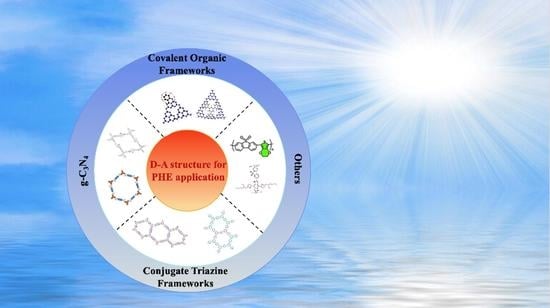
Recent Progress in Conjugated Polymers-Based Donor–Acceptor Semiconductor Materials for Photocatalytic Hydrogen Evolution from Water Splitting
Abstract
:1. Introduction
2. Fundamental Description and Mechanism of D–A Heterostructures
2.1. Fundamental Description of D–A Heterostructures
2.2. Mechanism of D–A Heterostructures
3. CPs-Based D–A Photocatalysts for Photocatalytic Hydrogen Evolution
3.1. D–A Structure Based on g-C3N4
3.2. Covalent Organic Frameworks (COFs)
3.3. Covalent Triazine-Based Frameworks (CTFs)
3.4. Other Photocatalysts
4. Conclusions and Outlook
- (i)
- D–A g-C3N4 is one of the most promising modification methods to improve the photocatalytic activity of g-C3N4 under visible light. In future works, further reasonable design of photocatalyst can be carried out in the following aspects. The separation and migration of photogenerated carriers can be further enhanced by loading suitable cocatalyst onto the electron acceptor of D–A g-C3N4. In addition, the combination of molecular structure design and morphological modulation can also greatly improve the PHE performance, including the preparation of porous structures and ultra-thin nanosheet structures. Finally, the appropriate ratio of D–A monomer and the extension of conjugate structure also have significant effects on the photocatalytic activity.
- (ii)
- The synthesis of highly crystalline D–A COFs requires many strict conditions, such as time, temperature, reaction rate, and preparation method. Therefore, the use of high throughput synthesis method is very promising. In addition, the active site of the reaction can be increased by anchoring uniformly dispersed metal atoms on D–A COFs. At the same time, the selection of appropriate skeleton and D–A repeating element are also extremely important to achieve the required geometric match between D and A elements.
- (iii)
- Unlike COFs, which are linked by reversible covalent bonds, CTFs are composed of stable C=N bonds, which is not conducive to dynamic association and dissociation of bonds during the reaction. In addition, the synthesis of D–A CTFs generally requires relatively harsher reaction conditions, such as high temperature, high pressure, and strong acids or bases. Therefore, it is necessary to explore an environmental-friendly synthesis of CTFs. Finally, most CTFs are amorphous or have poor crystallinity, so some design strategies such as improving crystallinity or even achieving crystal plane modulation should be further investigated.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nishiyama, H.; Yamada, T.; Nakabayashi, M.; Maehara, Y.; Yamaguchi, M.; Kuromiya, Y.; Nagatsuma, Y.; Tokudome, H.; Akiyama, S.; Watanabe, T.; et al. Photocatalytic solar hydrogen production from water on a 100-m2 scale. Nature 2021, 598, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Wang, Z.; Cao, L.; Zeng, L.; Zhang, C.; Lin, W.; Wang, C. Metal-organic frameworks embedded in a liposome facilitate overall photocatalytic water splitting. Nat. Chem. 2021, 13, 358–366. [Google Scholar] [CrossRef]
- Gutić, S.J.; Metarapi, D.; Jovanović, A.Z.; Gebremariam, G.K.; Dobrota, A.S.; Nedić Vasiljević, B.; Pašti, I.A. Redrawing HER Volcano with Interfacial Processes—The Role of Hydrogen Spillover in Boosting H2 Evolution in Alkaline Media. Catalysts 2023, 13, 89. [Google Scholar] [CrossRef]
- Otep, S.; Michinobu, T.; Zhang, Q. Pure Organic Semiconductor-Based Photoelectrodes for Water Splitting. Sol. RRL 2019, 4, 1900395. [Google Scholar] [CrossRef]
- Shahzad, W.; Badawi, A.K.; Rehan, Z.A.; Khan, A.M.; Khan, R.A.; Shah, F.; Ali, S.; Ismail, B. Enhanced visible light photocatalytic performance of Sr0.3(Ba, Mn)0.7ZrO3 perovskites anchored on graphene oxide. Ceram. Int. 2022, 48, 24979–24988. [Google Scholar] [CrossRef]
- Ni, L.; Xiao, Y.; Zhou, X.; Jiang, Y.; Liu, Y.; Zhang, W.; Zhang, J.; Liu, Z. Significantly Enhanced Photocatalytic Performance of the g-C3N4/Sulfur-Vacancy-Containing Zn3In2S6 Heterostructure for Photocatalytic H2 and H2O2 Generation by Coupling Defects with Heterojunction Engineering. Inorg. Chem. 2022, 61, 19552–19566. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, J.; Xiao, Y.; Jiang, Y.; Zhang, W.; Liu, Y.; Liu, Z.; Zhang, J. Boosting photoelectron transport in Zn0.5Cd0.5S/Sn3O4 heterostructure through close interface contact for enhancing photocatalytic H2 generation and degradation of tetracycline hydrochloride. Sep. Purif. Technol. 2023, 311, 123243. [Google Scholar] [CrossRef]
- Baaloudj, O.; Nasrallah, N.; Kenfoud, H.; Bourkeb, K.W.; Badawi, A.K. Polyaniline/Bi12TiO20 Hybrid System for Cefixime Removal by Combining Adsorption and Photocatalytic Degradation. ChemEngineering 2023, 7, 4. [Google Scholar] [CrossRef]
- Alasri, T.M.; Ali, S.L.; Salama, R.S.; Alshorifi, F.T. Band-Structure Engineering of TiO2 Photocatalyst by AuSe Quantum Dots for Efficient Degradation of Malachite Green and Phenol. J. Inorg. Organomet. P. 2023. [Google Scholar] [CrossRef]
- Saleh, T.S.; Badawi, A.K.; Salama, R.S.; Mostafa, M.M.M. Design and Development of Novel Composites Containing Nickel Ferrites Supported on Activated Carbon Derived from Agricultural Wastes and Its Application in Water Remediation. Materials 2023, 16, 2170. [Google Scholar] [CrossRef]
- Saber, A.F.; Elewa, A.M.; Chou, H.H.; El-Mahdy, A.F.M. Donor to Acceptor Charge Transfer in Carbazole-based Conjugated Microporous Polymers for Enhanced Visible-Light-Driven Photocatalytic Water Splitting. ChemCatChem 2023, 15, 202201287. [Google Scholar] [CrossRef]
- Fang, Y.; Chen, Y.; Li, J.; Dong, H.; Zhou, X.; Yin, X.; Li, C. Micro built-in electric field arrays created by embedding high-dispersed RuP3 quantum dots with ultra-small size on polymeric carbon nitride nanosheets for synergistically actuating photocatalytic hydrogen evolution. Sep. Purif. Technol. 2023, 308, 122891. [Google Scholar] [CrossRef]
- Li, C.; Su, N.; Wu, H.; Liu, C.; Che, G.; Dong, H. Synergies of Adjacent Sites in Atomically Dispersed Ruthenium toward Achieving Stable Hydrogen Evolution. Inorg. Chem. 2022, 61, 13453–13461. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhu, D.; Cheng, S.; Zuo, Y.; Wang, Y.; Ma, C.; Dong, H. Recent research progress of bimetallic phosphides-based nanomaterials as cocatalyst for photocatalytic hydrogen evolution. Chin. Chem. Lett. 2022, 33, 1141–1153. [Google Scholar] [CrossRef]
- Ruan, X.; Cui, X.; Jia, G.; Wu, J.; Zhao, J.; Singh, D.J.; Liu, Y.; Zhang, H.; Zhang, L.; Zheng, W. Intramolecular heterostructured carbon nitride with heptazine-triazine for enhanced photocatalytic hydrogen evolution. Chem. Eng. J. 2022, 428, 132579. [Google Scholar] [CrossRef]
- Tian, L.; Guan, X.; Zong, S.; Dai, A.; Qu, J. Cocatalysts for Photocatalytic Overall Water Splitting: A Mini Review. Catalysts 2023, 13, 355. [Google Scholar] [CrossRef]
- Ruan, X.; Cui, X.; Cui, Y.; Fan, X.; Li, Z.; Xie, T.; Ba, K.; Jia, G.; Zhang, H.; Zhang, L.; et al. Favorable Energy Band Alignment of TiO2 Anatase/Rutile Heterophase Homojunctions Yields Photocatalytic Hydrogen Evolution with Quantum Efficiency Exceeding 45.6%. Adv. Energy Mater. 2022, 12, 2200298. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, H.; Jiang, Y.; Zhang, W.; Zhang, J.; Wu, X.; Liu, Z.; Deng, W. Hierarchical Sb2S3/ZnIn2S4 core–shell heterostructure for highly efficient photocatalytic hydrogen production and pollutant degradation. J. Colloid Interf. Sci. 2022, 623, 109–123. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, L.; Jiang, Y.; Liu, Y.; Zhang, W.; Zhang, J.; Olayemi Macauley, A.L. Morphology-engineered carbon quantum dots embedded on octahedral CdIn2S4 for enhanced photocatalytic activity towards pollutant degradation and hydrogen evolution. Environ. Res. 2022, 209, 112800. [Google Scholar] [CrossRef]
- Ruan, X.; Huang, C.; Cheng, H.; Zhang, Z.; Cui, Y.; Li, Z.; Xie, T.; Ba, K.; Zhang, H.; Zhang, L.; et al. A Twin S-Scheme Artificial Photosynthetic System with Self-Assembled Heterojunctions Yields Superior Photocatalytic Hydrogen Evolution Rate. Adv. Mater. 2023, 35, 2209141. [Google Scholar] [CrossRef]
- Liras, M.; Barawi, M.; de la Pena O’Shea, V.A. Hybrid materials based on conjugated polymers and inorganic semiconductors as photocatalysts: From environmental to energy applications. Chem. Soc. Rev. 2019, 48, 5454–5487. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zhang, P.; Zhu, D.; Cheng, S.; Dong, H.; Wang, Y.; Che, G.; Niu, Y.; Yan, M.; Li, C. Synergistic effect triggered by skeleton delocalization and edge induction of carbon nitride expedites photocatalytic hydrogen evolution. Chem. Eng. J. 2022, 442, 136190. [Google Scholar] [CrossRef]
- Li, W.; Ding, X.; Yu, B.; Wang, H.; Gao, Z.; Wang, X.; Liu, X.; Wang, K.; Jiang, J. Tuning Molecular Chromophores of Isoreticular Covalent Organic Frameworks for Visible Light-Induced Hydrogen Generation. Adv. Funct. Mater. 2022, 32, 2207394. [Google Scholar] [CrossRef]
- Chen, R.; Wang, Y.; Ma, Y.; Mal, A.; Gao, X.Y.; Gao, L.; Qiao, L.; Li, X.B.; Wu, L.Z.; Wang, C. Rational design of isostructural 2D porphyrin-based covalent organic frameworks for tunable photocatalytic hydrogen evolution. Nat. Commun. 2021, 12, 1354. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Su, N.; Zhang, P.; Fang, Y.; Wang, J.; Zhou, X.; Dong, H.; Li, C. Coupling effect of (SCN)x nanoribbons on PCN nanosheets in the metal-free 2D/1D Van der Waals heterojunction for boosting photocatalytic hydrogen evolution from water splitting. Sep. Purif. Technol. 2023, 307, 122796. [Google Scholar] [CrossRef]
- Li, C.; Zhou, T.; Yan, M.; Cheng, S.; Wang, Y.; Sun, J.; Chen, G.; Dong, H. Intramolecular π-conjugated channel expansion achieved by doping cross-linked dopants into carbon nitride frameworks for propelling photocatalytic hydrogen evolution and mechanism insight. Inorg. Chem. Front. 2022, 9, 60–69. [Google Scholar] [CrossRef]
- Li, C.; Wu, H.; Zhu, D.; Zhou, T.; Yan, M.; Chen, G.; Sun, J.; Dai, G.; Ge, F.; Dong, H. High-efficient charge separation driven directionally by pyridine rings grafted on carbon nitride edge for boosting photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2021, 297, 120433. [Google Scholar] [CrossRef]
- Liu, Y.; Liao, Z.; Ma, X.; Xiang, Z. Ultrastable and Efficient Visible-Light-Driven Hydrogen Production Based on Donor–Acceptor Copolymerized Covalent Organic Polymer. ACS Appl. Mater. Interfaces 2018, 10, 30698–30705. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, J.; Che, H.; Wang, P.; Liu, B.; Ao, Y. Recent Advances in g-C3N4-Based Donor–Acceptor Photocatalysts for Photocatalytic Hydrogen Evolution: An Exquisite Molecular Structure Engineering. ACS Mater. Lett. 2022, 4, 2166–2186. [Google Scholar] [CrossRef]
- Di, Y.M.; Liu, J.Y.; Li, M.H.; Zhang, S.Q.; You, M.H.; Lin, M.J. Donor-Acceptor Hybrid Heterostructures: An Emerging Class of Photoactive Materials with Inorganic and Organic Semiconductive Components. Small 2022, 18, 2201159. [Google Scholar] [CrossRef]
- Yang, J.; Jing, J.; Zhu, Y. A Full-Spectrum Porphyrin-Fullerene D–A Supramolecular Photocatalyst with Giant Built-In Electric Field for Efficient Hydrogen Production. Adv. Mater. 2021, 33, 2101026. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Zhang, L.; Cheng, R.; Wang, M.; Li, M.; Zhou, Y.; Shi, J. Construction of Graphitic C3N4-Based Intramolecular Donor–Acceptor Conjugated Copolymers for Photocatalytic Hydrogen Evolution. ACS Catal. 2015, 5, 5008–5015. [Google Scholar] [CrossRef]
- Yu, F.; Wang, Z.; Zhang, S.; Ye, H.; Kong, K.; Gong, X.; Hua, J.; Tian, H. Molecular Engineering of Donor-Acceptor Conjugated Polymer/g-C3N4 Heterostructures for Significantly Enhanced Hydrogen Evolution Under Visible-Light Irradiation. Adv. Funct. Mater. 2018, 28, 1804512. [Google Scholar] [CrossRef]
- Zang, S.; Zhang, G.; Yang, P.; Zheng, D.; Wang, X. Polymeric Donor-Acceptor Heterostructures for Enhanced Photocatalytic H2 Evolution without Using Pt Cocatalysts. Chemistry 2019, 25, 6102–6107. [Google Scholar] [CrossRef]
- Zhao, H.; Dong, Y.; Sun, P.; Bai, Y.; Ru, C.; Wu, X.; Li, Z.; Han, X.; Wu, J.; Pan, X. Effect of D/A Ratio on Photocatalytic Hydrogen Evolution Performance of Conjugated Polymer Photocatalysts. ACS Appl. Energy Mater. 2022, 5, 4631–4640. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, J.; Wan, Y.; Chen, J.; Zhou, Y.; Zhang, J.; Wang, G.; Wang, R. Donor-Acceptor structural polymeric carbon nitride with in-plane electric field accelerating charge separation for efficient photocatalytic hydrogen evolution. Chem. Eng. J. 2022, 430, 132725. [Google Scholar] [CrossRef]
- Lan, Z.A.; Wu, M.; Fang, Z.; Chi, X.; Chen, X.; Zhang, Y.; Wang, X. A Fully Coplanar Donor–Acceptor Polymeric Semiconductor with Promoted Charge Separation Kinetics for Photochemistry. Angew. Chem. Int. Edit. 2021, 60, 16355–16359. [Google Scholar] [CrossRef]
- Huang, W.; Cheng, P.; Yang, Y.M.; Li, G.; Yang, Y. High-Performance Organic Bulk-Heterojunction Solar Cells Based on Multiple-Donor or Multiple-Acceptor Components. Adv. Mater. 2018, 30, 1705706. [Google Scholar] [CrossRef]
- Yao, L.; Zhang, Y.; Wang, H.-X.; Guo, Y.; Zhuang, Z.-M.; Wen, W.; Zhang, X.; Wang, S. An energy and charge transfer synergetic donor–acceptor heterostructure 2D-COF in photovoltaics. J. Mater. Chem. A 2020, 8, 8518–8526. [Google Scholar] [CrossRef]
- Facchetti, A. Polymer donor–polymer acceptor (all-polymer) solar cells. Mater. Today 2013, 16, 123–132. [Google Scholar] [CrossRef]
- Levine, A.M.; Biswas, S.; Braunschweig, A.B. Photoactive organic material discovery with combinatorial supramolecular assembly. Nanoscale Adv. 2019, 1, 3858–3869. [Google Scholar] [CrossRef] [PubMed]
- Zang, L. Interfacial Donor-Acceptor Engineering of Nanofiber Materials To Achieve Photoconductivity and Applications. Acc. Chem. Res. 2015, 48, 2705–2714. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Li, W.; Li, W.S. Construction of a long range p/n heterojunction with a pair of nanometre-wide continuous D/A phases. Nanoscale 2011, 3, 3447–3461. [Google Scholar] [CrossRef] [PubMed]
- Logsdon, J.L.; Hartnett, P.E.; Nelson, J.N.; Harris, M.A.; Marks, T.J.; Wasielewski, M.R. Charge Separation Mechanisms in Ordered Films of Self-Assembled Donor–Acceptor Dyad Ribbons. ACS Appl. Mater. Interfaces 2017, 9, 33493–33503. [Google Scholar] [CrossRef]
- Black, H.T.; Perepichka, D.F. Crystal engineering of dual channel p/n organic semiconductors by complementary hydrogen bonding. Angew. Chem. Int. Edit. 2014, 53, 2138–2142. [Google Scholar] [CrossRef]
- Li, Y.; Huang, W.; Zhao, D.; Wang, L.; Jiao, Z.; Huang, Q.; Wang, P.; Sun, M.; Yuan, G. Recent Progress in Organic Solar Cells: A Review on Materials from Acceptor to Donor. Molecules 2022, 27, 1800. [Google Scholar] [CrossRef]
- Reyes, Y.I.A.; Ting, L.Y.; Tu, X.; Chen, H.Y.T.; Chou, H.H.; Coluccini, C. Mechanistic Studies of Hydrogen Evolution Reaction on Donor-Acceptor Conjugated Polymer Photocatalysts. Appl. Sci. 2020, 10, 7017. [Google Scholar] [CrossRef]
- Liu, Y.; Li, B.; Xiang, Z. Pathways towards Boosting Solar-Driven Hydrogen Evolution of Conjugated Polymers. Small 2021, 17, 2007576. [Google Scholar] [CrossRef]
- Mullen, K.; Pisula, W. Donor-Acceptor Polymers. J. Am. Chem. Soc. 2015, 137, 9503–9505. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.M.; Chen, S.; Kumari, T.; Kang, S.H.; Cho, Y.; Yang, C. Organic Photovoltaics with Multiple Donor–Acceptor Pairs. Adv. Mater. 2018, 31, 1804762. [Google Scholar] [CrossRef]
- Pachfule, P.; Acharjya, A.; Roeser, J.; Sivasankaran, R.P.; Ye, M.Y.; Brückner, A.; Schmidt, J.; Thomas, A. Donor–acceptor covalent organic frameworks for visible light induced free radical polymerization. Chem. Sci. 2019, 10, 8316–8322. [Google Scholar] [CrossRef]
- Lan, Z.A.; Zhang, G.; Chen, X.; Zhang, Y.; Zhang, K.A.I.; Wang, X. Reducing the Exciton Binding Energy of Donor-Acceptor-Based Conjugated Polymers to Promote Charge-Induced Reactions. Angew. Chem. Int. Edit. 2019, 58, 10236–10240. [Google Scholar] [CrossRef] [PubMed]
- Worner, H.J.; Arrell, C.A.; Banerji, N.; Cannizzo, A.; Chergui, M.; Das, A.K.; Hamm, P.; Keller, U.; Kraus, P.M.; Liberatore, E.; et al. Charge migration and charge transfer in molecular systems. Struct. Dynam. 2017, 4, 061508. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Weerasinghe, K.C.; Sun, H.; Hu, X.; Lu, T.; Liu, D.; Hu, W.; Li, W.; Zhou, X.; Wang, L. Effect of Triplet State on the Lifetime of Charge Separation in Ambipolar D–A1-A2 Organic Semiconductors. J. Phys. Chem. C 2016, 120, 11338–11349. [Google Scholar] [CrossRef]
- Li, K.; Zhang, W.D. Creating Graphitic Carbon Nitride Based Donor-π-Acceptor-π-Donor Structured Catalysts for Highly Photocatalytic Hydrogen Evolution. Small 2018, 14, 1703599. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Jiang, Y.; Zeng, L.; Huang, L. Intramolecular Charge Transfer and Extended Conjugate Effects in Donor-π-Acceptor-Type Mesoporous Carbon Nitride for Photocatalytic Hydrogen Evolution. ChemSusChem 2019, 12, 1325–1333. [Google Scholar] [CrossRef]
- Che, H.; Liu, C.; Che, G.; Liao, G.; Dong, H.; Li, C.; Song, N.; Li, C. Facile construction of porous intramolecular g-C3N4-based donor-acceptor conjugated copolymers as highly efficient photocatalysts for superior H2 evolution. Nano Energy 2020, 67, 104273. [Google Scholar] [CrossRef]
- Che, H.; Li, C.; Li, C.; Liu, C.; Dong, H.; Song, X. Benzoyl isothiocyanate as a precursor to design of ultrathin and high-crystalline g-C3N4-based donor–acceptor conjugated copolymers for superior photocatalytic H2 production. Chem. Eng. J. 2021, 410, 127791. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, L.; Li, Y.; Wang, Y.; Chen, J.; Lin, B.; Zheng, Y.; Cheng, L.; Wang, S.; Chen, Y. Triptycene incorporated carbon nitride based donor-acceptor conjugated polymers with superior visible-light photocatalytic activities. J. Colloid Interf. Sci. 2022, 622, 675–689. [Google Scholar] [CrossRef]
- Song, H.; Liu, X.; Wang, Y.; Chen, L.; Zhang, J.; Zhao, C.; He, F.; Dong, P.; Li, B.; Wang, S.; et al. Synergy of intermolecular Donor-Acceptor and ultrathin structures in crystalline carbon nitride for efficient photocatalytic hydrogen evolution. J. Colloid Interf. Sci. 2022, 607, 1603–1612. [Google Scholar] [CrossRef]
- Ai, M.; Zhang, J.W.; Gao, R.; Pan, L.; Zhang, X.; Zou, J.J. MnOx-decorated 3D porous C3N4 with internal donor–acceptor motifs for efficient photocatalytic hydrogen production. Appl. Catal. B Environ. 2019, 256, 117805. [Google Scholar] [CrossRef]
- Zong, X.; Niu, L.; Jiang, W.; Yu, Y.; An, L.; Qu, D.; Wang, X.; Sun, Z. Constructing creatinine-derived moiety as donor block for carbon nitride photocatalyst with extended absorption and spatial charge separation. Appl. Catal. B Environ. 2021, 291, 120099. [Google Scholar] [CrossRef]
- Jiang, R.; Lu, G.; Liu, J.; Wu, D.; Yan, Z.; Wang, Y. Incorporation of π-conjugated molecules as electron donors in g-C3N4 enhances photocatalytic H2-production. Renew. Energ. 2021, 164, 531–540. [Google Scholar] [CrossRef]
- Xing, W.; Shao, W.; Tian, L.; Zhang, Y.; Yang, J.; Zhou, H.; Han, J.; Wu, G.; Chen, G. Realizing high-efficiency carrier separation in 3D hierarchical carbon nitride nanosheets via intramolecular donor-acceptor motifs strategy. Mater. Today Chem. 2022, 25, 100977. [Google Scholar] [CrossRef]
- Lai, J.Y.; Zhang, W.D.; Yu, Y.X. Building sp carbon-bridged g-C3N4-based electron donor-π-acceptor unit for efficient photocatalytic water splitting. Mol. Catal. 2021, 505, 111518. [Google Scholar] [CrossRef]
- Gao, S.; Wan, S.; Yu, J.; Cao, S. Donor–Acceptor Modification of Carbon Nitride for Enhanced Photocatalytic Hydrogen Evolution. Adv. Sustain. Syst. 2022, 7, 2200130. [Google Scholar] [CrossRef]
- Zhang, G.; Lin, L.; Li, G.; Zhang, Y.; Savateev, A.; Zafeiratos, S.; Wang, X.; Antonietti, M. Ionothermal Synthesis of Triazine-Heptazine-Based Copolymers with Apparent Quantum Yields of 60% at 420 nm for Solar Hydrogen Production from “Sea Water”. Angew. Chem. Int. Edit. 2018, 57, 9372–9376. [Google Scholar] [CrossRef]
- Yu, F.; Wang, Z.; Zhang, S.; Wu, W.; Ye, H.; Ding, H.; Gong, X.; Hua, J. Construction of polymeric carbon nitride and dibenzothiophene dioxide-based intramolecular donor-acceptor conjugated copolymers for photocatalytic H2 evolution. Nanoscale Adv. 2021, 3, 1699–1707. [Google Scholar] [CrossRef]
- Lu, Z.; Yang, C.; He, L.; Hong, J.; Huang, C.; Wu, T.; Wang, X.; Wu, Z.; Liu, X.; Miao, Z.; et al. Asymmetric Hydrophosphonylation of Imines to Construct Highly Stable Covalent Organic Frameworks with Efficient Intrinsic Proton Conductivity. J. Am. Chem. Soc. 2022, 144, 9624–9633. [Google Scholar] [CrossRef]
- Maschita, J.; Banerjee, T.; Savasci, G.; Haase, F.; Ochsenfeld, C.; Lotsch, B.V. Ionothermal Synthesis of Imide-Linked Covalent Organic Frameworks. Angew. Chem. Int. Edit. 2020, 59, 15750–15758. [Google Scholar] [CrossRef]
- Lanni, L.M.; Tilford, R.W.; Bharathy, M.; Lavigne, J.J. Enhanced hydrolytic stability of self-assembling alkylated two-dimensional covalent organic frameworks. J. Am. Chem. Soc. 2011, 133, 13975–13983. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Zhou, J.; Li, Z.; Sun, Y.; Kantorovich, L.N.; Fang, Q.; Besenbacher, F.; Yu, M. Graphene-Like Covalent Organic Framework with a Wide Band Gap Synthesized on Surface via Stepwise Reactions. Angew. Chem. Int. Edit. 2020, 59, 15958–15962. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H.; Yang, Z.; Li, Z.; Wu, J.R.; Yang, B.; Yang, Y.W. Construction of Hydrazone-Linked Macrocycle-Enriched Covalent Organic Frameworks for Highly Efficient Photocatalysis. Chem. Mater. 2022, 34, 5726–5739. [Google Scholar] [CrossRef]
- Wei, S.; Zhang, F.; Zhang, W.; Qiang, P.; Yu, K.; Fu, X.; Wu, D.; Bi, S.; Zhang, F. Semiconducting 2D Triazine-Cored Covalent Organic Frameworks with Unsubstituted Olefin Linkages. J. Am. Chem. Soc. 2019, 141, 14272–14279. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Luo, M.; Liu, K.; Cao, H.; Yan, H. Covalent organic frameworks for photocatalytic applications. Appl. Catal. B Environ. 2020, 276, 119174. [Google Scholar] [CrossRef]
- Zhao, J.; Ren, J.; Zhang, G.; Zhao, Z.; Liu, S.; Zhang, W.; Chen, L. Donor-Acceptor Type Covalent Organic Frameworks. Chemistry 2021, 27, 10781–10797. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Guo, J. Covalent Organic Frameworks for Photocatalytic Hydrogen Evolution: Design, Strategy, and Structure-to-Performance Relationship. Macromol. Rapid Commun. 2022. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jena, H.S.; Feng, X.; Leus, K.; Van Der Voort, P. Engineering Covalent Organic Frameworks as Heterogeneous Photocatalysts for Organic Transformations. Angew. Chem. Int. Edit. 2022, 61, 202204938. [Google Scholar]
- Zhao, Z.; Zheng, Y.; Wang, C.; Zhang, S.; Song, J.; Li, Y.; Ma, S.; Cheng, P.; Zhang, Z.; Chen, Y. Fabrication of Robust Covalent Organic Frameworks for Enhanced Visible-Light-Driven H2 Evolution. ACS Catal. 2021, 11, 2098–2107. [Google Scholar] [CrossRef]
- Chen, W.; Wang, L.; Mo, D.; He, F.; Wen, Z.; Wu, X.; Xu, H.; Chen, L. Modulating Benzothiadiazole-Based Covalent Organic Frameworks via Halogenation for Enhanced Photocatalytic Water Splitting. Angew. Chem. Int. Edit. 2020, 59, 16902–16909. [Google Scholar] [CrossRef]
- Yang, J.; Acharjya, A.; Ye, M.Y.; Rabeah, J.; Li, S.; Kochovski, Z.; Youk, S.; Roeser, J.; Gruneberg, J.; Penschke, C.; et al. Protonated Imine-Linked Covalent Organic Frameworks for Photocatalytic Hydrogen Evolution. Angew. Chem. Int. Edit. 2021, 60, 19797–19803. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, J.; Yan, X.; Wu, C.; Zhu, X.; Li, B.; Li, T.; Guo, Q.; Gao, J.; Hu, M.; et al. Donor–acceptor covalent organic framework hollow submicrospheres with a hierarchical pore structure for visible-light-driven H2 evolution. J. Mater. Chem. A 2022, 10, 11010–11018. [Google Scholar] [CrossRef]
- Wang, G.B.; Xu, H.P.; Xie, K.H.; Kan, J.L.; Fan, J.; Wang, Y.J.; Geng, Y.; Dong, Y.B. A covalent organic framework constructed from a donor–acceptor–donor motif monomer for photocatalytic hydrogen evolution from water. J. Mater. Chem. A 2023, 11, 4007–4012. [Google Scholar] [CrossRef]
- Lin, C.; Liu, X.; Yu, B.; Han, C.; Gong, L.; Wang, C.; Gao, Y.; Bian, Y.; Jiang, J. Rational Modification of Two-Dimensional Donor-Acceptor Covalent Organic Frameworks for Enhanced Visible Light Photocatalytic Activity. ACS Appl. Mater. Interfaces 2021, 13, 27041–27048. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; He, Y.; Liu, X.; Wang, Z.; Liu, H.L.; Zhu, X.; Hou, C.C.; Weng, Y.; Zhang, Q.; Chen, Y. Regulating Excitonic Effects in Covalent Organic Frameworks to Promote Free Charge Carrier Generation. ACS Catal. 2022, 12, 9494–9502. [Google Scholar] [CrossRef]
- Li, W.; Huang, X.; Zeng, T.; Liu, Y.A.; Hu, W.; Yang, H.; Zhang, Y.B.; Wen, K. Thiazolo [5,4-d] thiazole-Based Donor-Acceptor Covalent Organic Framework for Sunlight-Driven Hydrogen Evolution. Angew. Chem. Int. Edit. 2021, 60, 1869–1874. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, W.; Liu, H.; Chen, R.; Pan, Q.; Li, Z.; Zhao, Y. Facile construction of fully sp2-carbon conjugated two-dimensional covalent organic frameworks containing benzobisthiazole units. Nat. Commun. 2022, 13, 100. [Google Scholar] [CrossRef]
- Lv, M.; Ren, X.; Cao, R.; Chang, Z.; Chang, X.; Bai, F.; Li, Y. Zn (II) Porphyrin Built-in D–A Covalent Organic Framework for Efficient Photocatalytic H2 Evolution. Polymers 2022, 14, 4893. [Google Scholar] [CrossRef]
- Wang, G.B.; Zhu, F.C.; Lin, Q.Q.; Kan, J.L.; Xie, K.H.; Li, S.; Geng, Y.; Dong, Y.B. Rational design of benzodifuran-functionalized donor-acceptor covalent organic frameworks for photocatalytic hydrogen evolution from water. Chem. Commun. 2021, 57, 4464–4467. [Google Scholar] [CrossRef]
- Li, C.; Liu, J.; Li, H.; Wu, K.; Wang, J.; Yang, Q. Covalent organic frameworks with high quantum efficiency in sacrificial photocatalytic hydrogen evolution. Nat. Commun. 2022, 13, 2357. [Google Scholar] [CrossRef]
- Wang, F.D.; Yang, L.J.; Wang, X.X.; Rong, Y.; Yang, L.B.; Zhang, C.X.; Yan, F.Y.; Wang, Q.L. Pyrazine-Functionalized Donor-Acceptor Covalent Organic Frameworks for Enhanced Photocatalytic H2 Evolution with High Proton Transport. Small 2023. [Google Scholar] [CrossRef]
- Xie, J.; Fang, Z.; Wang, H. Modification of Covalent Triazine-Based Frameworks for Photocatalytic Hydrogen Generation. Polymers 2022, 14, 1363. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Shevlin, S.A.; Ruan, Q.; Moniz, S.J.A.; Liu, Y.; Liu, X.; Li, Y.; Lau, C.C.; Guo, Z.X.; Tang, J. Efficient visible light-driven water oxidation and proton reduction by an ordered covalent triazine-based framework. Energy Environ. Sci. 2018, 11, 1617–1624. [Google Scholar] [CrossRef]
- Meier, C.B.; Clowes, R.; Berardo, E.; Jelfs, K.E.; Zwijnenburg, M.A.; Sprick, R.S.; Cooper, A.I. Structurally Diverse Covalent Triazine-Based Framework Materials for Photocatalytic Hydrogen Evolution from Water. Chem. Mater. 2019, 31, 8830–8838. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Chen, T.; Ma, L.; Wang, G.; Hu, W.B.; Zou, Z.; Wen, K.; Yang, H. Covalent Triazine-Based Polymers with Controllable Band Alignment Matched with BiVO4 To Boost Photogeneration of Holes for Water Splitting. Chem. Mater. 2019, 31, 8062–8068. [Google Scholar] [CrossRef]
- Kong, D.; Han, X.; Xie, J.; Ruan, Q.; Windle, C.D.; Gadipelli, S.; Shen, K.; Bai, Z.; Guo, Z.; Tang, J. Tunable Covalent Triazine-Based Frameworks (CTF-0) for Visible-Light-Driven Hydrogen and Oxygen Generation from Water Splitting. ACS Catal. 2019, 9, 7697–7707. [Google Scholar] [CrossRef]
- Zhang, S.; Cheng, G.; Guo, L.; Wang, N.; Tan, B.; Jin, S. Strong-Base-Assisted Synthesis of a Crystalline Covalent Triazine Framework with High Hydrophilicity via Benzylamine Monomer for Photocatalytic Water Splitting. Angew. Chem. Int. Edit. 2020, 59, 6007–6014. [Google Scholar] [CrossRef]
- Cai, B.; Cao, L.; Zhang, R.; Xu, N.; Tang, J.; Wang, K.; Li, Q.; Xu, B.; Liu, Y.; Fan, Y. Construction of Benzodithiophene-Based Donor–Acceptor-Type Covalent Triazine Frameworks with Tunable Structure for Photocatalytic Hydrogen Evolution. ACS Appl. Energy Mater. 2022, 6, 930–938. [Google Scholar] [CrossRef]
- Huang, W.; He, Q.; Hu, Y.; Li, Y. Molecular Heterostructures of Covalent Triazine Frameworks for Enhanced Photocatalytic Hydrogen Production. Angew. Chem. Int. Edit. 2019, 58, 8676–8680. [Google Scholar] [CrossRef]
- Lan, Z.A.; Chi, X.; Wu, M.; Zhang, X.; Chen, X.; Zhang, G.; Wang, X. Molecular Design of Covalent Triazine Frameworks with Anisotropic Charge Migration for Photocatalytic Hydrogen Production. Small 2022, 18, 2200129. [Google Scholar] [CrossRef]
- Guo, L.; Niu, Y.; Razzaque, S.; Tan, B.; Jin, S. Design of D–A1–A2 Covalent Triazine Frameworks via Copolymerization for Photocatalytic Hydrogen Evolution. ACS Catal. 2019, 9, 9438–9445. [Google Scholar] [CrossRef]
- Jayakumar, J.; Chou, H.H. Recent Advances in Visible-Light-Driven Hydrogen Evolution from Water using Polymer Photocatalysts. ChemCatChem 2020, 12, 689–704. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, Z.; Shi, R.; Yang, X.; Zhang, T. Recent Advances in Conjugated Polymers for Visible-Light-Driven Water Splitting. Adv. Mater. 2020, 32, 1907296. [Google Scholar] [CrossRef] [PubMed]
- Mansha, M.; Ahmad, T.; Ullah, N.; Akram Khan, S.; Ashraf, M.; Ali, S.; Tan, B.; Khan, I. Photocatalytic Water-Splitting by Organic Conjugated Polymers: Opportunities and Challenges. Chem. Rec. 2022, 22, 202100336. [Google Scholar] [CrossRef]
- Wang, W.R.; Li, J.; Li, Q.; Xu, Z.W.; Liu, L.N.; Chen, X.Q.; Xiao, W.J.; Yao, J.; Zhang, F.; Li, W.S. Side-chain-extended conjugation: A strategy for improving the photocatalytic hydrogen production performance of a linear conjugated polymer. J. Mater. Chem. A 2021, 9, 8782–8791. [Google Scholar] [CrossRef]
- Tan, Z.R.; Xing, Y.Q.; Cheng, J.Z.; Zhang, G.; Shen, Z.Q.; Zhang, Y.J.; Liao, G.; Chen, L.; Liu, S.Y. EDOT-based conjugated polymers accessed via C-H direct arylation for efficient photocatalytic hydrogen production. Chem. Sci. 2022, 13, 1725–1733. [Google Scholar] [CrossRef]












| Photocatalyst | Light Source | Mass[g]/(Solution)/ Volume [mL] | H2 Evolution Activity (mmol g−1 h−1) | Quantum Efficiency (%) | Ref. | |
|---|---|---|---|---|---|---|
| T-CN-0.010 | 300 W Xe-lamp | > 420 nm | 0.05/10% TEOA/100 mL | 1.618 | 4.8% at 420 nm | [59] |
| UCCN | CEL-HXF300 | > 420 nm | 0.025/10% TEOA/100 mL | 9.7 | 73.6% at 420 nm | [60] |
| MSCN | 300 W Xe-lamp | > 420 nm | 0.05/10% TEOA/100 mL | 1.0856 | - | [61] |
| CDM | PLS-SEX300D | > 420 nm | 0.001/10% TEOA/100 mL | 9.454 | 8.41% at 420 nm | [62] |
| NP-CNx | 300 W Xe-lamp | > 420 nm | 0.05/20% TEOA/100 mL | 2.7914 | 6.4% at 400 nm | [63] |
| 3D CN | 300 W Xe-lamp | > 420 nm | 0.05/15% TEOA/100 mL | 2.5212 | 8.21% at 420 nm | [64] |
| UCN-BTD | 300 W Xe-lamp | > 420 nm | 0.05/10% TEOA/100 mL | 2.44 | 6.8% at 450 nm | [65] |
| CN-abIM0.03 | 300 W Xe-lamp | > 420 nm | 0.02/10% TEOA/80 mL | 2.566 | - | [66] |
| CN-NaK | LED | > 420 nm | 0.05/10% TEOA/38 mL | 11.72 | 60% at 420 nm | [67] |
| CNSO-20 | 300 W Xe-lamp | > 420 nm | 0.05/14.3% TEOA/70 mL | 5.02 | 10.16% at 420 nm | [68] |
| Photocatalyst | Light Source | Mass[g]/(Solution)/ Volume [mL] | H2 Evolution Activity (mmol g−1 h−1) | Quantum Efficiency (%) | Ref. | |
|---|---|---|---|---|---|---|
| DABT-Py-COF | 300 W Xe-lamp | > 420 nm | 0.005/0.1 M Aa/10 mL | 5.458 | - | [83] |
| HBT-COF | 300 W Xe-lamp | > 420 nm | 0.005/0.1 M Aa/20 mL | 0.019 | - | [84] |
| Tz-COF-3 | 300 W Xe-lamp | - | 0.05/10% TEOA/100 mL | 43.2 | 6.9% at 420 nm | [85] |
| PyTz-COF | 300 W Xe-lamp | - | 0.01/1 M Aa/20 mL | 2.0724 | - | [86] |
| BTH-3 | 300 W Xe-lamp | > 420 nm | 0.005/0.1 M Aa/- | 15.1 | 1.25% at 500 nm | [87] |
| Zn-Por-TT COF | 300 W Xe-lamp | > 420 nm | 0.005/1 M Aal/50 mL | 8.2 | - | [88] |
| BDF-TAPT-COF | - | > 420 nm | 0.01/1 M Aa/20 mL | 1.39 | 7.8% at 420 nm | [89] |
| CYANO-COF | 300 W Xe-lamp | > 420 nm | 0.02/0.1 M Aa/30 mL | 60.85 | 82.6% at 450 nm | [90] |
| PyTP-COF | 300 W Xe-lamp | > 420 nm | 0.02/0.1 M Aa/30 mL | 7.542 | 0.56% at 420 nm | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Sheng, J.; Zhao, X.; Mo, J.; Wang, J.; Chen, Z.; Dong, H.; Li, C. Recent Progress in Conjugated Polymers-Based Donor–Acceptor Semiconductor Materials for Photocatalytic Hydrogen Evolution from Water Splitting. Catalysts 2023, 13, 850. https://doi.org/10.3390/catal13050850
Zhao Y, Sheng J, Zhao X, Mo J, Wang J, Chen Z, Dong H, Li C. Recent Progress in Conjugated Polymers-Based Donor–Acceptor Semiconductor Materials for Photocatalytic Hydrogen Evolution from Water Splitting. Catalysts. 2023; 13(5):850. https://doi.org/10.3390/catal13050850
Chicago/Turabian StyleZhao, Yanhui, Jingfu Sheng, Xiaobo Zhao, Jian Mo, Jilong Wang, Zhuang Chen, Hongjun Dong, and Chunmei Li. 2023. "Recent Progress in Conjugated Polymers-Based Donor–Acceptor Semiconductor Materials for Photocatalytic Hydrogen Evolution from Water Splitting" Catalysts 13, no. 5: 850. https://doi.org/10.3390/catal13050850