Supported Ni Single-Atom Catalysts: Synthesis, Structure, and Applications in Thermocatalytic Reactions
Abstract
:1. Introduction
2. Synthesis of Supported Ni Single-Atom Catalysts
2.1. Wet-Chemistry Methods
2.2. Pyrolysis Methods
3. Structure of Supported Ni Single-Atom Catalysts
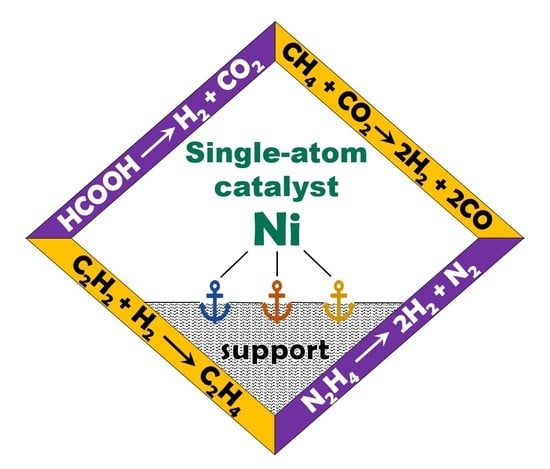
4. Application of Supported Ni Single-Atom Catalysts
4.1. C–H Activation
Dry Reforming of Methane
4.2. H–H Activation
4.2.1. CO2 Hydrogenation
4.2.2. C2H2 Hydrogenation
4.2.3. Other Hydrogenation Reactions
4.3. O–H Activation
4.3.1. Transfer Hydrogenation Reactions
4.3.2. H2 Production from Formic Acid
4.4. N–H Activation
5. Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abundance in Earth’s Crust for All the Elements in the Periodic Table. Available online: https://periodictable.com/Properties/A/CrustAbundance.html (accessed on 2 May 2023).
- Sabatier, P. Catalysis in Organic Chemistry; D. Van Nostrand Company: New York, NY, USA, 1922. [Google Scholar]
- Keim, W. Nickel: An Element with Wide Application in Industrial Homogeneous Catalysis. Angew. Chem. Int. Ed. Engl. 1990, 29, 235–244. [Google Scholar] [CrossRef]
- Schneider, C.; Leischner, T.; Ryabchuk, P.; Jackstell, R.; Junge, K.; Beller, M. Development of Bulk Organic Chemical Processes—History, Status, and Opportunities for Academic Research. CCS Chem. 2021, 3, 512–530. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, Z.-H.; Yuan, T.-Q.; Ren, X.; Rong, Z. Raney Ni as a Versatile Catalyst for Biomass Conversion. ACS Catal. 2021, 11, 10508–10536. [Google Scholar] [CrossRef]
- Jette, E.R.; Foote, F. Precision Determination of Lattice Constants. J. Chem. Phys. 1935, 3, 605–616. [Google Scholar] [CrossRef]
- Kaiser, S.K.; Chen, Z.; Faust Akl, D.; Mitchell, S.; Pérez-Ramírez, J. Single-Atom Catalysts across the Periodic Table. Chem. Rev. 2020, 120, 11703–11809. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Duan, X.; Wang, S.; Yue, Q.; Gao, B.; Xu, X. Carbon-Based Single Atom Catalyst: Synthesis, Characterization, DFT Calculations. Chin. Chem. Lett. 2022, 33, 663–673. [Google Scholar] [CrossRef]
- Qi, P.; Wang, J.; Djitcheu, X.; He, D.; Liu, H.; Zhang, Q. Techniques for the Characterization of Single Atom Catalysts. RSC Adv. 2022, 12, 1216–1227. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xiong, L.; Zhao, B.; Liu, M.; Huang, L. Densely Populated Single Atom Catalysts. Small Methods 2020, 4, 1900540. [Google Scholar] [CrossRef]
- Yang, J.; Li, W.; Wang, D.; Li, Y. Electronic Metal–Support Interaction of Single-Atom Catalysts and Applications in Electrocatalysis. Adv. Mater. 2020, 32, 2003300. [Google Scholar] [CrossRef]
- Qi, K.; Chhowalla, M.; Voiry, D. Single Atom Is Not Alone: Metal–Support Interactions in Single-Atom Catalysis. Mater. Today 2020, 40, 173–192. [Google Scholar] [CrossRef]
- Kottwitz, M.; Li, Y.; Wang, H.; Frenkel, A.I.; Nuzzo, R.G. Single Atom Catalysts: A Review of Characterization Methods. Chemistry–Methods 2021, 1, 278–294. [Google Scholar] [CrossRef]
- Liu, J.; Bunes, B.R.; Zang, L.; Wang, C. Supported Single-Atom Catalysts: Synthesis, Characterization, Properties, and Applications. Environ. Chem. Lett. 2018, 16, 477–505. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Nishchakova, A.D.; Trubina, S.V.; Stonkus, O.A.; Asanov, I.P.; Okotrub, A.V.; Bulusheva, L.G. Ni-N4 Sites in a Single-Atom Ni Catalyst on N-Doped Carbon for Hydrogen Production from Formic Acid. J. Catal. 2021, 402, 264–274. [Google Scholar] [CrossRef]
- Yang, H.; Lin, Q.; Zhang, C.; Yu, X.; Cheng, Z.; Li, G.; Hu, Q.; Ren, X.; Zhang, Q.; Liu, J.; et al. Carbon Dioxide Electroreduction on Single-Atom Nickel Decorated Carbon Membranes with Industry Compatible Current Densities. Nat. Commun. 2020, 11, 593. [Google Scholar] [CrossRef]
- Nishchakova, A.D.; Bulushev, D.A.; Trubina, S.V.; Stonkus, O.A.; Shubin, Y.V.; Asanov, I.P.; Kriventsov, V.V.; Okotrub, A.V.; Bulusheva, L.G. Highly Dispersed Ni on Nitrogen-Doped Carbon for Stable and Selective Hydrogen Generation from Gaseous Formic Acid. Nanomaterials 2023, 13, 545. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Z.; Lu, P.; Wang, X.; Liu, W.; Liu, Z.; Ma, J.; Ren, W.; Jiang, Z.; Bao, X. High-Valence Nickel Single-Atom Catalysts Coordinated to Oxygen Sites for Extraordinarily Activating Oxygen Evolution Reaction. Adv. Sci. 2020, 7, 1903089. [Google Scholar] [CrossRef]
- Li, Y.; Lu, X.F.; Xi, S.; Luan, D.; Wang, X.; Lou, X.W. (David) Synthesis of N-Doped Highly Graphitic Carbon Urchin-Like Hollow Structures Loaded with Single-Ni Atoms towards Efficient CO2 Electroreduction. Angew. Chem. Int. Ed. 2022, 61, e202201491. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, R.; Shang, L.; Waterhouse, G.I.N.; Zhao, J.; Zhang, Q.; Gu, L.; Zhang, T. High-Efficiency Oxygen Reduction to Hydrogen Peroxide Catalyzed by Nickel Single-Atom Catalysts with Tetradentate N2O2 Coordination in a Three-Phase Flow Cell. Angew. Chem. Int. Ed. 2020, 59, 13057–13062. [Google Scholar] [CrossRef]
- Büchele, S.; Martín, A.J.; Mitchell, S.; Krumeich, F.; Collins, S.M.; Xi, S.; Borgna, A.; Pérez-Ramírez, J. Structure Sensitivity and Evolution of Nickel-Bearing Nitrogen-Doped Carbons in the Electrochemical Reduction of CO2. ACS Catal. 2020, 10, 3444–3454. [Google Scholar] [CrossRef]
- Yang, H.; Shang, L.; Zhang, Q.; Shi, R.; Waterhouse, G.I.N.; Gu, L.; Zhang, T. A Universal Ligand Mediated Method for Large Scale Synthesis of Transition Metal Single Atom Catalysts. Nat. Commun. 2019, 10, 4585. [Google Scholar] [CrossRef]
- Mou, K.; Chen, Z.; Zhang, X.; Jiao, M.; Zhang, X.; Ge, X.; Zhang, W.; Liu, L. Highly Efficient Electroreduction of CO2 on Nickel Single-Atom Catalysts: Atom Trapping and Nitrogen Anchoring. Small 2019, 15, 1903668. [Google Scholar] [CrossRef]
- Liu, W.; Chen, Y.; Qi, H.; Zhang, L.; Yan, W.; Liu, X.; Yang, X.; Miao, S.; Wang, W.; Liu, C.; et al. A Durable Nickel Single-Atom Catalyst for Hydrogenation Reactions and Cellulose Valorization under Harsh Conditions. Angew. Chem. Int. Ed. 2018, 57, 7071–7075. [Google Scholar] [CrossRef]
- Wei, S.; Wang, Y.; Chen, W.; Li, Z.; Cheong, W.-C.; Zhang, Q.; Gong, Y.; Gu, L.; Chen, C.; Wang, D.; et al. Atomically Dispersed Fe Atoms Anchored on COF-Derived N-Doped Carbon Nanospheres as Efficient Multi-Functional Catalysts. Chem. Sci. 2019, 11, 786–790. [Google Scholar] [CrossRef]
- Ochoa-Gómez, J.R.; Roncal, T. Production of Sorbitol from Biomass. In Production of Platform Chemicals from Sustainable Resources; Biofuels and, Biorefineries; Fang, Z., Smith, R.L., Qi, X., Eds.; Springer: Singapore, 2017; pp. 265–309. ISBN 978-981-10-4171-6. [Google Scholar]
- Zhu, L.; Sun, H.; Fu, H.; Zheng, J.; Zhang, N.; Li, Y.; Chen, B.H. Effect of Ruthenium Nickel Bimetallic Composition on the Catalytic Performance for Benzene Hydrogenation to Cyclohexane. Appl. Catal. Gen. 2015, 499, 124–132. [Google Scholar] [CrossRef]
- Copéret, C.; Allouche, F.; Chan, K.W.; Conley, M.P.; Delley, M.F.; Fedorov, A.; Moroz, I.B.; Mougel, V.; Pucino, M.; Searles, K.; et al. Bridging the Gap between Industrial and Well-Defined Supported Catalysts. Angew. Chem. Int. Ed. 2018, 57, 6398–6440. [Google Scholar] [CrossRef]
- Finiels, A.; Fajula, F.; Hulea, V. Nickel-Based Solid Catalysts for Ethylene Oligomerization—A Review. Catal. Sci. Technol. 2014, 4, 2412–2426. [Google Scholar] [CrossRef]
- LaMer, V.K.; Dinegar, R.H. Theory, Production and Mechanism of Formation of Monodispersed Hydrosols. J. Am. Chem. Soc. 1950, 72, 4847–4854. [Google Scholar] [CrossRef]
- Lepre, E.; Heske, J.; Nowakowski, M.; Scoppola, E.; Zizak, I.; Heil, T.; Kühne, T.D.; Antonietti, M.; López-Salas, N.; Albero, J. Ni-Based Electrocatalysts for Unconventional CO2 Reduction Reaction to Formic Acid. Nano Energy 2022, 97, 107191. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, Z.L.; Dong, S.; He, D.; Lawrence, M.J.; Han, S.; Cai, C.; Xiang, S.; Rodriguez, P.; Xiang, B.; et al. Design of Active Nickel Single-Atom Decorated MoS2 as a PH-Universal Catalyst for Hydrogen Evolution Reaction. Nano Energy 2018, 53, 458–467. [Google Scholar] [CrossRef]
- Rong, X.; Wang, H.; Lu, X.; Si, R.; Lu, T. Controlled Synthesis of a Vacancy-Defect Single-Atom Catalyst for Boosting CO2 Electroreduction. Angew. Chem. Int. Ed. 2019, 59, 1961–1965. [Google Scholar] [CrossRef]
- Yan, C.; Li, H.; Ye, Y.; Wu, H.; Cai, F.; Si, R.; Xiao, J.; Miao, S.; Xie, S.; Yang, F.; et al. Coordinatively Unsaturated Nickel–Nitrogen Sites towards Selective and High-Rate CO2 Electroreduction. Energy Environ. Sci. 2018, 11, 1204–1210. [Google Scholar] [CrossRef]
- Wu, S.; Yi, F.; Ping, D.; Huang, S.; Zhang, Y.; Han, L.; Wang, S.; Wang, H.; Yang, X.; Guo, D.; et al. Constructing Single-Atomic Nickel Sites in Carbon Nanotubes for Efficient CO2 Electroreduction. Carbon 2022, 196, 1–9. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, S.; Johannessen, B.; Veder, J.; Saunders, M.; Rowles, M.R.; Cheng, M.; Liu, C.; Chisholm, M.F.; Marco, R.; et al. Atomically Dispersed Transition Metals on Carbon Nanotubes with Ultrahigh Loading for Selective Electrochemical Carbon Dioxide Reduction. Adv. Mater. 2018, 30, 1706287. [Google Scholar] [CrossRef]
- Fei, H.; Dong, J.; Feng, Y.; Allen, C.S.; Wan, C.; Volosskiy, B.; Li, M.; Zhao, Z.; Wang, Y.; Sun, H.; et al. General Synthesis and Definitive Structural Identification of MN4C4 Single-Atom Catalysts with Tunable Electrocatalytic Activities. Nat. Catal. 2018, 1, 63–72. [Google Scholar] [CrossRef]
- Jeong, H.; Balamurugan, M.; Choutipalli, V.S.K.; Jo, J.; Baik, H.; Subramanian, V.; Kim, M.; Sim, U.; Nam, K.T. Tris(2-benzimidazolylmethyl)Amine-Directed Synthesis of Single-Atom Nickel Catalysts for Electrochemical CO Production from CO2. Chem. Eur. J. 2018, 24, 18444–18454. [Google Scholar] [CrossRef]
- Bi, W.; Li, X.; You, R.; Chen, M.; Yuan, R.; Huang, W.; Wu, X.; Chu, W.; Wu, C.; Xie, Y. Surface Immobilization of Transition Metal Ions on Nitrogen-Doped Graphene Realizing High-Efficient and Selective CO2 Reduction. Adv. Mater. 2018, 30, 1706617. [Google Scholar] [CrossRef]
- Pan, F.; Deng, W.; Justiniano, C.; Li, Y. Identification of Champion Transition Metals Centers in Metal and Nitrogen-Codoped Carbon Catalysts for CO2 Reduction. Appl. Catal. B Environ. 2018, 226, 463–472. [Google Scholar] [CrossRef]
- Yang, H.B.; Hung, S.-F.; Liu, S.; Yuan, K.; Miao, S.; Zhang, L.; Huang, X.; Wang, H.-Y.; Cai, W.; Chen, R.; et al. Atomically Dispersed Ni(i) as the Active Site for Electrochemical CO2 Reduction. Nat. Energy 2018, 3, 140–147. [Google Scholar] [CrossRef]
- Su, H.; Gao, P.; Wang, M.; Zhai, G.; Zhang, J.; Zhao, T.; Su, J.; Antonietti, M.; Li, X.; Chen, J. Grouping Effect of Single Nickel−N4 Sites in Nitrogen-Doped Carbon Boosts Hydrogen Transfer Coupling of Alcohols and Amines. Angew. Chem. Int. Ed. 2018, 57, 15194–15198. [Google Scholar] [CrossRef]
- Zhao, S.; Cheng, Y.; Veder, J.-P.; Johannessen, B.; Saunders, M.; Zhang, L.; Liu, C.; Chisholm, M.F.; De Marco, R.; Liu, J.; et al. One-Pot Pyrolysis Method to Fabricate Carbon Nanotube Supported Ni Single-Atom Catalysts with Ultrahigh Loading. ACS Appl. Energy Mater. 2018, 10, 5286–5297. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, W.; Luo, J.; Chen, W.; Cao, T.; Zheng, L.; Dong, J.; Zhang, J.; Zhang, M.; Han, Y.; et al. A Cocoon Silk Chemistry Strategy to Ultrathin N-Doped Carbon Nanosheet with Metal Single-Site Catalysts. Nat. Commun. 2018, 9, 3861. [Google Scholar] [CrossRef] [PubMed]
- Fei, H.; Dong, J.; Wan, C.; Zhao, Z.; Xu, X.; Lin, Z.; Wang, Y.; Liu, H.; Zang, K.; Luo, J.; et al. Microwave-Assisted Rapid Synthesis of Graphene-Supported Single Atomic Metals. Adv. Mater. 2018, 30, 1802146. [Google Scholar] [CrossRef]
- Yang, J.; Qiu, Z.; Zhao, C.; Wei, W.; Chen, W.; Li, Z.; Qu, Y.; Dong, J.; Luo, J.; Li, Z.; et al. In Situ Thermal Atomization to Convert Supported Nickel Nanoparticles into Surface-Bound Nickel Single-Atom Catalysts. Angew. Chem. Int. Ed. 2018, 57, 14095–14100. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Huang, B.; Yi, Y.; Guo, Y.; Zuo, Z.; Li, Y.; Jia, Z.; Liu, H.; Li, Y. Anchoring Zero Valence Single Atoms of Nickel and Iron on Graphdiyne for Hydrogen Evolution. Nat. Commun. 2018, 9, 1460. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jia, Y.; Gao, G.; Yan, X.; Chen, N.; Chen, J.; Soo, M.T.; Wood, B.; Yang, D.; Du, A.; et al. Graphene Defects Trap Atomic Ni Species for Hydrogen and Oxygen Evolution Reactions. Chem 2018, 4, 285–297. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, L.; Chen, T.; Zhou, W.; Lou, X.W.D. Surface Modulation of Hierarchical MoS2 Nanosheets by Ni Single Atoms for Enhanced Electrocatalytic Hydrogen Evolution. Adv. Funct. Mater. 2018, 28, 1807086. [Google Scholar] [CrossRef]
- Fan, Q.; Hou, P.; Choi, C.; Wu, T.; Hong, S.; Li, F.; Soo, Y.; Kang, P.; Jung, Y.; Sun, Z. Activation of Ni Particles into Single Ni–N Atoms for Efficient Electrochemical Reduction of CO2. Adv. Energy Mater. 2019, 10, 1903068. [Google Scholar] [CrossRef]
- Hou, Y.; Qiu, M.; Kim, M.G.; Liu, P.; Nam, G.; Zhang, T.; Zhuang, X.; Yang, B.; Cho, J.; Chen, M.; et al. Atomically Dispersed Nickel–Nitrogen–Sulfur Species Anchored on Porous Carbon Nanosheets for Efficient Water Oxidation. Nat. Commun. 2019, 10, 1392. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, Z.; Lu, X.; Liang, Y.; Wang, H. Domino Electroreduction of CO2 to Methanol on a Molecular Catalyst. Nature 2019, 575, 639–642. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Chen, T.; Zhang, J.; Zhang, J.; Lou, X.W. (David) Unveiling the Activity Origin of Electrocatalytic Oxygen Evolution over Isolated Ni Atoms Supported on a N-Doped Carbon Matrix. Adv. Mater. 2019, 31, 1904548. [Google Scholar] [CrossRef]
- Lu, C.; Yang, J.; Wei, S.; Bi, S.; Xia, Y.; Chen, M.; Hou, Y.; Qiu, M.; Yuan, C.; Su, Y.; et al. Atomic Ni Anchored Covalent Triazine Framework as High Efficient Electrocatalyst for Carbon Dioxide Conversion. Adv. Funct. Mater. 2019, 29, 1806884. [Google Scholar] [CrossRef]
- Yang, F.; Wang, M.; Liu, W.; Yang, B.; Wang, Y.; Luo, J.; Tang, Y.; Hou, L.; Li, Y.; Li, Z.; et al. Atomically Dispersed Ni as the Active Site towards Selective Hydrogenation of Nitroarenes. Green Chem. 2019, 21, 704–711. [Google Scholar] [CrossRef]
- Lu, P.; Yang, Y.; Yao, J.; Wang, M.; Dipazir, S.; Yuan, M.; Zhang, J.; Wang, X.; Xie, Z.; Zhang, G. Facile Synthesis of Single-Nickel-Atomic Dispersed N-Doped Carbon Framework for Efficient Electrochemical CO2 Reduction. Appl. Catal. B Environ. 2019, 241, 113–119. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, D.; Muhammad, Z.; Wan, F.; Xie, W.; Wang, Y.; Song, L.; Niu, Z.; Chen, J. Single Nickel Atoms on Nitrogen-Doped Graphene Enabling Enhanced Kinetics of Lithium–Sulfur Batteries. Adv. Mater. 2019, 31, 1903955. [Google Scholar] [CrossRef]
- Jeong, H.-Y.; Balamurugan, M.; Choutipalli, V.S.K.; Jeong, E.; Subramanian, V.; Sim, U.; Nam, K.T. Achieving Highly Efficient CO2 to CO Electroreduction Exceeding 300 MA Cm−2 with Single-Atom Nickel Electrocatalysts. J. Mater. Chem. A 2019, 7, 10651–10661. [Google Scholar] [CrossRef]
- Tang, Y.; Wei, Y.; Wang, Z.; Zhang, S.; Li, Y.; Nguyen, L.; Li, Y.; Zhou, Y.; Shen, W.; Tao, F.F.; et al. Synergy of Single-Atom Ni1 and Ru1 Sites on CeO2 for Dry Reforming of CH4. J. Am. Chem. Soc. 2019, 141, 7283–7293. [Google Scholar] [CrossRef]
- Akri, M.; Zhao, S.; Li, X.; Zang, K.; Lee, A.F.; Isaacs, M.A.; Xi, W.; Gangarajula, Y.; Luo, J.; Ren, Y.; et al. Atomically Dispersed Nickel as Coke-Resistant Active Sites for Methane Dry Reforming. Nat. Commun. 2019, 10, 5181. [Google Scholar] [CrossRef]
- Li, Y.; Hao, J.; Song, H.; Zhang, F.; Bai, X.; Meng, X.; Zhang, H.; Wang, S.; Hu, Y.; Ye, J. Selective Light Absorber-Assisted Single Nickel Atom Catalysts for Ambient Sunlight-Driven CO2 Methanation. Nat. Commun. 2019, 10, 2359. [Google Scholar] [CrossRef]
- Xiong, W.; Li, H.; Wang, H.; Yi, J.; You, H.; Zhang, S.; Hou, Y.; Cao, M.; Zhang, T.; Cao, R. Hollow Mesoporous Carbon Sphere Loaded Ni–N4 Single-Atom: Support Structure Study for CO2 Electrocatalytic Reduction Catalyst. Small 2020, 16, 2003943. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, J.; Fang, B.; Xuan, X.; Liu, N.; Yang, X.; Zhou, J. Single Ni Atoms with Higher Positive Charges Induced by Hydroxyls for Electrocatalytic CO2 Reduction. Nanoscale 2020, 12, 18437–18445. [Google Scholar] [CrossRef]
- Zhou, M.; Jiang, Y.; Wang, G.; Wu, W.; Chen, W.; Yu, P.; Lin, Y.; Mao, J.; Mao, L. Single-Atom Ni-N4 Provides a Robust Cellular NO Sensor. Nat. Commun. 2020, 11, 3188. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhuang, C.; Li, S.; Wang, Y.; Zou, X.; Liu, X.; Huang, W.; Zhu, G. Efficient Single-Atom Ni for Catalytic Transfer Hydrogenation of Furfural to Furfuryl Alcohol. J. Mater. Chem. A 2020, 9, 1110–1118. [Google Scholar] [CrossRef]
- Sa, Y.J.; Jung, H.; Shin, D.; Jeong, H.Y.; Ringe, S.; Kim, H.; Hwang, Y.J.; Joo, S.H. Thermal Transformation of Molecular Ni2+–N4 Sites for Enhanced CO2 Electroreduction Activity. ACS Catal. 2020, 10, 10920–10931. [Google Scholar] [CrossRef]
- Liu, S.; Yang, H.B.; Hung, S.; Ding, J.; Cai, W.; Liu, L.; Gao, J.; Li, X.; Ren, X.; Kuang, Z.; et al. Elucidating the Electrocatalytic CO2 Reduction Reaction over a Model Single-Atom Nickel Catalyst. Angew. Chem. Int. Ed. 2020, 59, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Koshy, D.M.; Chen, S.; Lee, D.U.; Stevens, M.B.; Abdellah, A.M.; Dull, S.M.; Chen, G.; Nordlund, D.; Gallo, A.; Hahn, C.; et al. Understanding the Origin of Highly Selective CO2 Electroreduction to CO on Ni,N-doped Carbon Catalysts. Angew. Chem. Int. Ed. 2020, 59, 4043–4050. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Y.; Gu, M.; Wang, M.; Zhang, Z.; Pan, W.; Jiang, Z.; Zheng, H.; Lucero, M.; Wang, H.; et al. Molecular Engineering of Dispersed Nickel Phthalocyanines on Carbon Nanotubes for Selective CO2 Reduction. Nat. Energy 2020, 5, 684–692. [Google Scholar] [CrossRef]
- Gong, Y.; Jiao, L.; Qian, Y.; Pan, C.; Zheng, L.; Cai, X.; Liu, B.; Yu, S.; Jiang, H. Regulating the Coordination Environment of MOF-Templated Single-Atom Nickel Electrocatalysts for Boosting CO2 Reduction. Angew. Chem. Int. Ed. 2020, 59, 2705–2709. [Google Scholar] [CrossRef]
- Li, S.; Ceccato, M.; Lu, X.; Frank, S.; Lock, N.; Roldan, A.; Hu, X.-M.; Skrydstrup, T.; Daasbjerg, K. Incorporation of Nickel Single Atoms into Carbon Paper as Self-Standing Electrocatalyst for CO2 Reduction. J. Mater. Chem. A 2020, 9, 1583–1592. [Google Scholar] [CrossRef]
- Zhang, C.; Fu, Z.; Zhao, Q.; Du, Z.; Zhang, R.; Li, S. Single-Atom-Ni-Decorated, Nitrogen-Doped Carbon Layers for Efficient Electrocatalytic CO2 Reduction Reaction. Electrochem. Commun. 2020, 116, 106758. [Google Scholar] [CrossRef]
- Chen, J.; Li, H.; Fan, C.; Meng, Q.; Tang, Y.; Qiu, X.; Fu, G.; Ma, T. Dual Single-Atomic Ni-N4 and Fe-N4 Sites Constructing Janus Hollow Graphene for Selective Oxygen Electrocatalysis. Adv. Mater. 2020, 32, 2003134. [Google Scholar] [CrossRef]
- Cai, Z.; Du, P.; Liang, W.; Zhang, H.; Wu, P.; Cai, C.; Yan, Z. Single-Atom-Sized Ni–N 4 Sites Anchored in Three-Dimensional Hierarchical Carbon Nanostructures for the Oxygen Reduction Reaction. J. Mater. Chem. A 2020, 8, 15012–15022. [Google Scholar] [CrossRef]
- Luo, F.; Zhu, J.; Ma, S.; Li, M.; Xu, R.; Zhang, Q.; Yang, Z.; Qu, K.; Cai, W.; Chen, Z. Regulated Coordination Environment of Ni Single Atom Catalyst toward High-Efficiency Oxygen Electrocatalysis for Rechargeable Zinc-Air Batteries. Energy Storage Mater. 2021, 35, 723–730. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, S.; Chen, Z.; Lu, C.; Han, S.; Ke, C.; Zhu, J.; Zhang, J.; Tranca, D.; Zhuang, X. Carbon Nanosheets Supporting Ni–N3S Single-Atom Sites for Efficient Electrocatalytic CO2 Reduction. Carbon 2021, 178, 488–496. [Google Scholar] [CrossRef]
- Zhang, S.; Ao, X.; Huang, J.; Wei, B.; Zhai, Y.; Zhai, D.; Deng, W.; Su, C.; Wang, D.; Li, Y. Isolated Single-Atom Ni–N5 Catalytic Site in Hollow Porous Carbon Capsules for Efficient Lithium–Sulfur Batteries. Nano Lett. 2021, 21, 9691–9698. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiao, L.; Yang, W.; Xie, C.; Jiang, H. Rational Fabrication of Low-Coordinate Single-Atom Ni Electrocatalysts by MOFs for Highly Selective CO2 Reduction. Angew. Chem. Int. Ed. 2021, 60, 7607–7611. [Google Scholar] [CrossRef]
- Feng, Y.; Long, S.; Chen, B.; Jia, W.; Xie, S.; Sun, Y.; Tang, X.; Yang, S.; Zeng, X.; Lin, L. Inducing Electron Dissipation of Pyridinic N Enabled by Single Ni–N4 Sites for the Reduction of Aldehydes/Ketones with Ethanol. ACS Catal. 2021, 11, 6398–6405. [Google Scholar] [CrossRef]
- Jia, C.; Li, S.; Zhao, Y.; Hocking, R.K.; Ren, W.; Chen, X.; Su, Z.; Yang, W.; Wang, Y.; Zheng, S.; et al. Nitrogen Vacancy Induced Coordinative Reconstruction of Single-Atom Ni Catalyst for Efficient Electrochemical CO2 Reduction. Adv. Funct. Mater. 2021, 31, 2107072. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, L.; Liu, X.; Qi, H.; Liu, Q.; Yang, J.; Su, Y.; Ma, J.; Yin, J.; Wang, A. Tuning the Coordination Environment of Single-Atom Catalyst M-N-C towards Selective Hydrogenation of Functionalized Nitroarenes. Nano Res. 2021, 15, 519–527. [Google Scholar] [CrossRef]
- Svalova, A.; Brusko, V.; Sultanova, E.; Kirsanova, M.; Khamidullin, T.; Vakhitov, I.; Dimiev, A.M. Individual Ni Atoms on Reduced Graphene Oxide as Efficient Catalytic System for Reduction of 4-Nitrophenol. Appl. Surf. Sci. 2021, 565, 150503. [Google Scholar] [CrossRef]
- Mei, B.; Liu, C.; Sun, F.; Lu, S.; Du, X.; Li, X.; Song, F.; Xu, W.; Jiang, Z. Unraveling the Potential-Dependent Volcanic Selectivity Changes of an Atomically Dispersed Ni Catalyst During CO2 Reduction. ACS Catal. 2022, 12, 8676–8686. [Google Scholar] [CrossRef]
- Li, Y.; Adli, N.M.; Shan, W.; Wang, M.; Zachman, M.J.; Hwang, S.; Tabassum, H.; Karakalos, S.; Feng, Z.; Wang, G.; et al. Atomically Dispersed Single Ni Site Catalysts for High-Efficiency CO2 Electroreduction at Industrial-Level Current Densities. Energy Environ. Sci. 2022, 15, 2108–2119. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, X.; Ke, T.; Jia, M.; Jin, B.; Li, Y.; Yang, Q.; Ren, L.; Ren, Y.; Cheng, D.; et al. Nickel Single Atom Overcoordinated Active Sites to Accelerate the Electrochemical Reaction Kinetics for Li-S Cathode. J. Energy Chem. 2022, 78, 203–210. [Google Scholar] [CrossRef]
- Chen, K.; Cao, M.; Lin, Y.; Fu, J.; Liao, H.; Zhou, Y.; Li, H.; Qiu, X.; Hu, J.; Zheng, X.; et al. Ligand Engineering in Nickel Phthalocyanine to Boost the Electrocatalytic Reduction of CO2. Adv. Funct. Mater. 2022, 32, 2111322. [Google Scholar] [CrossRef]
- Huang, M.; Deng, B.; Zhao, X.; Zhang, Z.; Li, F.; Li, K.; Cui, Z.; Kong, L.; Lu, J.; Dong, F.; et al. Template-Sacrificing Synthesis of Well-Defined Asymmetrically Coordinated Single-Atom Catalysts for Highly Efficient CO2 Electrocatalytic Reduction. ACS Nano 2022, 16, 2110–2119. [Google Scholar] [CrossRef] [PubMed]
- Xi, D.; Li, J.; Low, J.; Mao, K.; Long, R.; Li, J.; Dai, Z.; Shao, T.; Zhong, Y.; Li, Y.; et al. Limiting the Uncoordinated N Species in M–Nx Single-Atom Catalysts toward Electrocatalytic CO2 Reduction in Broad Voltage Range. Adv. Mater. 2022, 34, 2104090. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xie, Z.; Jiang, L.; Zhao, W.; Cao, S.; Wang, B.; Si, R.; Zhang, R.; Liu, Y.; Zhao, Z. Selective Transfer Hydrogenation Coupling of Nitroaromatics to Azoxy/Azo Compounds by Electron-Enriched Single Ni-N4 Sites on Mesoporous N-Doped Carbon. Chem. Eng. J. 2022, 443, 136416. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.L.; Cheng, W.; Chen, Y.; Luan, D.; Gao, S.; Lou, X.W. (David) Loading Single-Ni Atoms on Assembled Hollow N-Rich Carbon Plates for Efficient CO2 Electroreduction. Adv. Mater. 2022, 34, 2105204. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zeng, Y.; Chen, Y.; Luan, D.; Gao, S.; Lou, X.W. (David) Mesoporous N-rich Carbon with Single-Ni Atoms as a Multifunctional Sulfur Host for Li-S Batteries. Angew. Chem. Int. Ed. 2022, 61, e202212680. [Google Scholar] [CrossRef]
- Huang, J.; Qiu, X.; Zhao, Z.; Zhu, H.; Liu, Y.; Shi, W.; Liao, P.; Chen, X. Single-Product Faradaic Efficiency for Electrocatalytic of CO2 to CO at Current Density Larger than 1.2 A Cm−2 in Neutral Aqueous Solution by a Single-Atom Nanozyme. Angew. Chem. Int. Ed. 2022, 61, e202210985. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, J.; Lv, H.; Yang, X.; Ding, L.; Xu, Y.; Zhang, K.; Sun, W.; Zhou, J. Sulfur-Doped Unsaturated Ni-N3 Coordination for Efficient Electroreduction of CO2. Chem. Eng. J. 2022, 450, 137950. [Google Scholar] [CrossRef]
- Leverett, J.; Yuwono, J.A.; Kumar, P.; Tran-Phu, T.; Qu, J.; Cairney, J.; Wang, X.; Simonov, A.N.; Hocking, R.K.; Johannessen, B.; et al. Impurity Tolerance of Unsaturated Ni-N-C Active Sites for Practical Electrochemical CO2 Reduction. ACS Energy Lett. 2022, 7, 920–928. [Google Scholar] [CrossRef]
- Liu, X.; Liao, L.; Xia, G.; Yu, F.; Zhang, G.; Shu, M.; Wang, H. An Accurate “Metal Pre-Buried” Strategy for Constructing Ni–N2C2 Single-Atom Sites with High Metal Loadings toward Electrocatalytic CO2 Reduction. J. Mater. Chem. A 2022, 10, 25047–25054. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, X.F.; Fan, G.; Luan, D.; Gu, X.; Lou, X.W. (David) Surface-Exposed Single-Ni Atoms with Potential-Driven Dynamic Behaviors for Highly Efficient Electrocatalytic Oxygen Evolution. Angew. Chem. Int. Ed. 2022, 61, e202212542. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Li, S.; Liu, S.; Cheong, W.-C.; Peng, C.; Yao, K.; Li, Y.; Wang, J.; Jiang, B.; Chen, Z.; et al. Biomass-Assisted Approach for Large-Scale Construction of Multi-Functional Isolated Single-Atom Site Catalysts. Nano Res. 2022, 15, 3980–3990. [Google Scholar] [CrossRef]
- Sun, X.; Chen, C.; Xiong, C.; Zhang, C.; Zheng, X.; Wang, J.; Gao, X.; Yu, Z.-Q.; Wu, Y. Surface Modification of MoS2 Nanosheets by Single Ni Atom for Ultrasensitive Dopamine Detection. Nano Res. 2022, 16, 917–924. [Google Scholar] [CrossRef]
- Ling, Y.; Ge, H.; Chen, J.; Zhang, Y.; Duan, Y.; Liang, M.; Guo, Y.; Wu, T.; Soo, Y.; Yin, X.; et al. General Strategy toward Hydrophilic Single Atom Catalysts for Efficient Selective Hydrogenation. Adv. Sci. 2022, 9, 2202144. [Google Scholar] [CrossRef]
- Jiang, M.; Chen, X.; Wang, L.; Liang, J.; Wei, X.; Nong, W. Anchoring Single Ni Atoms on CeO2 Nanospheres as an Efficient Catalyst for the Hydrogenolysis of Lignin to Aromatic Monomers. Fuel 2022, 324, 124499. [Google Scholar] [CrossRef]
- Ma, R.; Gao, J.; Kou, J.; Dean, D.P.; Breckner, C.J.; Liang, K.; Zhou, B.; Miller, J.T.; Zou, G. Insights into the Nature of Selective Nickel Sites on Ni/Al2O3 Catalysts for Propane Dehydrogenation. ACS Catal. 2022, 12, 12607–12616. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Bulusheva, L.G. Catalysts with Single Metal Atoms for the Hydrogen Production from Formic Acid. Catal. Rev. 2022, 64, 835–874. [Google Scholar] [CrossRef]
- Avakyan, L.A.; Manukyan, A.S.; Mirzakhanyan, A.A.; Sharoyan, E.G.; Zubavichus, Y.V.; Trigub, A.L.; Kolpacheva, N.A.; Bugaev, L.A. Atomic Structure of Nickel Phthalocyanine Probed by X-Ray Absorption Spectroscopy and Density Functional Simulations. Opt. Spectrosc. 2013, 114, 347–352. [Google Scholar] [CrossRef]
- Jentzen, W.; Turowska-Tyrk, I.; Scheidt, W.R.; Shelnutt, J.A. Planar Solid-State and Solution Structures of (Porphinato)Nickel(II) As Determined by X-Ray Diffraction and Resonance Raman Spectroscopy. Inorg. Chem. 1996, 35, 3559–3567. [Google Scholar] [CrossRef]
- Yamada, Y.; Suzuki, Y.; Yasuda, H.; Uchizawa, S.; Hirose-Takai, K.; Sato, Y.; Suenaga, K.; Sato, S. Functionalized Graphene Sheets Coordinating Metal Cations. Carbon 2014, 75, 81–94. [Google Scholar] [CrossRef]
- Chernyshev, V.M.; Ananikov, V.P. Nickel and Palladium Catalysis: Stronger Demand than Ever. ACS Catal. 2022, 12, 1180–1200. [Google Scholar] [CrossRef]
- Yang, Y.; Li, W.; Xu, H. A New Explanation for the Carbon Deposition and Elimination over Supported Ni, Ni-Ce and Ni-Co Catalysts for CO2-Reforming of Methane. React. Kinet. Catal. Lett. 2002, 77, 155–162. [Google Scholar] [CrossRef]
- Vogt, C.; Kranenborg, J.; Monai, M.; Weckhuysen, B.M. Structure Sensitivity in Steam and Dry Methane Reforming over Nickel: Activity and Carbon Formation. ACS Catal. 2020, 10, 1428–1438. [Google Scholar] [CrossRef]
- Khairudin, N.F.; Sukri, M.F.F.; Khavarian, M.; Mohamed, A.R. Understanding the Performance and Mechanism of Mg-Containing Oxides as Support Catalysts in the Thermal Dry Reforming of Methane. Beilstein J. Nanotechnol. 2018, 9, 1162–1183. [Google Scholar] [CrossRef]
- Baharudin, L.; Rahmat, N.; Othman, N.H.; Shah, N.; Syed-Hassan, S.S.A. Formation, Control, and Elimination of Carbon on Ni-Based Catalyst during CO2 and CH4 Conversion via Dry Reforming Process: A Review. J. CO2 Util. 2022, 61, 102050. [Google Scholar] [CrossRef]
- Wei, J.; Iglesia, E. Isotopic and Kinetic Assessment of the Mechanism of Reactions of CH4 with CO2 or H2O to Form Synthesis Gas and Carbon on Nickel Catalysts. J. Catal. 2004, 224, 370–383. [Google Scholar] [CrossRef]
- Lustemberg, P.G.; Ramírez, P.J.; Liu, Z.; Gutiérrez, R.A.; Grinter, D.G.; Carrasco, J.; Senanayake, S.D.; Rodriguez, J.A.; Ganduglia-Pirovano, M.V. Room-Temperature Activation of Methane and Dry Re-Forming with CO2 on Ni-CeO2 (111) Surfaces: Effect of Ce3+ Sites and Metal–Support Interactions on C–H Bond Cleavage. ACS Catal. 2016, 6, 8184–8191. [Google Scholar] [CrossRef]
- Pantha, N.; Ulman, K.; Narasimhan, S. Adsorption of Methane on Single Metal Atoms Supported on Graphene: Role of Electron Back-Donation in Binding and Activation. J. Chem. Phys. 2020, 153, 244701. [Google Scholar] [CrossRef]
- Wu, J.; Gao, J.; Lian, S.; Li, J.; Sun, K.; Zhao, S.; Kim, Y.D.; Ren, Y.; Zhang, M.; Liu, Q.; et al. Engineering the Oxygen Vacancies Enables Ni Single-Atom Catalyst for Stable and Efficient C-H Activation. Appl. Catal. B Environ. 2022, 314, 121516. [Google Scholar] [CrossRef]
- Akri, M.; El Kasmi, A.; Batiot-Dupeyrat, C.; Qiao, B. Highly Active and Carbon-Resistant Nickel Single-Atom Catalysts for Methane Dry Reforming. Catalysts 2020, 10, 630. [Google Scholar] [CrossRef]
- Bellido, J.D.A.; Assaf, E.M. Effect of the Y2O3–ZrO2 Support Composition on Nickel Catalyst Evaluated in Dry Reforming of Methane. Appl. Catal. Gen. 2009, 352, 179–187. [Google Scholar] [CrossRef]
- Paladino Lino, A.V.; Rodella, C.B.; Assaf, E.M.; Assaf, J.M. Methane Tri-Reforming for Synthesis Gas Production Using Ni/CeZrO2/MgAl2O4 Catalysts: Effect of Zr/Ce Molar Ratio. Int. J. Hydrog. Energy 2020, 45, 8418–8432. [Google Scholar] [CrossRef]
- Wang, N.; Yu, X.; Shen, K.; Chu, W.; Qian, W. Synthesis, Characterization and Catalytic Performance of MgO-Coated Ni/SBA-15 Catalysts for Methane Dry Reforming to Syngas and Hydrogen. Int. J. Hydrog. Energy 2013, 38, 9718–9731. [Google Scholar] [CrossRef]
- Zuo, Z.; Liu, S.; Wang, Z.; Liu, C.; Huang, W.; Huang, J.; Liu, P. Dry Reforming of Methane on Single-Site Ni/MgO Catalysts: Importance of Site Confinement. ACS Catal. 2018, 8, 9821–9835. [Google Scholar] [CrossRef]
- Juan-Juan, J.; Román-Martínez, M.C.; Illán-Gómez, M.J. Effect of Potassium Content in the Activity of K-Promoted Ni/Al2O3 Catalysts for the Dry Reforming of Methane. Appl. Catal. Gen. 2006, 301, 9–15. [Google Scholar] [CrossRef]
- Zapata, B.; Valenzuela, M.A.; Palacios, J.; Torres-Garcia, E. Effect of Ca, Ce or K Oxide Addition on the Activity of Ni/SiO2 Catalysts for the Methane Decomposition Reaction. Int. J. Hydrog. Energy 2010, 35, 12091–12097. [Google Scholar] [CrossRef]
- Kim, S.M.; Abdala, P.M.; Margossian, T.; Hosseini, D.; Foppa, L.; Armutlulu, A.; van Beek, W.; Comas-Vives, A.; Copéret, C.; Müller, C. Cooperativity and Dynamics Increase the Performance of NiFe Dry Reforming Catalysts. J. Am. Chem. Soc. 2017, 139, 1937–1949. [Google Scholar] [CrossRef]
- Al-Fatesh, A. Suppression of Carbon Formation in CH4–CO2 Reforming by Addition of Sr into Bimetallic Ni–Co/γ-Al2O3 Catalyst. J. King Saud Univ. Eng. Sci. 2015, 27, 101–107. [Google Scholar] [CrossRef]
- Alipour, Z.; Rezaei, M.; Meshkani, F. Effect of Alkaline Earth Promoters (MgO, CaO, and BaO) on the Activity and Coke Formation of Ni Catalysts Supported on Nanocrystalline Al2O3 in Dry Reforming of Methane. J. Ind. Eng. Chem. 2014, 20, 2858–2863. [Google Scholar] [CrossRef]
- Roh, H.-S.; Jun, K.-W. Carbon Dioxide Reforming of Methane over Ni Catalysts Supported on Al2O3 Modified with La2O3, MgO, and CaO. Catal. Surv. Asia 2008, 12, 239–252. [Google Scholar] [CrossRef]
- Ma, Q.; Sun, J.; Gao, X.; Zhang, J.; Zhao, T.; Yoneyama, Y.; Tsubaki, N. Ordered Mesoporous Alumina-Supported Bimetallic Pd–Ni Catalysts for Methane Dry Reforming Reaction. Catal. Sci. Technol. 2016, 6, 6542–6550. [Google Scholar] [CrossRef]
- Huang, S.; Xu, H.; Li, H.; Guo, Y.; Sun, Z.; Du, Y.; Li, H.; Zhang, J.; Pang, R.; Dong, Q.; et al. Preparation and Characterization of Char Supported NiCu Nanoalloy Catalyst for Biomass Tar Cracking Together with Syngas-Rich Gas Production. Fuel Process. Technol. 2021, 218, 106858. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, L. Selective Hydrogenation of Acetylene Catalyzed by Nickel and Nitrogen-Doped C34: A Density Functional Theory Study. Chem. Phys. Lett. 2020, 757, 137871. [Google Scholar] [CrossRef]
- Poldorn, P.; Wongnongwa, Y.; Mudchimo, T.; Jungsuttiwong, S. Theoretical Insights into Catalytic CO2 Hydrogenation over Single-Atom (Fe or Ni) Incorporated Nitrogen-Doped Graphene. J. CO2 Util. 2021, 48, 101532. [Google Scholar] [CrossRef]
- Xue, M.; Jia, J.; Wu, H. A Density Functional Theory Study on the Catalytic Performance of Metal (Ni, Pd) Single Atom, Dimer and Trimer for H2 Dissociation. Chem. Phys. 2022, 552, 111336. [Google Scholar] [CrossRef]
- Zhuo, H.-Y.; Yu, X.; Yu, Q.; Xiao, H.; Zhang, X.; Li, J. Selective Hydrogenation of Acetylene on Graphene-Supported Non-Noble Metal Single-Atom Catalysts. Sci. China Mater. 2020, 63, 1741–1749. [Google Scholar] [CrossRef]
- Sun, M.; Nelson, A.; Adjaye, J. Ab Initio DFT Study of Hydrogen Dissociation on MoS2, NiMoS, and CoMoS: Mechanism, Kinetics, and Vibrational Frequencies. J. Catal. 2005, 233, 411–421. [Google Scholar] [CrossRef]
- Bing, Q.; Liu, W.; Yi, W.; Liu, J. Ni Anchored C2N Monolayers as Low-Cost and Efficient Catalysts for Hydrogen Production from Formic Acid. J. Power Sources 2019, 413, 399–407. [Google Scholar] [CrossRef]
- Prins, R. Hydrogen Spillover. Facts and Fiction. Chem. Rev. 2012, 112, 2714–2738. [Google Scholar] [CrossRef] [PubMed]
- Vogt, C.; Monai, M.; Kramer, G.J.; Weckhuysen, B.M. The Renaissance of the Sabatier Reaction and Its Applications on Earth and in Space. Nat. Catal. 2019, 2, 188–197. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, H.; Fang, X.; Zhai, S.; Zhai, D.; Sun, L.; Deng, W. Theoretical Studies on the Catalytic Hydrogenation of Carbon Dioxide by 3d Transition Metals Single-Atom Catalyst Supported on Covalent Triazine Frameworks. Mol. Catal. 2021, 508, 111581. [Google Scholar] [CrossRef]
- Homlamai, K.; Maihom, T.; Choomwattana, S.; Sawangphruk, M.; Limtrakul, J. Single-Atoms Supported (Fe, Co, Ni, Cu) on Graphitic Carbon Nitride for CO2 Adsorption and Hydrogenation to Formic Acid: First-Principles Insights. Appl. Surf. Sci. 2020, 499, 143928. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Sharifi, F.; Dinparast, L. Catalytic Hydrogenation of CO2 over Pt- and Ni-Doped Graphene: A Comparative DFT Study. J. Mol. Graph. Model. 2017, 77, 143–152. [Google Scholar] [CrossRef]
- Ren, Y.; Sun, X.; Qi, K.; Zhao, Z. Single Atom Supported on MoS2 as Efficient Electrocatalysts for the CO2 Reduction Reaction: A DFT Study. Appl. Surf. Sci. 2022, 602, 154211. [Google Scholar] [CrossRef]
- Alonso, G.; López, E.; Huarte-Larrañaga, F.; Sayós, R.; Prats, H.; Gamallo, P. Zeolite-Encapsulated Single-Atom Catalysts for Efficient CO2 Conversion. J. CO2 Util. 2021, 54, 101777. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, C.; Sun, K.; Jia, X.; Ye, J.; Liu, C. Advances in Studies of the Structural Effects of Supported Ni Catalysts for CO2 Hydrogenation: From Nanoparticle to Single Atom Catalyst. J. Mater. Chem. A 2022, 10, 5792–5812. [Google Scholar] [CrossRef]
- Millet, M.-M.; Algara-Siller, G.; Wrabetz, S.; Mazheika, A.; Girgsdies, F.; Teschner, D.; Seitz, F.; Tarasov, A.; Levchenko, S.V.; Schlögl, R.; et al. Ni Single Atom Catalysts for CO2 Activation. J. Am. Chem. Soc. 2019, 141, 2451–2461. [Google Scholar] [CrossRef]
- Farlow, M.W.; Adkins, H. The Hydrogenation of Carbon Dioxide and a Correction of the Reported Synthesis of Urethans. J. Am. Chem. Soc. 1935, 57, 2222–2223. [Google Scholar] [CrossRef]
- Wang, T.; Ren, D.; Huo, Z.; Song, Z.; Jin, F.; Chen, M.; Chen, L. A Nanoporous Nickel Catalyst for Selective Hydrogenation of Carbonates into Formic Acid in Water. Green Chem. 2017, 19, 716–721. [Google Scholar] [CrossRef]
- Cen, Y.; Yue, Y.; Wang, S.; Lu, J.; Wang, B.; Jin, C.; Guo, L.; Hu, Z.-T.; Zhao, J. Adsorption Behavior and Electron Structure Engineering of Pd-Based Catalysts for Acetylene Hydrochlorination. Catalysts 2019, 10, 24. [Google Scholar] [CrossRef]
- Riley, C.; Zhou, S.; Kunwar, D.; De La Riva, A.; Peterson, E.; Payne, R.; Gao, L.; Lin, S.; Guo, H.; Datye, A. Design of Effective Catalysts for Selective Alkyne Hydrogenation by Doping of Ceria with a Single-Atom Promotor. J. Am. Chem. Soc. 2018, 140, 12964–12973. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Chen, Z.; Yao, T.; Zheng, L.; Lin, Y.; Liu, W.; Ju, H.; Zhu, J.; Hong, X.; Wei, S.; et al. Single Ni Sites Distributed on N-Doped Carbon for Selective Hydrogenation of Acetylene. Chem Commun 2017, 53, 11568–11571. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.; Liao, S.; Li, H.; Tong, R.; Dong, C.; Zhang, M.; Gu, W.; Liu, X. Carbon-Based Materials with Tunable Morphology Confined Ni(0) and Ni-Nx Active Sites: Highly Efficient Selective Hydrogenation Catalysts. Carbon 2019, 154, 48–57. [Google Scholar] [CrossRef]
- Wang, F.-F.; Guo, R.; Jian, C.; Zhang, W.; Xue, R.; Chen, D.-L.; Zhang, F.; Zhu, W. Mechanism of Catalytic Transfer Hydrogenation for Furfural Using Single Ni Atom Catalysts Anchored to Nitrogen-Doped Graphene Sheets. Inorg. Chem. 2022, 61, 9138–9146. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Ross, J.R.H. Towards Sustainable Production of Formic Acid. ChemSusChem 2018, 11, 821–836. [Google Scholar] [CrossRef]
- Gharib, A.; Arab, A. Decomposition of Formic Acid via Carboxyl Mechanism on the Graphene Nanosheet Decorated by Cr, Mn, Fe, Co, Ni, Pd, Ag, and Cd Metals: A DFT Study. Int. J. Hydrog. Energy 2023, 48, 566–575. [Google Scholar] [CrossRef]
- Akça, A.; Karaman, O. Electrocatalytic Decomposition of Formic Acid Catalyzed by M-Embedded Graphene (M = Ni and Cu): A DFT Study. Top. Catal. 2022, 65, 1–13. [Google Scholar] [CrossRef]
- Bing, Q.; Liu, J. Transition Metal Single Atom Anchored C3N for Highly Efficient Formic Acid Dehydrogenation: A DFT Study. Appl. Surf. Sci. 2021, 562, 150186. [Google Scholar] [CrossRef]
- Zhong, W.; Liu, Y.; Deng, M.; Zhang, Y.; Jia, C.; Prezhdo, O.V.; Yuan, J.; Jiang, J. C2N-Supported Single Metal Ion Catalysts for HCOOH Dehydrogenation. J. Mater. Chem. A 2018, 6, 11105–11112. [Google Scholar] [CrossRef]
- Feng, B.; Guo, R.; Cai, Q.; Song, Y.; Li, N.; Fu, Y.; Chen, D.-L.; Zhang, J.; Zhu, W.; Zhang, F. Construction of Isolated Ni Sites on Nitrogen-Doped Hollow Carbon Spheres with Ni–N3 Configuration for Enhanced Reduction of Nitroarenes. Nano Res. 2022, 15, 6001–6009. [Google Scholar] [CrossRef]
- Genç, A.E.; Küçük, H.; Alp, I.O.; Akça, A. Hydrazine Decomposition on Nickel-Embedded Graphene. Int. J. Hydrog. Energy 2020, 45, 33407–33418. [Google Scholar] [CrossRef]













| Year | Support Material | Ni Content | Ni Coordination | Bond Length a, Å | Reference |
|---|---|---|---|---|---|
| 2018 | N–doped holey graphene framework | 0.05 at% b | Ni–N4 | 1.89 | [37] |
| N–doped graphene | 0.41 at% c | Ni–N4O1 | 1.87 and 2.19 | [38] | |
| N–doped graphene | 0.8 wt% b | Ni–N4 | 1.86 | [39] | |
| N–doped carbon | 2.83 wt% d | Ni–N4 | Not given | [40] | |
| N–doped graphene | 4.6 wt% b | Ni–N4 | 1.861 | [41] | |
| N–doped porous carbon | 5.44 wt% e | Ni–N2V2 | 1.88 | [34] | |
| N–doped carbon | 7.5 wt% b | Ni–N5 | 1.90 and 2.14 | [24] | |
| N–doped carbon | 9.5 wt% f | Ni–N4 | 1.838 | [42] | |
| N–doped carbon nanotubes | 20 wt% g | Ni–N4 | 1.86 | [36] | |
| N–doped carbon nanotubes | 20.3 wt% | Ni–N4 | 1.86 | [43] | |
| N–doped carbon nanosheet | Not given | Ni–N4 | Not given | [44] | |
| N–doped graphene | Not given | Ni–N3O1 | 1.87 and 2.10 | [45] | |
| N–doped carbon | Not given | Ni–N4 | 1.89 | [46] | |
| Graphdiyne | 0.278 wt% d | Ni–C12 | 2.05 | [47] | |
| Defective graphene | 1.24 wt% | Ni–C4 and Ni–C5 | 1.78 and 1.99 | [48] | |
| MoS2 nanosheets array on carbon cloth | 1.8 at% b | Ni–Sx | Not given | [32] | |
| Hierarchical MoS2 nanosheets supported on carbon matrix nanofibers | 2.7 wt% e | Ni–S5 | 2.19 | [49] | |
| 2019 | N–doped carbon cloth | 0.48 μg·cm−2 | Ni–N3V1 | 1.84 | [33] |
| 0.52 μg·cm−2 | Ni–N4 | 1.88 | |||
| N-doped carbon matrix on carbon nanotubes | 0.087 wt% b | Ni–N2C2 | 1.86 and 2.73 | [50] | |
| Porous carbon nanosheets | 0.2 wt% e | Ni–N3S1 | 1.85 and 2.33 | [51] | |
| Carbon nanotubes | 0.27 wt% d | Ni–N4 | Not given | [52] | |
| N atoms decorated hollow carbon matrix | 1.27 wt% d | Ni–N4 | 1.91 | [53] | |
| Covalent triazine framework | 2.4 wt% c | Ni–N4 | 1.845 | [54] | |
| N–doped graphene aerogel | 2.6 wt% | Ni–Nx<4 | Not given | [23] | |
| N–doped porous carbon | 4.4 wt% e | Ni–N3 | 1.84 | [55] | |
| 4.9 wt% e | Ni–N4 | 1.86 | |||
| N–doped black carbon | 5.32 wt% e | Ni–N4 | Not given | [22] | |
| N–doped carbon nanotubes | 6.63 wt% e | Ni–Nx | Not given | [56] | |
| N–doped graphene | Not given | Ni–N4 | 1.81 and 1.97 | [57] | |
| N–doped carbon | Not given | Ni–N4 | 1.864 | [58] | |
| CeO2 | 2.5 wt% | Ni–O3Ce3 | 1.89 and 2.94 | [59] | |
| Hydroxyapatite | 2.58 wt% b | Ni–O6 | 2.05 | [60] | |
| Amorphous Y2O3 nanosheets | 3.9 wt% b | Ni–O3 | Not given | [61] | |
| 2020 | Carbon spheres | 0.37 % f | Ni–N4 | 2.02 | [62] |
| Honeycomb–like carbon | 0.77 at% c | Ni–N4 | 1.96 | [63] | |
| N–doped hollow carbon | 0.10 wt% | Ni–N4 | 1.92 | [64] | |
| N–doped carbon | 0.12 wt% e | Ni–N4 | 1.98 | [65] | |
| Carbon nanotubes | 0.17 wt% e | Ni–N3V1 | 1.844 | [66] | |
| 0.21 wt% e | Ni–N4 | 1.898 | |||
| Carbon nanotubes | 0.27 wt% e | Ni–N4 | Not given | [67] | |
| N–doped carbon | 0.48 wt% e | Ni–N4–5 | 2.05 | [21] | |
| N–doped graphitic carbon | 0.5 wt% h | Ni–N4 | 1.876 | [68] | |
| Carbon nanotubes | 0.76 wt% d | Ni–N4 | 1.91 | [69] | |
| N–doped carbon | 0.872 wt% b | Ni–N4C1 | 1.91 and 2.13 | [70] | |
| 0.889 wt% b | Ni–N2C2 | 1.87 and 2.13 | |||
| 0.917 wt% b | Ni–N3C1 | 1.86 and 2.11 | |||
| Carbon paper | 1.04 wt% e | Ni–N3S | 1.879 and 1.939 | [71] | |
| Carbon membrane | 1.3 wt% e | Ni–N4 | 1.93 | [16] | |
| N–doped carbon layers | 1.61 wt% b | Ni–N3 | 1.87 | [72] | |
| Janus hollow graphene | 1.9 wt% b | Ni–N4 | 2.09 | [73] | |
| N–doped carbon | 2 wt% e | Ni–N2O2 | 1.85 | [20] | |
| 2.2 wt% e | Ni–N4 | Not given | |||
| Three-dimensional hierarchical carbon | 4.2 wt% e | Ni–N4 | 1.87 | [74] | |
| Graphene–like carbon | 3.1 wt% | Ni–O4(OH)2 | 2.05 | [18] | |
| 2021 | N–doped carbon | 0.24 wt% d | Ni–N4O1 | 1.87 | [75] |
| Not given | Ni–N4 | 1.87 | |||
| Porous carbon nanosheets | 0.5 wt% e | Ni–N1N2S1 | 1.91, 2.06, and 2.03 | [76] | |
| N–doped porous carbon | 0.6 wt% e | Ni–N5 | 1.92 | [77] | |
| N–doped carbon | 0.85 wt % b | Ni–N3 | 1.86 | [78] | |
| 1.06 wt% b | Ni–N4 | 1.88 | |||
| N–doped porous carbon | 1 wt% | Ni–N4 | 2.00 | [15] | |
| N–doped carbon | 2.1 wt% e | Ni–N4 | 1.89 | [79] | |
| N–doped graphene | 2.14 wt% e | Ni–N2 | 1.83 | [80] | |
| N–doped carbon | 3.3 wt% b | Ni–N3,4 | 1.85 | [81] | |
| Reduced graphene oxide | 1.4 at% | Ni–Ox | Not given | [82] | |
| 2022 | N–doped carbon | 0.51 at% g | Ni–N4 | 1.93 | [83] |
| N–rich carbon hosts | 1.1 at% c | Ni–N2O2 | 1.85 and 1.99 | [84] | |
| N-doped carbon | Not given | Ni–N4O1 | 1.96 and 2.27 | [85] | |
| Carbon nanotubes | 0.4553 wt% e | Ni–N4 | 1.887 | [86] | |
| N–doped carbon matrix | 0.51 wt% e | Ni–N4O1 | 1.94 and 1.90 | [87] | |
| N–doped carbon | 1.0 wt% b | Ni–N4 | 1.84 | [88] | |
| N–doped carbon | 1.5 wt% b | Ni–N5 | 1.91 and 2.11 | [89] | |
| 3.3 wt% b | Ni–N4 | 1.85 | |||
| N–rich carbon | 1.6 wt% e | Ni–N4 | 1.88 | [90] | |
| N–rich carbon | 1.66 wt% e | Ni–N4 | Not given | [91] | |
| N–doped carbon | Not given | Ni–N4 | 1.91 | [92] | |
| 1.89 wt% b | Ni–N5 | 1.88 | |||
| Three-dimensional carbon material | 2.20 wt% d | Ni–N3S1 | 1.89 and 1.94 | [93] | |
| N–doped carbon | 2.37 wt% e | Ni–N4 | 1.86 | [19] | |
| N–doped carbon | 3.7 wt% e | Ni–C3N1 | 1.88 and 2.00 | [94] | |
| N–doped carbon | 4 wt% e | Ni–C1N1O2 | 1.777, 2.045, and 2.925 | [31] | |
| N–doped carbon | 9.15 wt% f | Ni-C2N2 | 1.90 and 2.07 | [95] | |
| S/N–doped carbon spheres | 1.1 wt% e | Ni–N4 | 1.88 | [96] | |
| N,B co–doped porous carbon | 1.5 wt% e | Ni–N(B)4 | 1.89 | [97] | |
| MoS2 nanosheets | 1.4 wt% | Ni–S3 | 2.23 | [98] | |
| TiO2 | 0.4 wt% b | Ni–O3–4 | 2.04 | [99] | |
| CeO2 nanospheres | 1.0 wt% e | Ni–O3 | 2.02 | [100] | |
| γ–Al2O3 | 2.5 wt% | Ni–O4 | 2.02 | [101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishchakova, A.D.; Bulusheva, L.G.; Bulushev, D.A. Supported Ni Single-Atom Catalysts: Synthesis, Structure, and Applications in Thermocatalytic Reactions. Catalysts 2023, 13, 845. https://doi.org/10.3390/catal13050845
Nishchakova AD, Bulusheva LG, Bulushev DA. Supported Ni Single-Atom Catalysts: Synthesis, Structure, and Applications in Thermocatalytic Reactions. Catalysts. 2023; 13(5):845. https://doi.org/10.3390/catal13050845
Chicago/Turabian StyleNishchakova, Alina D., Lyubov G. Bulusheva, and Dmitri A. Bulushev. 2023. "Supported Ni Single-Atom Catalysts: Synthesis, Structure, and Applications in Thermocatalytic Reactions" Catalysts 13, no. 5: 845. https://doi.org/10.3390/catal13050845