 10, 44756–44767. [Google Scholar] [CrossRef] [PubMed]
- Lyu, F.; Bai, Y.; Li, Z.; Xu, W.; Wang, Q.; Mao, J.; Wang, L.; Zhang, X.; Yin, Y. Self-Templated Fabrication of CoO–MoO2 Nanocages for Enhanced Oxygen Evolution. Adv. Funct. Mater. 2017, 27, 1702324. [Google Scholar] [CrossRef]
- Huang, L.; Chen, D.; Luo, G.; Lu, Y.-R.; Chen, C.; Zou, Y.; Dong, C.-L.; Li, Y.; Wang, S. Zirconium-Regulation-Induced Bifunctionality in 3D Cobalt–Iron Oxide Nanosheets for Overall Water Splitting. Adv. Mater. 2019, 31, 1901439. [Google Scholar] [CrossRef]
- Jin, Y.; Huang, S.; Yue, X.; Du, H.; Shen, P.K. Mo- and Fe-Modified Ni(OH)2/NiOOH Nanosheets as Highly Active and Stable Electrocatalysts for Oxygen Evolution Reaction. ACS Catal. 2018, 8, 2359–2363. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, Y.; Zhao, G.; Lin, Y.; Yu, Z.; Xu, X.; Wang, X.; Liu, H.K.; Sun, W.; Dou, S.X. Active-Site-Enriched Iron-Doped Nickel/Cobalt Hydroxide Nanosheets for Enhanced Oxygen Evolution Reaction. ACS Catal. 2018, 8, 5382–5390. [Google Scholar] [CrossRef]
- Niu, S.; Jiang, W.-J.; Wei, Z.; Tang, T.; Ma, J.; Hu, J.-S.; Wan, L.-J. Se-Doping Activates FeOOH for Cost-Effective and Efficient Electrochemical Water Oxidation. J. Am. Chem. Soc. 2019, 141, 7005–7013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Bedford, N.M.; Pan, J.; Lu, X.; Amal, R. A Fully Reversible Water Electrolyzer Cell Made Up from FeCoNi (Oxy)hydroxide Atomic Layers. Adv. Energy Mater. 2019, 9, 1901312. [Google Scholar] [CrossRef]
- Ye, S.-H.; Shi, Z.-X.; Feng, J.-X.; Tong, Y.-X.; Li, G.-R. Activating CoOOH Porous Nanosheet Arrays by Partial Iron Substitution for Efficient Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2018, 57, 2672–2676. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Wang, M.; Zhao, L.; Jiang, J.; Liu, H.; Liu, J. Self-Supported Stainless Steel Nanocone Array Coated with a Layer of Ni–Fe Oxides/(Oxy)hydroxides as a Highly Active and Robust Electrode for Water Oxidation. ACS Appl. Mater. Interfaces 2018, 10, 8786–8796. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yu, F.; Zhu, Q.; Sun, J.; Qin, F.; Yu, L.; Bao, J.; Yu, Y.; Chen, S.; Ren, Z. Water splitting by electrolysis at high current densities under 1.6 volts. Energy Environ. Sci. 2018, 11, 2858–2864. [Google Scholar] [CrossRef]
- Hu, E.; Feng, Y.; Nai, J.; Zhao, D.; Hu, Y.; Lou, X.W. Construction of hierarchical Ni–Co–P hollow nanobricks with oriented nanosheets for efficient overall water splitting. Energy Environ. Sci. 2018, 11, 872–880. [Google Scholar] [CrossRef]
- Suryawanshi, M.P.; Ghorpade, U.V.; Shin, S.W.; Suryawanshi, U.P.; Jo, E.; Kim, J.H. Hierarchically Coupled Ni:FeOOH Nanosheets on 3D N-Doped Graphite Foam as Self-Supported Electrocatalysts for Efficient and Durable Water Oxidation. ACS Catal. 2019, 9, 5025–5034. [Google Scholar] [CrossRef]
- Kuang, Y.; Kenney, M.J.; Meng, Y.T.; Hung, W.H.; Liu, Y.J.; Huang, J.E.; Prasanna, R.; Li, P.S.; Li, Y.P.; Wang, L.; et al. Solar-driven, highly sustained splitting of seawater into hydrogen and oxygen fuels. Proc. Natl. Acad. Sci. USA 2019, 116, 6624–6629. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Y.; Weng, C.-C.; Ren, J.-T.; Yuan, Z.-Y. An overview and recent advances in electrocatalysts for direct seawater splitting. Front. Chem. Sci. Eng. 2021, 15, 1408–1426. [Google Scholar] [CrossRef]
- Honarparvar, S.; Zhang, X.; Chen, T.; Alborzi, A.; Afroz, K.; Reible, D. Frontiers of Membrane Desalination Processes for Brackish Water Treatment: A Review. Membranes 2021, 11, 246. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, L.; Lu, W. Corrigendum to “Synergistic effect: Hierarchical Ni3S2@Co(OH)2 heterostructure as efficient bifunctional electrocatalyst for overall water splitting” Applied Surface Science, 405(2017)267–272. Appl. Surf. Sci. 2018, 462, 1044. [Google Scholar] [CrossRef]
- Yu, L.; Ren, Z. Systematic study of the influence of iR compensation on water electrolysis. Mater. Today Phys. 2020, 14, 100253. [Google Scholar] [CrossRef]
- Oelßner, W.; Berthold, F.; Guth, U. The iR drop–well-known but often underestimated in electrochemical polarization measurements and corrosion testing. Mater. Corros. 2006, 57, 455–466. [Google Scholar] [CrossRef]






| Electrocatalysts | Synthesis Process | Substrate | Current Density | Electrocatalysts | Classification of Synthetic Methods |
|---|---|---|---|---|---|
| (Ni/Fe/Mo)OOH | One-step solution-immersion synthesis, 5 min at room temperature. | Ni foam | 100 | 265 | One-step solution-immersion synthesis |
| S-(Ni,Fe)OOH [27] | One-pot solution-phase method: 5 min at room temperature. | Ni foam | 100 | 281 | |
| Ni/NiFeMoOx [28] | Three steps: hydrothermal for 10 h at 120 °C, 10 h at 120 °C using different solution and 2 h at 400 °C | Ni foam | 100 | 289 | Hydrothermal method |
| NiFeOH [34] | Two steps: hydrothermal for 10 h at 120 °C and 12 h at 50 °C. | Glassy carbon | 100 | 350 | |
| Se-doped FeOOH [35] | Two steps: hydrothermal for 12 h at 140 °C and 4 h at room temperature. | Fe foam | 100 | 279 | |
| FeCoNiOOH [36] | Hydrothermal for 6 h at 190 °C. | Ni foam | 100 | 330 | |
| FexCo1−xOOH [37] | A two-step electrochemical method: 1 h and 30 min at room temperature. | Carbon fiber cloth | 100 | 300 | Electrodeposition method |
| Ni–Fe–Mo [20] | Electrodeposition for 2 min. | Ni foam | 10 | 306 | |
| NiFeOx/NiFeOOH [38] | Two steps: 1 h for acid corrosion and 10 h of electrodeposition at room temperature. | Stainless steel | 100 | 280 | Multi-step multi-method mixed-use |
| NiCo-LDH@NiCoV-LDH [12] | Hydrothermal for 12 h at 110 °C, then electrodeposition for 15 min. | Ni foam | 100 | 260 | |
| (Ni,Fe)OOH [39] | Two steps, including a strong mechanically stirred process: 18 h at room temperature. | Ni foam | 100 | 220 | |
| NiCoP [40] | Four steps: using microwave refluxing system 400 w for 20 min, hydrothermal for 10 h at 90 °C, 4 h at 400 °C, and using the expensive reagent AgNO3. | Ni foam | 100 | 345 | |
| Ni:FeOOH [41] | Using hydrothermal process for 24 h at 180 °C, then using electrodeposition for 1 h at room temperature. | N-doped graphite foam | 100 | 270 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Dong, Y.; Xu, W.; Zhang, W. Ultrafast and Facile Synthesis of (Ni/Fe/Mo)OOH on Ni Foam for Oxygen Evolution Reaction in Seawater Electrolysis. Catalysts 2023, 13, 924. https://doi.org/10.3390/catal13060924
Xu L, Dong Y, Xu W, Zhang W. Ultrafast and Facile Synthesis of (Ni/Fe/Mo)OOH on Ni Foam for Oxygen Evolution Reaction in Seawater Electrolysis. Catalysts. 2023; 13(6):924. https://doi.org/10.3390/catal13060924
Chicago/Turabian StyleXu, Li, Yuxuan Dong, Wei Xu, and Wen Zhang. 2023. "Ultrafast and Facile Synthesis of (Ni/Fe/Mo)OOH on Ni Foam for Oxygen Evolution Reaction in Seawater Electrolysis" Catalysts 13, no. 6: 924. https://doi.org/10.3390/catal13060924