Immobilization of TiO2 Photocatalysts for Water Treatment in Geopolymer Based Coatings
Abstract
:1. Introduction
2. Results
2.1. XRD
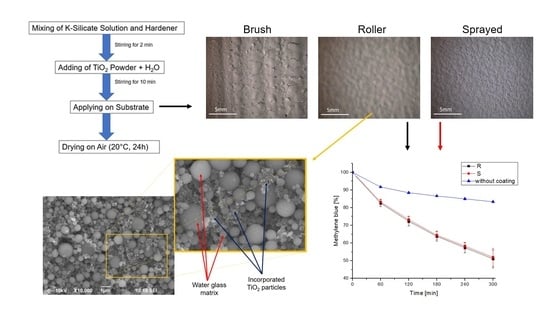
2.2. Optical Properties: Surface and Cross Section
2.3. EDX (Energy-Dispersive X-ray Spectroscopy)
2.4. Photocatalytic Activity
3. Discussion
4. Materials and Methods
4.1. Application of Photocatalytic Coatings
4.2. Powder and Coating Characterization
4.2.1. X-ray Diffraction (XRD)
4.2.2. SEM, EDX and Optical Microscope
4.3. Photocatalytic Activity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ismael, M. A review and recent advances in solar-to-hydrogen energy conversion based on photocatalytic water splitting over doped-TiO2 nanoparticles. Sol. Energy 2020, 211, 522–546. [Google Scholar] [CrossRef]
- Omar, A.; Ali, M.S.; Abd Rahim, N. Electron transport properties analysis of titanium dioxide dye-sensitized solar cells (TiO2-DSSCs) based natural dyes using electrochemical impedance spectroscopy concept: A review. Sol. Energy 2020, 207, 1088–1121. [Google Scholar] [CrossRef]
- Ochiai, T.; Fujishima, A. Photoelectrochemical properties of TiO2 photocatalyst and its applications for environmental purification. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 247–262. [Google Scholar] [CrossRef]
- Schoenell, E.K.; Otto, N.; Rodrigues, M.A.S.; Metzger, J.W. Removal of Organic Micropollutants from Treated Municipal Wastewater by O3/UV/H2O2 in a UVA-LED Reactor. Ozone Sci. Eng. 2022, 44, 172–181. [Google Scholar] [CrossRef]
- Dionysiou, D.D.; Bahnemann, D.; Puma, G.L.; Ye, J.; Schneider, J. (Eds.) Photocatalysis: Applications; Royal Society of Chemistry: Cambridge, UK, 2016; ISBN 978-1-78262-709-8. [Google Scholar]
- Pichat, P. (Ed.) Photocatalysis and Water Purification: From Fundamentals to Recent Applications; Wiley-VCH-Verl: Weinheim, Germany, 2013; ISBN 978-3-527-33187-1. [Google Scholar]
- Schneider, J.; Bahnemann, D.; Ye, J.; Li Puma, G.; Dionysiou, D.D. (Eds.) Photocatalysis; Royal Society of Chemistry: Cambridge, UK, 2016; ISBN 978-1-78262-041-9. [Google Scholar]
- Bojinova, A.; Kralchevska, R.; Poulios, I.; Dushkin, C. Anatase/rutile TiO2 composites: Influence of the mixing ratio on the photocatalytic degradation of Malachite Green and Orange II in slurry. Mater. Chem. Phys. 2007, 106, 187–192. [Google Scholar] [CrossRef]
- Zaleska, A. Doped-TiO2: A Review. ENG 2008, 2, 157–164. [Google Scholar] [CrossRef]
- Ollis, D.F.; Al-Ekabi, H. (Eds.) Photocatalytic Purification and Treatment of Water and Air: Proceedings of the 1st International Conference on TiO2 Photocatalytic Purification and Treatment of Water and Air, London, ON, Canada, 8–13 November 1992; Elsevier Science Limited: Amsterdam, The Netherlands, 1993. [Google Scholar]
- Mills, A.; Le Hunte, S. An overview of semiconductor photocatalysis. J. Photochem. Photobiol. A Chem. 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Ide, Y.; Inami, N.; Hattori, H.; Saito, K.; Sohmiya, M.; Tsunoji, N.; Komaguchi, K.; Sano, T.; Bando, Y.; Golberg, D.; et al. Remarkable Charge Separation and Photocatalytic Efficiency Enhancement through Interconnection of TiO2 Nanoparticles by Hydrothermal Treatment. Angew. Chem. Int. Ed. Engl. 2016, 55, 3600–3605. [Google Scholar] [CrossRef]
- Ohtani, B.; Prieto-Mahaney, O.O.; Li, D.; Abe, R. What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. J. Photochem. Photobiol. A Chem. 2010, 216, 179–182. [Google Scholar] [CrossRef]
- Tobaldi, D.M.; Pullar, R.C.; Seabra, M.P.; Labrincha, J.A. Fully quantitative X-ray characterisation of Evonik Aeroxide TiO2 P25®. Mater. Lett. 2014, 122, 345–347. [Google Scholar] [CrossRef]
- Salkic, S.; Eckler, L.H.; Nee, M.J. Noninvasive monitoring of photocatalytic degradation of X-ray contrast media using Raman spectrometry. J. Raman Spectrosc. 2013, 44, 1746–1752. [Google Scholar] [CrossRef]
- Rachel, A.; Subrahmanyam, M.; Boule, P. Comparison of photocatalytic efficiencies of TiO2 in suspended and immobilised form for the photocatalytic degradation of nitrobenzenesulfonic acids. Appl. Catal. B Environ. 2002, 37, 301–308. [Google Scholar] [CrossRef]
- Cohen-Yaniv, V.; Narkis, N.; Armon, R. Photocatalytic inactivation of Flavobacterium and E. coli in water by a continuous stirred tank reactor (CSTR) fed with suspended/immobilised TiO2 medium. Water Sci. Technol. 2008, 58, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Pozzo, R.L.; Baltanás, M.A.; Cassano, A.E. Supported titanium oxide as photocatalyst in water decontamination: State of the art. Catal. Today 1997, 39, 219–231. [Google Scholar] [CrossRef]
- Singh, S.; Mahalingam, H.; Singh, P.K. Polymer-supported titanium dioxide photocatalysts for environmental remediation: A review. Appl. Catal. A Gen. 2013, 462, 178–195. [Google Scholar] [CrossRef]
- Espíndola, J.C.; Cristóvão, R.O.; Mendes, A.; Boaventura, R.A.; Vilar, V.J. Photocatalytic membrane reactor performance towards oxytetracycline removal from synthetic and real matrices: Suspended vs immobilized TiO2-P25. Chem. Eng. J. 2019, 378, 122114. [Google Scholar] [CrossRef]
- Alrousan, D.; Polo-López, M.I.; Dunlop, P.; Fernández-Ibáñez, P.; Byrne, J.A. Solar photocatalytic disinfection of water with immobilised titanium dioxide in re-circulating flow CPC reactors. Appl. Catal. B Environ. 2012, 128, 126–134. [Google Scholar] [CrossRef]
- Khan, S.J.; Reed, R.H.; Rasul, M.G. Thin-film fixed-bed reactor for solar photocatalytic inactivation of Aeromonas hydrophila: Influence of water quality. BMC Microbiol. 2012, 12, 285. [Google Scholar] [CrossRef]
- Mehos, M.S.; Turchi, C.S. Field testing solar photocatalytic detoxification on TCE-contaminated groundwater. Environ. Prog. 1993, 12, 194–199. [Google Scholar] [CrossRef]
- Oelgemöller, M.; Healy, N.; de Oliveira, L.; Jung, C.; Mattay, J. Green photochemistry: Solar-chemical synthesis of Juglone with medium concentrated sunlight. Green Chem. 2006, 8, 831–834. [Google Scholar] [CrossRef]
- Martín-Sómer, M.; Moreno-SanSegundo, J.; Álvarez-Fernández, C.; van Grieken, R.; Marugán, J. High-performance low-cost solar collectors for water treatment fabricated with recycled materials, open-source hardware and 3d-printing technologies. Sci. Total Environ. 2021, 784, 147119. [Google Scholar] [CrossRef] [PubMed]
- Akpan, U.G.; Hameed, B.H. The advancements in sol–gel method of doped-TiO2 photocatalysts. Appl. Catal. A Gen. 2010, 375, 1–11. [Google Scholar] [CrossRef]
- Arconada, N.; Durán, A.; Suárez, S.; Portela, R.; Coronado, J.M.; Sánchez, B.; Castro, Y. Synthesis and photocatalytic properties of dense and porous TiO2-anatase thin films prepared by sol–gel. Appl. Catal. B Environ. 2009, 86, 1–7. [Google Scholar] [CrossRef]
- Toma, F.-L.; Bertrand, G.; Klein, D.; Meunier, C.; Begin, S. Development of Photocatalytic Active TiO2 Surfaces by Thermal Spraying of Nanopowders. J. Nanomater. 2008, 2008, 1–8. [Google Scholar] [CrossRef]
- Toma, F.; Bertrand, G.; Begin, S.; Meunier, C.; Barres, O.; Klein, D.; Coddet, C. Microstructure and environmental functionalities of TiO2-supported photocatalysts obtained by suspension plasma spraying. Appl. Catal. B Environ. 2006, 68, 74–84. [Google Scholar] [CrossRef]
- Toma, F.-L.; Bertrand, G.; Klein, D.; Coddet, C.; Meunier, C. Nanostructured Photocatalytic Titania Coatings Formed by Suspension Plasma Spraying. J. Therm. Spray Tech. 2006, 15, 587–592. [Google Scholar] [CrossRef]
- Bolelli, G.; Cannillo, V.; Gadow, R.; Killinger, A.; Lusvarghi, L.; Rauch, J. Properties of High Velocity Suspension Flame Sprayed (HVSFS) TiO2 coatings. Surf. Coat. Technol. 2009, 203, 1722–1732. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, M.; Lei, L. Preparation of anatase TiO2 supported on alumina by different metal organic chemical vapor deposition methods. Appl. Catal. A Gen. 2005, 282, 285–293. [Google Scholar] [CrossRef]
- Kim, B.-H.; Lee, J.-Y.; Choa, Y.-H.; Higuchi, M.; Mizutani, N. Preparation of TiO2 thin film by liquid sprayed mist CVD method. Mater. Sci. Eng. B 2004, 107, 289–294. [Google Scholar] [CrossRef]
- Ghorai, T.K.; Dhak, D.; Biswas, S.K.; Dalai, S.; Pramanik, P. Photocatalytic oxidation of organic dyes by nano-sized metal molybdate incorporated titanium dioxide (MxMoxTi1−xO6) (M=Ni, Cu, Zn) photocatalysts. J. Mol. Catal. A Chem. 2007, 273, 224–229. [Google Scholar] [CrossRef]
- Babelon, P.; Dequiedt, A.; Mostéfa-Sba, H.; Bourgeois, S.; Sibillot, P.; Sacilotti, M. SEM and XPS studies of titanium dioxide thin films grown by MOCVD. Thin Solid Film. 1998, 322, 63–67. [Google Scholar] [CrossRef]
- Karuppuchamy, S.; Suzuki, N.; Ito, S.; Endo, T. A novel one-step electrochemical method to obtain crystalline titanium dioxide films at low temperature. Curr. Appl. Phys. 2009, 9, 243–248. [Google Scholar] [CrossRef]
- Robayo-Salazar, R.A.; Mejía-Arcila, J.M.; Mejía de Gutiérrez, R. Eco-efficient alkali-activated cement based on red clay brick wastes suitable for the manufacturing of building materials. J. Clean. Prod. 2017, 166, 242–252. [Google Scholar] [CrossRef]
- Provis, J.L.; van Deventer, J.S.J. Geopolymers; Elsevier: Amsterdam, The Netherlands, 2009; ISBN 9781845694494. [Google Scholar]
- McLellan, B.C.; Williams, R.P.; Lay, J.; van Riessen, A.; Corder, G.D. Costs and carbon emissions for geopolymer pastes in comparison to ordinary portland cement. J. Clean. Prod. 2011, 19, 1080–1090. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Pacheco Torgal, F.; Jalali, S. Eco-Efficient Construction and Building Materials; Springer: London, UK, 2011; ISBN 978-0-85729-892-8. [Google Scholar]
- Mao, Y.; Biasetto, L.; Colombo, P. Metakaolin-based geopolymer coatings on metals by airbrush spray deposition. J. Coat Technol. Res. 2020, 17, 991–1002. [Google Scholar] [CrossRef]
- Zheng, K.; Chen, L.; Gbozee, M. Thermal stability of geopolymers used as supporting materials for TiO2 film coating through sol-gel process: Feasibility and improvement. Constr. Build. Mater. 2016, 125, 1114–1126. [Google Scholar] [CrossRef]
- Strini, A.; Roviello, G.; Ricciotti, L.; Ferone, C.; Messina, F.; Schiavi, L.; Corsaro, D.; Cioffi, R. TiO₂-Based Photocatalytic Geopolymers for Nitric Oxide Degradation. Materials 2016, 9, 513. [Google Scholar] [CrossRef]
- Otto, N. Evaluierung Eines UV-A-LED Paneelreaktorkonzepts für Photo-Oxidationsverfahren in der (Ab-) Wasserreinigung; Forschungs- und Entwicklungsinstitut für Industrie- und Siedlungswasserbau e.V.: Stuttgart, Germany, 2022. [Google Scholar]
- Din, M.I.; Khalid, R.; Najeeb, J.; Hussain, Z. Fundamentals and photocatalysis of methylene blue dye using various nanocatalytic assemblies- a critical review. J. Clean. Prod. 2021, 298, 126567. [Google Scholar] [CrossRef]
- Lachheb, H.; Puzenat, E.; Houas, A.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.-M. Photocatalytic degradation of various types of dyes (Alizarin S, Crocein Orange G, Methyl Red, Congo Red, Methylene Blue) in water by UV-irradiated titania. Appl. Catal. B Environ. 2002, 39, 75–90. [Google Scholar] [CrossRef]
- Hindryawati, N.; Maniam, G.P.; Karim, M.R.; Chong, K.F. Transesterification of used cooking oil over alkali metal (Li, Na, K) supported rice husk silica as potential solid base catalyst. Eng. Sci. Technol. Int. J. 2014, 17, 95–103. [Google Scholar] [CrossRef]
- Vu, P.; Otto, N.; Vogel, A.; Kern, F.; Killinger, A.; Gadow, R. Efficiently quantifying the anatase content and investigating its effect on the photocatalytic activity of titania coatings by suspension plasma spraying. Surf. Coat. Technol. 2019, 371, 117–123. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Deutsches Institut für Normung. Photocatalytic Activity of Surfaces-Determination of Photocatalytic Activity by Degradation of Methylene Blue: DIN 52980. Beuth, 2008 (52980). Available online: https://www.beuth.de/de/norm/din-52980/110195678 (accessed on 1 February 2023).









| P25-TiO2 | Crystallite Size [nm] | Phase Content [%] |
|---|---|---|
| Anatase | 21.9 | 87.4 |
| Rutile | 41.0 | 12.6 |
| Sample Name | Coating Process |
|---|---|
| B | Application with a paint brush |
| R | Application with a roller |
| S | Application with a spray gun |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dufner, L.; Ott, F.; Otto, N.; Lembcke, T.; Kern, F. Immobilization of TiO2 Photocatalysts for Water Treatment in Geopolymer Based Coatings. Catalysts 2023, 13, 898. https://doi.org/10.3390/catal13050898
Dufner L, Ott F, Otto N, Lembcke T, Kern F. Immobilization of TiO2 Photocatalysts for Water Treatment in Geopolymer Based Coatings. Catalysts. 2023; 13(5):898. https://doi.org/10.3390/catal13050898
Chicago/Turabian StyleDufner, Lukas, Felix Ott, Nikolai Otto, Tom Lembcke, and Frank Kern. 2023. "Immobilization of TiO2 Photocatalysts for Water Treatment in Geopolymer Based Coatings" Catalysts 13, no. 5: 898. https://doi.org/10.3390/catal13050898