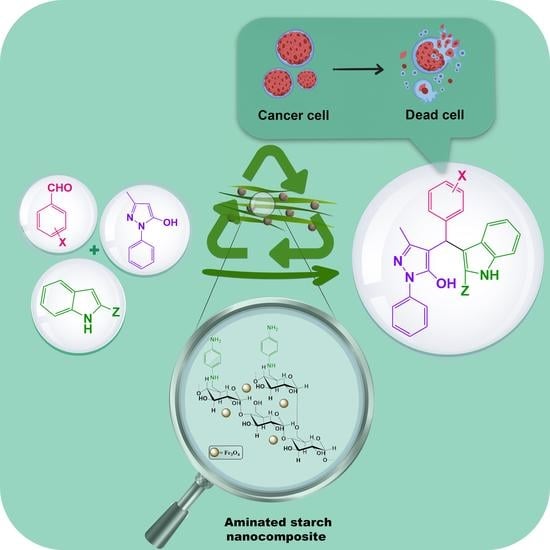
Synthesis, and Anticancer Evaluation of 4-[(Indol-3-yl)-arylmethyl]-1-phenyl-3-methyl-5-pyrazolone Derivatives via a Magnetic Aminated Starch Biocatalyst
Abstract
:1. Introduction
2. Results and Discussions
2.1. Characterization of Biocatalyst
2.2. Investigation of the Catalytic Activity of the MAST
2.2.1. Proposed Mechanism
2.2.2. Recovery
2.3. In-Vitro Anticancer Study
3. Materials and Methods
3.1. Materials and Instruments
3.2. Synthesis of Aminated Starch
3.3. Preparation of Magnetic Aminated Starch
3.4. Synthesis of 3-Methyl-1-phenyl-1H-pyrazole5-ol: A General Procedure
3.5. Synthesis of 4-[(Indol-3-yl)-arylmethyl]-1-phenyl-3-methyl-5-pyrazolones
3.6. In Vitro Cell Viability Assay
4. Conclusions
5. Spectroscopic Data
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Heidarizadeh, F.; Rahimi, E. Synthesis of 3-substituted Indoles through Yonemitsu Reaction with Copper Benzene-1,3,5-tricarboxylate acid Catalyst. Mater. Chem. Horizons 2022, 1, 169–176. [Google Scholar] [CrossRef]
- Asadi Nasr, M.; Deinavizadeh, M.; Kiasat, A.R. Fe3O4@SiO2/DABCO(OH) Core-Shell Hybrid Nanocomposite: Efficient Nanomagnetic and Basic Reusable Catalyst in the One-pot Synthesis of Trithiocarbonate Derivatives. Mater. Chem. Horizons. 2023; in press. [Google Scholar] [CrossRef]
- Reddy, K.R.; Rajgopal, K.; Kantam, M.L. Copper-alginates: A biopolymer supported Cu(II) catalyst for 1,3-dipolar cycloaddition of alkynes with azides and oxidative coupling of 2-naphthols and phenols in water. Catal. Letters 2007, 114, 36–40. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Motamedi, A.; Hosseini, S.H.; Nazari, M. Magnetic starch nanocomposite as a green heterogeneous support for immobilization of large amounts of copper ions: Heterogeneous catalyst for click synthesis of 1,2,3-triazoles. RSC Adv. 2016, 6, 19128–19135. [Google Scholar] [CrossRef]
- Zareh, E.N.; Moghadam, P.N.; Azariyan, E.; Sharifian, I. Conductive and biodegradable polyaniline/starch blends and their composites with polystyrene. Iran. Polym. J. 2011, 20, 319–328. [Google Scholar]
- Sharma, B.; Malik, P.; Jain, P. Biopolymer reinforced nanocomposites: A comprehensive review. Mater. Today Commun. 2018, 16, 353–363. [Google Scholar] [CrossRef]
- Alizadeh, E.; Baseri, H. Photocatalytic degradation of Sumatriptan Succinate by ZnO, Fe doped ZnO and TiO2-ZnO nanocatalysts. Mater. Chem. Horizons 2022, 1, 7–21. [Google Scholar] [CrossRef]
- Heidari, G.; Hassanpour, M.; Nejaddehbashi, F.; Sarfjoo, M.R.; Yousefiasl, S.; Sharifi, E.; Bigham, A.; Agarwal, T.; Borzacchiello, A.; Lagreca, E.; et al. Biosynthesized Nanomaterials with Antioxidant and Antimicrobial Properties. Mater. Chem. Horizons 2022, 1, 35–48. [Google Scholar] [CrossRef]
- Ghorbanipour, F.; Nezhad, S.M.; Pourmousavi, S.A.; Zare, E.N.; Heidari, G. Superparamagnetic polymer nanocomposite as a catalyst for the synthesis of pyrano[3,2-c]chromene, pyrano[2,3-c]pyrazole, and benzylpyrazolyl coumarin. Inorg. Chem. Commun. 2023, 147, 110271. [Google Scholar] [CrossRef]
- Nakagawa, H.; Ohyama, R.; Kimata, A.; Suzuki, T.; Miyata, N. Hydroxyl radical scavenging by edaravone derivatives: Efficient scavenging by 3-methyl-1-(pyridin-2-yl)-5-pyrazolone with an intramolecular base. Bioorg. Med. Chem. Lett. 2006, 16, 5939–5942. [Google Scholar] [CrossRef]
- Alam, M.A. Antibacterial pyrazoles: Tackling resistant bacteria. Future Med. Chem. 2022, 14, 343–362. [Google Scholar] [CrossRef] [PubMed]
- Vijesh, A.M.; Isloor, A.M.; Isloor, S.; Shivananda, K.N.; Shyma, P.C.; Arulmoli, T. Synthesis of some new pyrazolone derivatives as potent antimicrobial agents. Der Pharma Chem. 2011, 3, 454–463. [Google Scholar]
- Ramajayam, R.; Tan, K.P.; Liu, H.G.; Liang, P.H. Synthesis and evaluation of pyrazolone compounds as SARS-coronavirus 3C-like protease inhibitors. Bioorg. Med. Chem. 2010, 18, 7849–7854. [Google Scholar] [CrossRef]
- Marković, V.; Erić, S.; Stanojković, T.; Gligorijević, N.; Aranelović, S.; Todorović, N.; Trifunović, S.; Manojlović, N.; Jelić, R.; Joksović, M.D. Antiproliferative activity and QSAR studies of a series of new 4-aminomethylidene derivatives of some pyrazol-5-ones. Bioorg. Med. Chem. Lett. 2011, 21, 4416–4421. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, P.; Perumal, S.; Yogeeswari, P.; Sriram, D. A facile four-component sequential protocol in the expedient synthesis of novel 2-aryl-5-methyl-2,3-dihydro-1H-3-pyrazolones in water and their antitubercular evaluation. Eur. J. Med. Chem. 2011, 46, 4530–4536. [Google Scholar] [CrossRef]
- Ragab, F.A.F.; Abdel-Gawad, N.M.; Georgey, H.H.; Said, M.F. Pyrazolone derivatives: Synthesis, anti-inflammatory, analgesic quantitative structure-activity relationship and in vitro studies. Chem. Pharm. Bull. 2013, 61, 834–845. [Google Scholar] [CrossRef]
- Rao, D.J.; Nagaraju, K.; Maddila, S. Microwave irradiated mild, rapid, one-pot and multi-component synthesis of isoxazole-5(4H)-ones. Chem. Data Collect. 2021, 32, 100669. [Google Scholar] [CrossRef]
- Sivakumar, K.K.; Rajasekaran, A.; Senthilkumar, P.; Wattamwar, P.P. Conventional and microwave assisted synthesis of pyrazolone Mannich bases possessing anti-inflammatory, analgesic, ulcerogenic effect and antimicrobial properties. Bioorg. Med. Chem. Lett. 2014, 24, 2940–2944. [Google Scholar] [CrossRef]
- Sarfjoo, M.R.; Shad, A.; Hassanpour, M.; Varma, R. An Overview on New Anticancer Drugs Approved by Food and Drug Administration: Impending Economic and Environmental Challenges. Mater. Chem. Horizons 2022, 1, 189–198. [Google Scholar] [CrossRef]
- Ma, R.; Zhu, J.; Liu, J.; Chen, L.; Shen, X.; Jiang, H.; Li, J. Microwave-assisted one-pot synthesis of pyrazolone derivatives under solvent-free conditions. Molecules 2010, 15, 3593–3601. [Google Scholar] [CrossRef]
- Malle, A.; Yehualawork, A. This is an electronic reprint of the original article. This reprint may differ from the original in pagination and typographic detail. Int. J. Spec. Educ. 2015, 30, 70–84. [Google Scholar]
- Mousa, S.A.S.; Ishak, E.A.; Bakheet, M.E.M.; Abu-shanab, F.A. New Route for the Synthesis of Pyrazolone Derivatives. Elixir Org. Chem. 2015, 89, 36854–36859. [Google Scholar]
- Yang, Z.; Wang, Z.; Bai, S.; Liu, X.; Lin, L.; Feng, X. Asymmetric α-amination of 4-substituted pyrazolones catalyzed by a chiral Gd(OTf)3/ N,N′-dioxide complex: Highly enantioselective synthesis of 4-amino-5-pyrazolone derivatives. Org. Lett. 2011, 13, 596–599. [Google Scholar] [CrossRef]
- Isaad, J.; El Achari, A. Synthesis and spectroscopic characterization of azoic dyes based on pyrazolone derivatives catalyzed by an acidic ionic liquid supported on silica-coated magnetite nanoparticle. J. Mol. Struct. 2018, 1154, 557–564. [Google Scholar] [CrossRef]
- Ekbote, S.S.; Gadge, S.T.; Bhanage, B.M. Synthesis of Pyrazole by Using Polyvinylsulfonic Acid (PVSA) as a Novel Bronsted Acid Catalyst. Curr. Catal. 2016, 5, 4–10. [Google Scholar] [CrossRef]
- Mojtahedi, M.M.; Javadpour, M.; Abaee, M.S. Convenient ultrasound mediated synthesis of substituted pyrazolones under solvent-free conditions. Ultrason. Sonochem. 2008, 15, 828–832. [Google Scholar] [CrossRef]
- Nouri, A.; Jelkmann, M.; Khoee, S.; Bernkop-Schnürch, A. Diaminated Starch: A Competitor of Chitosan with Highly Mucoadhesive Properties due to Increased Local Cationic Charge Density. Biomacromolecules 2020, 21, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Lu, Y. Solid-state synthesis of 4-[(indol-3-yl)-arylmethyl]-1-phenyl-3-methyl-5- pyrazolones by catalysis of molecular iodine. Synth. Commun. 2006, 36, 2389–2399. [Google Scholar] [CrossRef]
- Li, X.L.; Wang, Y.M.; Tian, B.; Matsuura, T.; Meng, J. Ben The Solid-State Michael Addition of 3-Methyl-1-phenyl-5-pyrazolone. J. Heterocycl. Chem. 1998, 35, 129–134. [Google Scholar] [CrossRef]
- Nezhad, S.M.; Pourmousavi, S.A.; Zare, E.N.; Heidari, G.; Makvandi, P. Magnetic sulfonated melamine-Formaldehyde Resin as an efficient catalyst for the synthesis of antioxidant and antimicrobial pyrazolone derivatives. Catalysts 2022, 12, 626. [Google Scholar] [CrossRef]
- Yusuf, H.; Satria, D.; Suryawati, S.; Fahriani, M. Combination therapy of eurycomanone and doxorubicin as anticancer on T47D and MCF-7 Cell Lines. Syst. Rev. Pharm. 2020, 11, 335–341. [Google Scholar]
- Fang, X.J.; Jiang, H.; Zhu, Y.Q.; Zhang, L.Y.; Fan, Q.H.; Tian, Y. Doxorubicin induces drug resistance and expression of the novel CD44st via NF-κB in human breast cancer MCF-7 cells. Oncol. Rep. 2014, 31, 2735–2742. [Google Scholar] [CrossRef] [PubMed]
- Lovitt, C.J.; Shelper, T.B.; Avery, V.M. Doxorubicin resistance in breast cancer cells is mediated by extracellular matrix proteins. BMC Cancer 2018, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- Rouibah, H.; Kebsa, W.; Lahouel, M.; Zihlif, M.; Ahram, M.; Aburmaileh, B.; Mansour Al Shhab, M.A.; Al-Ameer, H.J.; Mustafa, E. Algerian propolis: Between protection of normal cells and potentialisation of the anticancer effects of doxorubicin against breast cancer cells via P-glycoprotein inhibition and cell cycle arrest in the S phase. J. Physiol. Pharm. 2021, 72, 10–26402. [Google Scholar]









| Entry | Solvent | Catalyst (g) | Temp. (°C) | Time (Min.) | Yield (%) b |
|---|---|---|---|---|---|
| 1 | EtOH/H2O | 0.04 | 40 | 120 | 55 |
| 2 | H2O | 0.04 | 40 | 180 | 30 |
| 3 | THF | 0.04 | 40 | 180 | 30 |
| 4 | EtOH | 0.04 | 40 | 90 | 75 |
| 5 | MeOH | 0.04 | 40 | 90 | 70 |
| 5 | CHCl3 | 0.04 | 40 | 180 | 30 |
| 6 | Hexane | 0.04 | 40 | 180 | 10 |
| 7 | Solvent-free | 0.04 | 40 | 180 | 30 |
| 8 | EtOH | 0.04 | r.t | 100 | 60 |
| 9 | EtOH | 0.04 | 60 | 100 | 55 |
| 10 | EtOH | 0.04 | 80 | 100 | 30 |
| 11 | EtOH | 0.06 | 40 | 80 | 87 |
| 12 | EtOH | 0.08 | 40 | 80 | 88 |
| 13 | EtOH | - | 40 | 120 | 45 |
| Entry | Product | Code | Time (min.) | Yield (%) b | M.P. (°C) | Ref. | |
|---|---|---|---|---|---|---|---|
| Observed | Reported | ||||||
| 1 |  | 6a | 80 | 87 | 233–234 | 235–236 | [28] |
| 2 |  | 6b | 60 | 90 | 178–179 | 173–175 | [28] |
| 3 |  | 6c | 70 | 87 | 182–183 | 180–182 | [28] |
| 4 |  | 6d | 65 | 88 | 170–173 | 170–171 | [28] |
| 5 |  | 6e | 35 | 93 | 184–186 | 184–186 | [28] |
| 6 |  | 6f | 40 | 90 | 239–241 | 242–244 | [28] |
| 7 |  | 6g | 65 | 89 | 195–197 | 206 | [29] |
| 8 |  | 6h | 70 | 89 | 162–164 | 161–163 | [28] |
| 9 |  | 6i | 60 | 91 | 194–195 | 191–193 | [28] |
| 10 |  | 6j | 50 | 90 | 241–243 | 242–244 | [30] |
| 11 |  | 6k | 90 | 85 | 245–246 | 246 | [29] |
| 12 |  | 6l | 80 | 86 | 149–151 | NR | |
| 13 |  | 6m | 70 | 90 | 213–215 | NR | |
| 14 |  | 6n | 90 | 88 | 187–190 | NR | |
| 15 |  | 6o | 70 | 87 | 211–213 | 210–212 | [30] |
| 16 |  | 6p | 50 | 85 | 195–196 | 193–195 | [30] |
| 17 |  | 6q | 30 | 90 | 232–234 | 231–233 | [30] |
| 18 |  | 6r | 40 | 91 | 187–188 | 185–187 | [30] |
| 19 |  | 6s | 50 | 85 | 188–189 | 185–187 | [30] |
| 20 |  | 6t | 65 | 86 | 182–185 | NR | |
| 21 |  | 6u | 90 | 89 | 194–197 | NR | |
| 22 |  | 6v | 35 | 92 | 180–182 | NR | |
| 23 |  | 6w | 70 | 89 | 200–203 | NR | |
| 24 |  | 6x | 60 | 90 | 161–163 | NR | |
| 25 |  | 6y | 50 | 92 | 225–228 | NR | |
| Sample | Cell | Time (h) | |
|---|---|---|---|
| 24 | 48 | ||
| 6l | MCF-7 | 39.2 | 41.3 |
| Fibroblast | 392.1 | 409.4 | |
| 6p | MCF-7 | 38.2 | 23.1 |
| Fibroblast | 70.07 | 150.1 | |
| 6r | MCF-7 | 34.6 | 52.5 |
| Fibroblast | 81.9 | 115.4 | |
| 6n | MCF-7 | 25.8 | 21.3 |
| Fibroblast | 226.8 | 96.6 | |
| 6j | MCF-7 | 1660.5 | 11,647.4 |
| Fibroblast | 498,041.6 | 597,030.5 | |
| 6y | MCF-7 | 48.5 | 52.0 |
| Fibroblast | 92.5 | 135.8 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramshini, A.; Nezhad, S.M.; Pourmousavi, S.A.; Nazarzadeh Zare, E.; Pourjafar, M.; Sharifi, E. Synthesis, and Anticancer Evaluation of 4-[(Indol-3-yl)-arylmethyl]-1-phenyl-3-methyl-5-pyrazolone Derivatives via a Magnetic Aminated Starch Biocatalyst. Catalysts 2023, 13, 908. https://doi.org/10.3390/catal13050908
Ramshini A, Nezhad SM, Pourmousavi SA, Nazarzadeh Zare E, Pourjafar M, Sharifi E. Synthesis, and Anticancer Evaluation of 4-[(Indol-3-yl)-arylmethyl]-1-phenyl-3-methyl-5-pyrazolone Derivatives via a Magnetic Aminated Starch Biocatalyst. Catalysts. 2023; 13(5):908. https://doi.org/10.3390/catal13050908
Chicago/Turabian StyleRamshini, Ali, Shefa Mirani Nezhad, Seied Ali Pourmousavi, Ehsan Nazarzadeh Zare, Mona Pourjafar, and Esmaeel Sharifi. 2023. "Synthesis, and Anticancer Evaluation of 4-[(Indol-3-yl)-arylmethyl]-1-phenyl-3-methyl-5-pyrazolone Derivatives via a Magnetic Aminated Starch Biocatalyst" Catalysts 13, no. 5: 908. https://doi.org/10.3390/catal13050908