Effects of Mott–Schottky Frequency Selection and Other Controlling Factors on Flat-Band Potential and Band-Edge Position Determination of TiO2
Abstract
:1. Introduction
Theory on Band Edges Construction from MS-SF Analysis
2. Experiment
2.1. Sample Preparation
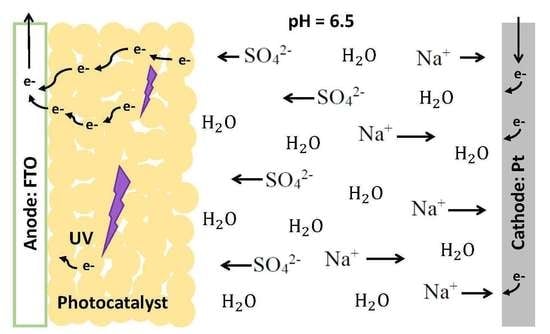
2.2. Experimental Setup and Electrochemical Method
3. Results and Discussion
3.1. Sample Characterization
3.2. Frequency Determination on MS-SF Analysis
3.3. Influence of Different Irradiation Sources
3.4. Influence of pH
3.5. Band Gap
3.6. Band-Edge positions for Photocatalytic Activity Prediction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martinez-Oviedo, A.; Kshetri, Y.K.; Joshi, B.; Lee, S.W. Surface modification of blue TiO2 with silane coupling agent for NOx abatement. Prog. Nat. Sci. Mater. Int. 2021, 31, 230–238. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, F.; Ko, S.; Wang, C.; Liu, H.; Tang, X.; Yi, H.; Zhou, Y. Superior catalytic performance within H2O-vapor of W-modified CoMn2O4/TiO2 catalyst for selective catalytic reduction of NOx with NH3. Chem. Eng. Sci. 2022, 434, 134770. [Google Scholar] [CrossRef]
- Khan, H.; Alalm, M.G.; Lalonde-Lavoie, M.; Ordonez, M.F.; Sartirana, M.; Giordana, A.; Cerrato, G.; Bianchi, C.L.; Boffito, D.C. Photocatalytic degradation of NOx and ethanol in the gas phase by spray dried Ce-TiO2. J. Environ. Chem. Eng. 2021, 9, 106813. [Google Scholar] [CrossRef]
- Jimenez-Relinque, E.; Lee, S.F.; Plaza, L.; Castellote, M. Synergetic adsorption–photocatalysis process for water treatment using TiO2 supported on waste stainless steel slag. Environ. Sci. Pollut. Res. 2022, 29, 39712–39722. [Google Scholar] [CrossRef]
- Zhao, D.; Yang, C.-F. Recent advances in the TiO2/CdS nanocomposite used for photocatalytic hydrogen production and quantum-dot-sensitized solar cells. Renew. Sustain. Energy Rev. 2016, 54, 1048–1059. [Google Scholar] [CrossRef]
- Xie, Z.; Chen, J.; Chen, Y.; Wang, T.; Jiang, X.; Xie, Y.; Lu, C.Z. A Z-scheme Pd modified ZnIn2S4/P25 heterojunction for enhanced photocatalytic hydrogen evolution. Appl. Surf. Sci. 2022, 579, 152003. [Google Scholar] [CrossRef]
- Mao, J.X.; Wang, J.C.; Gao, H.; Shi, W.; Jiang, H.P.; Hou, Y.; Li, R.; Zhang, W.; Liu, L. S-scheme heterojunction of CuBi2O4 supported Na doped P25 for enhanced photocatalytic H2 evolution. Int. J. Hydrogen Energy 2022, 47, 8214–8223. [Google Scholar] [CrossRef]
- Qin, M.; Chen, L.; Zhang, H.; Humayun, M.; Fu, Y.; Xu, X.; Xue, X.; Wang, C. Achieving highly efficient pH-universal hydrogen evolution by Mott-Schottky heterojunction of Co2P/Co4N. J. Chem. Eng. 2023, 454, 140230. [Google Scholar] [CrossRef]
- Sun, H.; Li, L.; Humayun, M.; Zhang, H.; Bo, Y.; Ao, X.; Xu, X.; Chen, K.; Ostrikov, K.; Huo, K.; et al. Achieving highly efficient pH-universal hydrogen evolution by superhydrophilic amorphous/crystalline Rh(OH)3/NiTe coaxial nanorod array electrode. Appl. Catal. B Environ. 2022, 305, 121088. [Google Scholar] [CrossRef]
- Chen, B.; Humayun, M.; Li, Y.; Zhang, H.; Sun, H.; Wu, Y.; Wang, C. Constructing hierarchical fluffy CoO−Co4N@NiFe-LDH nanorod arrays for highly effective overall water splitting and urea electrolysis. ACS Sustain. Chem. Eng. 2021, 9, 14180–14192. [Google Scholar] [CrossRef]
- Lee, S.F.; Jimenez-Relinque, E.; Martinez, I.; Castellote, M. Photoelectrochemical global approach to the behaviour of nanostructured anatase under different irradiation conditions. Catal. Today. 2022, 397–399, 286–295. [Google Scholar] [CrossRef]
- Naatz, H.; Hoffmann, R.; Hartwig, A.; la Mantia, F.; Pokhrel, S.; Mädler, L. Determination of the flat band potential of nanoparticles in porous electrodes by blocking the substrate−electrolyte contact. J. Phys. Chem. C 2018, 122, 2796–2805. [Google Scholar] [CrossRef]
- Hankin, A.; Bedoya-Lora, F.E.; Alexander, J.C.; Regoutz, A.; Kelsall, G.H. Flat band potential determination: Avoiding the pitfalls. J. Mater. Chem. A 2019, 7, 26162–26176. [Google Scholar] [CrossRef] [Green Version]
- Sivula, K. Mott−Schottky Analysis of Photoelectrodes: Sanity Checks Are Needed. ACS Energy Lett. 2021, 6, 2549–2551. [Google Scholar] [CrossRef]
- Krysova, H.; Mazzolini, P.; Casari, C.S.; Russo, V.; Bassi, A.L.; Kavana, L. Electrochemical properties of transparent conducting films of tantalum-doped titanium dioxide. Electrochim. Acta. 2017, 232, 44–53. [Google Scholar] [CrossRef]
- Sanz-Navarro, C.F.; Lee, S.F.; Yap, S.S.; Nee, C.H.; Yap, S.L. Electrochemical stability and corrosion mechanism of fluorine-doped tin oxide film under cathodic polarization in near neutral electrolyte. Thin Solid Films. 2023, 768, 139697. [Google Scholar] [CrossRef]
- Li, H.; Wu, S.; Hood, Z.D.; Sun, J.; Hu, B.; Liang, C.; Yang, S.; Xu, Y.; Jiang, B. Atomic defects in ultra-thin mesoporous TiO2 enhance photocatalytic hydrogen evolution from water splitting. Appl. Surf. Sci. 2020, 513, 145723. [Google Scholar] [CrossRef]
- Grochowska, K.; Nedyalkov, N.; Karczewski, J.; Haryński, Ł.; Śliwiński, G.; Siuzdak, K. Anodic titania nanotubes decorated with gold nanoparticles produced by laser-induced dewetting of thin metallic films. Sci. Rep. 2020, 10, 20506. [Google Scholar] [CrossRef]
- Lim, Y.; Lee, S.Y.; Kim, D.; Han, M.-K.; Han, H.S.; Kang, S.H.; Kim, J.K.; Sim, U.; Park, Y.I. Expanded solar absorption spectrum to improve photoelectrochemical oxygen evolution reaction: Synergistic effect of upconversion nanoparticles and ZnFe2O4/TiO2. J. Chem. Eng. 2022, 438, 135503. [Google Scholar] [CrossRef]
- Giannakopoulou, T.; Papailias, I.; Todorova, N.; Boukos, N.; Liu, Y.; Yu, J.; Trapalis, C. Tailoring the energy band gap and edges’ potentials of g-C3N4/TiO2 composite photocatalysts for NOx removal. Chem. Eng. J. 2017, 310, 571–580. [Google Scholar] [CrossRef]
- Sudhagar, P.; Devadoss, A.; Nakata, K.; Terashima, C.; Fujishima, A. Enhanced photoelectrocatalytic water splitting at hierarchical Gd3+:TiO2 nanostructures through amplifying light reception and surface states passivation. J. Electrochem. Soc. 2015, 162, 108–114. [Google Scholar] [CrossRef]
- Far, V.B.; Jafarzadeh, K.; Gugtapeh, H.S.; Mirali, S.M. A study on electrical properties of thermally grown TiO2 film at the interface of Ti/RuO2–IrO2–TiO2 anode using Mott-Schottky and electrochemical impedance spectroscopy techniques. Mater. Chem. Phys. 2020, 256, 123756. [Google Scholar]
- Chen, H.; Liu, G.; Wang, L. Switched photocurrent direction in Au/TiO2 bilayer thin films. Sci. Rep. 2015, 5, 10852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denisov, N.; Zhou, X.; Cha, G.; Schmuki, P. Photocurrent conversion efficiency of TiO2 nanotube photoanodes in dependence of illumination intensity. Electrochim. Acta. 2021, 377, 137988. [Google Scholar] [CrossRef]
- Radecka, M.; Rekas, M.; Trenczek-Zajac, A.; Zakrzewska, K. Importance of the band gap energy and flat band potential for application of modified TiO2 photoanodes in water photolysis. J. Power Sources. 2008, 181, 46–55. [Google Scholar] [CrossRef]
- Radecka, M.; Wierzbicka, M.; Komornicki, S.; Rekas, M. Influence of Cr on photoelectrochemical properties of TiO2 thin films. Phys. B Condens. Matter. 2004, 348, 160–168. [Google Scholar] [CrossRef]
- Gerischer, H. Neglected problems in the pH dependence of the flatband potential of semiconducting oxides and semiconductors covered with oxide layers. Electrochim. Acta. 1989, 34, 1005–1009. [Google Scholar] [CrossRef]
- Kong, D.-S.; Zhang, X.-D.; Wang, J.; Wang, C.; Zhao, X.; Feng, Y.-Y.; Li, W.-J. A photoelectrochemical study on the features of carbonate-catalyzed water oxidation at illuminated TiO2/Solution interface. J. Solid State Electrochem. 2013, 17, 69–77. [Google Scholar] [CrossRef]
- Van de Krol, R.; Goossens, A.; Schoonman, J. Mott-Schottky analysis of nanometer-scale thin-film anatase TiO2. J. Electrochem. Soc. 1997, 144, 1723–1727. [Google Scholar] [CrossRef]
- Bisquert, J. Physical electrochemistry of nanostructured devices. Phys. Chem. Chem. Phys. 2008, 10, 49–72. [Google Scholar] [CrossRef]
- Vafaei, M.; Mohammadi, M.R. Impact of chromium doping on physical, optical, electronic and photovoltaic properties of nanoparticle TiO2 photoanodes in dye-sensitized solar cells. New J. Chem. 2017, 41, 14516–14527. [Google Scholar] [CrossRef]
- Matsumoto, Y. Energy positions of oxide semiconductors and photocatalysis with iron complex oxides. J. Solid State Chem. 1996, 126, 227–234. [Google Scholar] [CrossRef]
- Kavan, L.; Grätzel, M.; Gilbert, S.E.; Klemenz, C.; Scheel, H.J. Electrochemical and photoelectrochemical investigation of single-crystal anatase. J. Am. Chem. Soc. 1996, 118, 6716–6723. [Google Scholar] [CrossRef]
- Doustkhah, E.; Assadi, M.H.N.; Komaguchi, K.; Tsunoji, N.; Esmat, M.; Fukata, N.; Tomita, O.; Abe, R.; Ohtani, B.; Ide, Y. In situ blue titania via band shape engineering for exceptional solar H2 production in rutile TiO2. Appl. Catal. B Environ. 2021, 297, 120380. [Google Scholar] [CrossRef]
- Sakai, N.; Ebina, Y.; Takada, K.; Sasaki, T. Electronic band structure of titania semiconductor nanosheets revealed by electrochemical and photoelectrochemical studies. J. Am. Chem. Soc. 2004, 126, 5851–5858. [Google Scholar] [CrossRef]
- Ohtani, B.; Prieto-Mahaney, O.O.; Li, D.; Abe, R. What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. J. Photochem. Photobiol. A Chem. 2010, 216, 179–182. [Google Scholar] [CrossRef] [Green Version]
- Nazeeruddin, M.K.; Kay, A.; Rodicio, I.; Humphry-Baker, R.; Muller, E.; Liska, P.; Vlachopoulos, N.; Grätzel, M. Conversion of light to electricity by cis-X2Bis(2,2′-bipyridyl-4,4′-dicarboxylate)ruthenium(II) charge-transfer sensitizers (X = Cl−, Br−, I−, CN−, and SCN−) on nanocrystalline TiO2 electrodes. J. Am. Chem. Soc. 1993, 115, 6382–6390. [Google Scholar] [CrossRef]
- de Jongh, P.E.; Vanmaekelbergh, D. Trap-limited electronic transport in assemblies of nanometer-size TiO2 particles. Phys. Rev. Lett. 1996, 77, 3427–3430. [Google Scholar] [CrossRef]
- El-Deen, S.S.; Hashem, A.M.; Abdel Ghany, A.E.; Indris, S.; Ehrenberg, H.; Mauger, A.; Julien, C.M. Anatase TiO2 nanoparticles for lithium-ion batteries. Ionics 2018, 24, 2925–2934. [Google Scholar] [CrossRef]
- Masood, M.H.; Haleem, N.; Shakeel, I.; Jamal, Y. Carbon dioxide conversion into the reaction intermediate sodium formate for the synthesis of formic acid. Res. Chem. Intermed. 2020, 46, 5165–5180. [Google Scholar] [CrossRef]
- Slavkova, Z.; Kostadinova, O.; Avdeev, G.; Petkova, T. Structure and thermal behaviour of lithium sodium sulphate. In Advanced Nanotechnologies for Detection and Defence against CBRN Agents; Petkov, P., Tsiulyanu, D., Popov, C., Kulisch, W., Eds.; Springer: Cham, The Netherlands, 2017; pp. 87–93. [Google Scholar]
- Lee, K.-T.; Lu, S.-Y. Porous FTO thin layers created with a facile one-step Sn4+-based anodic deposition process and their potential applications in ion sensing. J. Mater. Sci. 2012, 22, 16259–16268. [Google Scholar] [CrossRef]
- He, J.; Du, Y.-E.; Bai, Y.; An, J.; Cai, X.; Chen, Y.; Wang, P.; Yang, X.; Feng, Q. Facile formation of anatase/rutile TiO2 nanocomposites with enhanced photocatalytic activity. Molecules 2019, 24, 2996. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Navarro, C.; Doehne, E.; Sebastian, E. How does sodium sulfate crystallize? Implications for the decay and testing of building materials. Cem. Concr. Res. 2000, 30, 1527–1534. [Google Scholar] [CrossRef] [Green Version]
- Sequeda-Pico, I.N.; Joya-Herrera, L.M.; Meléndez, A.M.; Ospina, R.O.; Henao Martínez, J.A.; Niño-Gómez, M.E. Enhanced photoelectrochemical performance of iron and carbon self-doped TiO2 photoanodes modified with nitrogen. Thin Solid Films. 2018, 653, 326–332. [Google Scholar] [CrossRef]
- Beranek, R. (Photo)electrochemical methods for the determination of the band edge positions of TiO2-based nanomaterials. Adv. Phys. Chem. 2011, 2011, 786759. [Google Scholar] [CrossRef] [Green Version]
- Bukackova, M.; Marsalek, R. Interaction of BSA with ZnO, TiO2, and CeO2 nanoparticles. Biophys. Chem. 2020, 267, 106475. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Cheng, Y.; Yang, C.; Zeng, G.; Zhu, C.; Yan, Z. Influences of anion concentration and valence on dispersion and aggregation of titanium dioxide nanoparticles in aqueous solutions. J. Environ. Sci. 2017, 54, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Ding, Y. Formulation of nanofluids for natural convective heat transfer applications. Int. J. Heat Fluid Flow. 2005, 26, 855–864. [Google Scholar] [CrossRef]
- Gumy, D.; Morais, C.; Bowen, P.; Pulgarin, C.; Giraldo, S.; Hajdu, R.; Kiwi, J. Catalytic activity of commercial of TiO2 powders for the abatement of the bacteria (E. coli) under solar simulated light: Influence of the isoelectric point. Appl. Catal. B 2006, 63, 76–84. [Google Scholar] [CrossRef]
- Cheng, Y.; Jiang, S.P. Advances in electrocatalysts for oxygen evolution reaction of water electrolysis—From metal oxides to carbon nanotubes. Prog. Nat. Sci. Mater. Int. 2015, 25, 545–553. [Google Scholar] [CrossRef] [Green Version]
- Ghernaout, D. Advanced oxidation phenomena in electrocoagulation process: A myth or a reality? Desalin. Water Treat. 2013, 51, 7536–7554. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, K.; Hasija, V.; Sharma, V.; Sharma, S.; Raizada, P.; Singh, M.; Saini, A.K.; Hosseini-Bandegharaei, A.; Thakur, V.K. Systematic review on applicability of magnetic iron oxides-integrated photocatalysts for degradation of organic pollutants in water. Mater. Today Chem. 2019, 14, 100186. [Google Scholar] [CrossRef]
- Bard, A.J.; Parsons, R.; Jordan, J. Standard Potentials in Aqueous Solution; Marcel Dekker, Inc.: New York, NY, USA; Basel, Switzerland, 1985. [Google Scholar]
- Prolo, C.; Estrada, D.; Piacenza, L.; Benítez, D.; Comini, M.A.; Radi, R.; Álvarez, M.N. NOx2-derived superoxide radical is crucial to control acute Trypanosoma cruzi infection. Redox Biol. 2021, 46, 102085. [Google Scholar] [CrossRef]
- Lv, X.; Lam, F.L.-Y.; Hu, X. Developing SrTiO3/TiO2 heterostructure nanotube array for photocatalytic fuel cells with improved efficiency and elucidating the effects of organic substrates. Chem. Eng. J. 2022, 427, 131602. [Google Scholar] [CrossRef]
- Sheikhmohammadi, A.; Asgari, E.; Nourmoradi, H.; Fazli, M.M.; Yeganeh, M. Ultrasound-assisted decomposition of metronidazole by synthesized TiO2/Fe3O4 nanocatalyst: Influencing factors and mechanisms. J. Environ. Chem. Eng. 2021, 9, 105844. [Google Scholar] [CrossRef]
- Koppenol, W.H.; Stanbury, D.M.; Bounds, P.L. Electrode potentials of partially reduced oxygen species, from dioxygen to water. Free Radic. Biol. Med. 2010, 49, 317–322. [Google Scholar] [CrossRef]

















| Sample’s Label | Description |
|---|---|
| Ana(400)-fs&fs | Anatase calcined at 400 °C, fresh sample, and fresh solution for each frequency tested |
| Ana(400)-ss&ss | Anatase calcined at 400 °C, same sample, and same solution throughout the test |
| Ana-ss&ss | Anatase in as-received, same sample, and same solution throughout the test |
| P25-fs&fs | P25 in as-received, fresh sample, and fresh solution for each frequency tested |
| P25(400)-fs&fs | P25 calcined at 400 °C, fresh sample, and fresh solution for each frequency tested |
| P25(400)-ss&ss | P25 calcined at 400 °C, same sample, and same solution throughout the test |
| P25(400)-ss&ss + stir | P25 calcined at 400 °C, same sample, and same solution plus solution stirring by magnet throughout the test |
| Mean of Vfb | Standard Deviation, σ | Upper Limit of Vfb at +1σ | Lower Limit of Vfb at −1σ | Range of Frequency (Hz) Corresponding to Vfb ± 1σ | |
|---|---|---|---|---|---|
| Ana(400)- fs&fs | −0.516 | ±0.035 | −0.481 | −0.551 | 200–3000 |
| Ana(400)-ss&ss | −0.709 | ±0.172 | −0.538 | −0.881 | 75–3000 |
| Ana-ss&ss | −0.856 | ±0.091 | −0.765 | −0.945 | 75–3000 |
| P25-fs&fs | −0.823 | ±0.055 | −0.768 | −0.877 | 200–2000 |
| P25(400)-fs&fs | −0.584 | ±0.063 | −0.521 | −0.647 | 100–3000 |
| P25(400)-ss&ss | −0.749 | ±0.137 | −0.611 | −0.886 | 50–4000 |
| P25(400)-ss&ss + stir | −0.781 | ±0.092 | −0.689 | −0.872 | 25–4000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.F.; Jimenez-Relinque, E.; Martinez, I.; Castellote, M. Effects of Mott–Schottky Frequency Selection and Other Controlling Factors on Flat-Band Potential and Band-Edge Position Determination of TiO2. Catalysts 2023, 13, 1000. https://doi.org/10.3390/catal13061000
Lee SF, Jimenez-Relinque E, Martinez I, Castellote M. Effects of Mott–Schottky Frequency Selection and Other Controlling Factors on Flat-Band Potential and Band-Edge Position Determination of TiO2. Catalysts. 2023; 13(6):1000. https://doi.org/10.3390/catal13061000
Chicago/Turabian StyleLee, Siaw Foon, Eva Jimenez-Relinque, Isabel Martinez, and Marta Castellote. 2023. "Effects of Mott–Schottky Frequency Selection and Other Controlling Factors on Flat-Band Potential and Band-Edge Position Determination of TiO2" Catalysts 13, no. 6: 1000. https://doi.org/10.3390/catal13061000