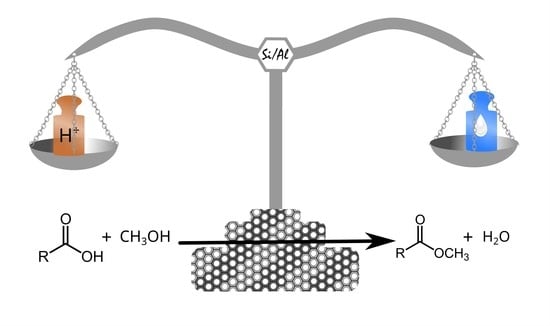
Balance between Catalyst Acidity and Hydrophilicity in Biofuel Production from Fatty Acid Esterification over Al-SBA-15
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
2.2. Catalyst Performance
3. Materials and Methods
3.1. Materials
3.2. Synthesis of SBA-15 and Isomorphically Substituted Al-SBA-15 Catalysts
3.3. Catalyst Characterization
3.4. Catalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Esters Market-Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2020–2030, TMR. Available online: https://www.transparencymarketresearch.com/esters-market.html (accessed on 20 March 2021).
- Blanco-Sánchez, M.; Franco, A.; Pineda, A.; Balu, A.; Romero, A.; Luque, R. NH4F Modified Al-SBA-15 Materials for Esterification of Valeric Acid to Alkyl Valerates. Proceedings 2019, 3, 4. [Google Scholar]
- do Nascimento, L.A.S.; Tito, L.M.Z.; Angélica, R.S.; da Costa, C.E.F.; Zamian, J.R.; da Rocha Filho, G.N. Esterification of oleic acid over solid acid catalysts prepared from Amazon flint kaolin. Appl. Catal. B Environ. 2011, 101, 495–503. [Google Scholar] [CrossRef]
- Mostafa Marzouk, N.; Abo El Naga, A.O.; Younis, S.A.; Shaban, S.A.; EL Torgoman, A.M.; EL Kady, F.Y. Process optimization of biodiesel production via esterification of oleic acid using sulfonated hierarchical mesoporous ZSM-5 as an efficient heterogeneous catalyst. J. Environ. Chem. Eng. 2021, 9, 105035. [Google Scholar] [CrossRef]
- Dal Pozzo, D.M.; dos Santos, J.A.; Júnior, E.S.; Santos, R.F.; Feiden, A.; de Souza, S.N. Free fatty acids esterification catalyzed by acid Faujasite type zeolite. RSC Adv. 2019, 9, 4900–4907. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.; Sakthivel, A. Zeolite-β based molecular sieves: A potential catalyst for esterification of biomass derived model compound levulinic acid. Mater. Sci. Energy Technol. 2021, 4, 307–316. [Google Scholar] [CrossRef]
- Gomes, G.J.; Costa, M.B.; Bittencourt, P.R.S.; Zalazar, M.F.; Arroyo, P.A. Catalytic improvement of biomass conversion: Effect of adding mesoporosity on MOR zeolite for esterification with oleic acid. Renew. Energy 2021, 178, 1–12. [Google Scholar] [CrossRef]
- Prinsen, P.; Luque, R.; González-Arellano, C. Zeolite catalyzed palmitic acid esterification. Microporous Mesoporous Mater. 2018, 262, 133–139. [Google Scholar] [CrossRef]
- Fawaz, E.G.; Salam, D.A.; Daou, T.J. Esterification of linoleic acid using HZSM-5 zeolites with different Si/Al ratios. Microporous Mesoporous Mater. 2020, 294, 109855. [Google Scholar] [CrossRef]
- Mohebbi, S.; Rostamizadeh, M.; Kahforoushan, D. Efficient sulfated high silica ZSM-5 nanocatalyst for esterification of oleic acid with methanol. Microporous Mesoporous Mater. 2020, 294, 109845. [Google Scholar] [CrossRef]
- Canhaci, S.J.; Perez, R.F.; Borges, L.E.P.; Fraga, M.A. Direct conversion of xylose to furfuryl alcohol on single organic–inorganic hybrid mesoporous silica-supported catalysts. Appl. Catal. B Environ. 2017, 207, 279–285. [Google Scholar] [CrossRef]
- Tututi-Ríos, E.; González, H.; Cabrera-Munguia, D.A.; Gutiérrez-Alejandre, A.; Rico, J.L. Acid properties of Sn-SBA-15 and Sn-SBA-15-PrSO3H materials and their role on the esterification of oleic acid. Catal. Today 2022, 394, 235–246. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, X.; Zhang, Y.; Tu, H.; Pan, P.; Li, S. Phosphotungstic acid and propylsulfonic acid bifunctionalized ordered mesoporous silica: A highly efficient and reusable catalysts for esterification of oleic acid. Chem. Eng. J. 2022, 430, 133059. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, H.; Fan, M.; Sun, W.; Jiang, P.; Dong, Y. Direct and postsynthesis of tin-incorporated SBA-15 functionalized with sulfonic acid for efficient biodiesel production. Fuel 2019, 235, 426–432. [Google Scholar] [CrossRef]
- Mbaraka, I.K.; Radu, D.R.; Lin, V.S.Y.; Shanks, B.H. Organosulfonic acid-functionalized mesoporous silicas for the esterification of fatty acid. J. Catal. 2003, 219, 329–336. [Google Scholar] [CrossRef]
- Pečar, D.; Goršek, A. Synthesis and characterization of sulfonic acid functionalized mesoporous SBA-15: Application in esterification reaction. React. Kinet. Mechan. Catal. 2019, 128, 991–1003. [Google Scholar] [CrossRef]
- Wang, Y.; You, J.; Liu, B. Preparation of mesoporous silica supported sulfonic acid and evaluation of the catalyst in esterification reactions. React. Kinet. Mechan. Catal. 2019, 128, 493–505. [Google Scholar]
- Tuel, A. Modification of mesoporous silicas by incorporation of heteroelements in the framework. Microporous Mesoporous Mater. 1999, 27, 151–169. [Google Scholar] [CrossRef]
- Carmo, A.C.; de Souza, L.K.C.; da Costa, C.E.F.; Longo, E.; Zamian, J.R.; da Rocha Filho, G.N. Production of biodiesel by esterification of palmitic acid over mesoporous aluminosilicate Al-MCM-41. Fuel 2009, 88, 461–468. [Google Scholar] [CrossRef]
- Talha, Z.; Bachir, C.; Ziri, S.; Bellahouel, S.; Bengueddach, A.; Villièras, F. Al-Rich Ordered Mesoporous Silica SBA-15 Materials: Synthesis, Surface Characterization and Acid Properties. Catal. Lett. 2017, 147, 2116–2126. [Google Scholar] [CrossRef]
- Sahel, F.; Sebih, F.; Bellahouel, S.; Bengueddach, A.; Hamacha, R. Synthesis and characterization of highly ordered mesoporous nanomaterials Al-MCM-41 and Al-SBA-15 from bentonite as efficient catalysts for the production of biodiesel MELA and EELA. Res. Chem. Intermed. 2020, 46, 133–148. [Google Scholar] [CrossRef]
- Cabrera-Munguia, D.A.; González, H.; Tututi-Ríos, E.; Gutiérrez-Alejandre, A.; Rico, J.L. Acid properties of M-SBA-15 and M-SBA-15-SO3H (M = Al, Ti) materials and their role on esterification of oleic acid. J. Mater. Res. 2018, 33, 3634–3645. [Google Scholar] [CrossRef]
- Perez, R.F.; Albuquerque, E.M.; Borges, L.E.P.; Hardacre, C.; Fraga, M.A. Aqueous-phase tandem catalytic conversion of xylose to furfuryl alcohol over [Al]-SBA-15 molecular sieves. Catal. Sci. Technol. 2019, 9, 5350–5358. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Benamor, T.; Vidal, L.; Lebeau, B.; Marichal, C. Influence of synthesis parameters on the physico-chemical characteristics of SBA-15 type ordered mesoporous silica. Microporous Mesoporous Mater. 2012, 153, 100–114. [Google Scholar] [CrossRef]
- Gallo, J.M.R.; Bisio, C.; Gatti, G.; Marchese, L.; Pastore, H.O. Physicochemical Characterization and Surface Acid Properties of Mesoporous [Al]-SBA-15 Obtained by Direct Synthesis. Langmuir 2010, 26, 5791–5800. [Google Scholar] [CrossRef]
- Xing, S.; Lv, P.; Fu, J.; Wang, J.; Fan, P.; Yang, L. Direct synthesis and characterization of pore-broadened Al-SBA-15. Microporous Mesoporous Mater. 2017, 239, 316–327. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Han, Y.; Xiao, F.S. Direct Observation of Nanorange Ordered Microporosity within Mesoporous Molecular Sieves. Chem. Mater. 2002, 14, 2536–2540. [Google Scholar] [CrossRef]
- Bhange, P.; Bhange, D.S.; Pradhan, S.; Ramaswamy, V. Direct synthesis of well-ordered mesoporous Al-SBA-15 and its correlation with the catalytic activity. Appl. Catal. A Gen. 2011, 400, 176–184. [Google Scholar] [CrossRef]
- Hu, W.; Luo, Q.; Su, Y.; Chen, L.; Yue, Y.; Ye, C. Acid sites in mesoporous Al-SBA-15 material as revealed by solid-state NMR spectroscopy. Microporous Mesoporous Mater. 2006, 92, 22–30. [Google Scholar] [CrossRef]
- Luan, Z.; Cheng, C.F.; Zhou, W.; Klinowski, J. Mesopore Molecular Sieve MCM-41 Containing Framework Aluminum. J. Phys. Chem. 1995, 99, 1018–1024. [Google Scholar] [CrossRef]
- Ravi, M.; Sushkevich, V.L.; van Bokhoven, J.A. Towards a better understanding of Lewis acidic aluminium in zeolites. Nat. Mater. 2020, 19, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Campos, G.P.; Albuquerque, E.M.; Fraga, M.A.; Pastore, H.O. Continuous Cellobiose Hydrolysis over Lamellar Aluminosilicates—Unveiling [Al]-magadiite Water-Tolerant Acid Sites. Ind. Eng. Chem. Res. 2021, 60, 4794–4805. [Google Scholar] [CrossRef]
- Busca, G. Acidity and basicity of zeolites: A fundamental approach. Microporous Mesoporous Mater. 2017, 254, 3–16. [Google Scholar] [CrossRef]
- Sandoval-Díaz, L.E.; González-Amaya, J.A.; Trujillo, C.A. General aspects of zeolite acidity characterization. Microporous Mesoporous Mater. 2015, 215, 229–243. [Google Scholar] [CrossRef]
- Socci, J.; Saraeian, A.; Stefanidis, S.D.; Banks, S.W.; Shanks, B.H.; Bridgwater, T. The role of catalyst acidity and shape selectivity on products from the catalytic fast pyrolysis of beech wood. J. Anal. Appl. Pyrolysis 2022, 162, 104710. [Google Scholar] [CrossRef]
- Milina, M.; Mitchell, S.; Michels, N.L.; Kenvin, J.; Pérez-Ramírez, J. Interdependence between porosity, acidity, and catalytic performance in hierarchical ZSM-5 zeolites prepared by post-synthetic modification. J. Catal. 2013, 308, 398–407. [Google Scholar] [CrossRef]
- Kresnawahjuesa, O.; Gorte, R.J.; de Oliveira, D.; Lau, L.Y. A Simple, Inexpensive, and Reliable Method for Measuring Brønsted-Acid Site Densities in Solid Acids. Catal. Lett. 2002, 82, 155–160. [Google Scholar] [CrossRef]
- Socci, J.; Osatiashtiani, A.; Kyriakou, G.; Bridgwater, T. The catalytic cracking of sterically challenging plastic feedstocks over high acid density Al-SBA-15 catalysts. Appl. Catal. A Gen. 2019, 570, 218–227. [Google Scholar] [CrossRef]
- Serrano, D.P.; Calleja, G.; Botas, J.A.; Gutierrez, F.J. Adsorption and Hydrophobic Properties of Mesostructured MCM-41 and SBA-15 Materials for Volatile Organic Compound Removal. Ind. Eng. Chem. Res. 2004, 43, 7010–7018. [Google Scholar] [CrossRef]
- Gounder, R. Hydrophobic microporous and mesoporous oxides as Brønsted and Lewis acid catalysts for biomass conversion in liquid water. Catal. Sci. Technol. 2014, 4, 2877–2886. [Google Scholar] [CrossRef]
- Doyle, A.M.; Albayati, T.M.; Abbas, A.S.; Alismaeel, Z.T. Biodiesel production by esterification of oleic acid over zeolite Y prepared from kaolin. Renew. Energy 2016, 97, 19–23. [Google Scholar] [CrossRef]
- Tarach, K.A.; Góra-Marek, K.; Martinez-Triguero, J.; Melián-Cabrera, I. Acidity and accessibility studies of desilicated ZSM-5 zeolites in terms of their effectiveness as catalysts in acid-catalyzed cracking processes. Catal. Sci. Technol. 2017, 7, 858–873. [Google Scholar] [CrossRef]
- Palčić, A.; Valtchev, V. Analysis and control of acid sites in zeolites. Appl. Catal. A Gen. 2020, 606, 117795. [Google Scholar] [CrossRef]
- Barthomeuf, D. Framework induced basicity in zeolites. Microporous Mesoporous Mater. 2003, 66, 1–14. [Google Scholar] [CrossRef]
- Rade, L.L.; Lemos, C.O.T.; Barrozo, M.A.S.; Ribas, R.M.; Monteiro, R.S.; Hori, C.E. Optimization of continuous esterification of oleic acid with ethanol over niobic acid. Renew. Energy 2018, 115, 208–216. [Google Scholar] [CrossRef]
- Liu, F.; Huang, K.; Zheng, A.; Xiao, F.S.; Dai, S. Hydrophobic Solid Acids and Their Catalytic Applications in Green and Sustainable Chemistry. ACS Catal. 2018, 8, 372–391. [Google Scholar] [CrossRef]
- Chung, K.H.; Chang, D.R.; Park, B.G. Removal of free fatty acid in waste frying oil by esterification with methanol on zeolite catalysts. Bioresour. Technol. 2008, 99, 7438–7443. [Google Scholar] [CrossRef]
- Chung, K.H.; Park, B.G. Esterification of oleic acid in soybean oil on zeolite catalysts with different acidity. J. Ind. Eng. Chem. 2009, 15, 388–392. [Google Scholar] [CrossRef]
- Sun, K.; Lu, J.; Ma, L.; Han, Y.; Fu, Z.; Ding, J. A comparative study on the catalytic performance of different types of zeolites for biodiesel production. Fuel 2015, 158, 848–854. [Google Scholar] [CrossRef]
- Hawash, S.A.; Ebrahiem, E.E.; Farag, H.A. Kinetics of esterification of oleic and linoleic free fatty acids. J. Adv. Eng. Trends 2020, 39, 25–36. [Google Scholar] [CrossRef]
- Hawash, S.A.; Ebrahiem, E.E.; Farag, H.A. Kinetic study of the esterification of unsaturated free fatty acids. Proc. Inst. Civ. Eng. —Energy 2019, 172, 105–114. [Google Scholar] [CrossRef]
- Joelianingsih, J.; Tambunan, A.H.; Nabetani, H. Reactivity of Palm Fatty Acids for the Non-catalytic Esterification in a Bubble Column Reactor at Atmospheric Pressure. Procedia Chem. 2014, 9, 182–193. [Google Scholar] [CrossRef]
- Kusdiana, D.; Saka, S. Methyl Esterification of Free Fatty Acids of Rapeseed Oil as Treated in Supercritical Methanol. J. Chem. Eng. Japan 2001, 34, 383–387. [Google Scholar] [CrossRef]
- Aranda, D.A.G.; Santos, R.T.P.; Tapanes, N.C.O.; Ramos, A.L.D.; Antunes, O.A.C. Acid-Catalyzed Homogeneous Esterification Reaction for Biodiesel Production from Palm Fatty Acids. Catal. Lett. 2008, 122, 20–25. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Rattanaphra, D.; Harvey, A.P.; Thanapimmetha, A.; Srinophakun, P. Kinetic of myristic acid esterification with methanol in the presence of triglycerides over sulfated zirconia. Renew. Energy 2011, 36, 2679–2686. [Google Scholar] [CrossRef]
- Lapuerta, M.; Rodríguez-Fernández, J.; Armas, O. Correlation for the estimation of the density of fatty acid esters fuels and its implications. A proposed Biodiesel Cetane Index. Chem. Phys. Lipids 2010, 163, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Gan, S.; Ng, H.K.; Chan, P.H.; Leong, F.L. Heterogeneous free fatty acids esterification in waste cooking oil using ion-exchange resins. Fuel Proc. Technol. 2012, 102, 67–72. [Google Scholar] [CrossRef]







| Catalyst | Si/Al | SBET (m2 g−1) | Vp (cm3 g−1) | Dp (nm) | AlTd (%) | AlOct (%) | BAS (μmol g−1) | H2O Adsorbed (μmol g−1) |
|---|---|---|---|---|---|---|---|---|
| SBA-15 | - | 944 | 0.96 | 4.2 | - | - | 11 | 315 |
| Al-SBA-15(13) | 13 | 699 | 1.08 | 5.6 | 48 | 35 | 195 | 842 |
| Al-SBA-15(19) | 19 | 567 | 0.88 | 5.9 | 50 | 29 | 136 | 784 |
| Al-SBA-15(33) | 33 | 824 | 1.16 | 5.2 | n.d. | n.d. | 165 | 693 |
| Al-SBA-15(70) | 70 | 744 | 0.90 | 4.6 | 32 | 48 | 71 | 390 |
| Al-SBA-15(174) | 174 | 740 | 0.55 | 3.6 | 27 | 59 | 45 | 333 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canhaci, S.J.; Albuquerque, E.M.; Lopes, C.C.; Faria, V.W.; Chinelatto Junior, L.S.; Duarte de Farias, A.M.; Quitete, C.B.; Fraga, M.A. Balance between Catalyst Acidity and Hydrophilicity in Biofuel Production from Fatty Acid Esterification over Al-SBA-15. Catalysts 2023, 13, 827. https://doi.org/10.3390/catal13050827
Canhaci SJ, Albuquerque EM, Lopes CC, Faria VW, Chinelatto Junior LS, Duarte de Farias AM, Quitete CB, Fraga MA. Balance between Catalyst Acidity and Hydrophilicity in Biofuel Production from Fatty Acid Esterification over Al-SBA-15. Catalysts. 2023; 13(5):827. https://doi.org/10.3390/catal13050827
Chicago/Turabian StyleCanhaci, Simone J., Elise M. Albuquerque, Camila C. Lopes, Vinícius W. Faria, Luiz Silvino Chinelatto Junior, Andréa M. Duarte de Farias, Cristina B. Quitete, and Marco A. Fraga. 2023. "Balance between Catalyst Acidity and Hydrophilicity in Biofuel Production from Fatty Acid Esterification over Al-SBA-15" Catalysts 13, no. 5: 827. https://doi.org/10.3390/catal13050827