Engineering of a Hybrid g-C3N4/ZnO-W/Cox Heterojunction Photocatalyst for the Removal of Methylene Blue Dye
Abstract
:1. Introduction
2. Results and Discussion
2.1. XRD Analysis
2.2. FTIR Analysis
2.3. SEM and EDS Analysis
2.4. Optical Analysis/Band Gap Tuning
2.5. Photocatalytic Activity
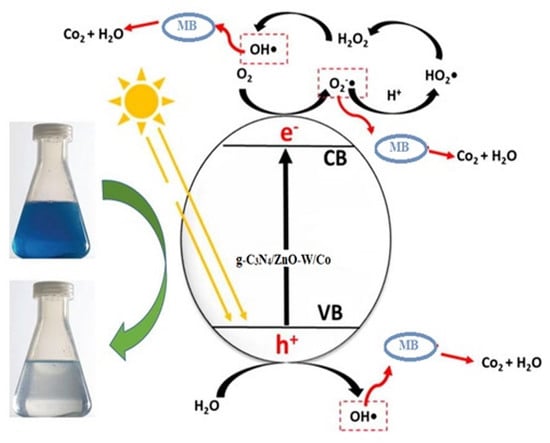
2.6. Photodegradation Mechanism
2.7. Active Species Analysis and Reusability Test
2.8. Reusability Test
3. Materials and Methods
3.1. Materials
3.2. Synthesis of g-C3N4
3.3. Synthesis of ZnO (NPs)
3.4. Synthesis of the g-C3N4/ZnO-W Composite
3.5. Synthesis of the g-C3N4/ZnO-W/Cox Nanocomposites
3.6. Photocatalytic Activity
3.7. Characterisation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Das, R. Application photocatalysis for treatment of industrial waste water—A short review. Open Access Libr. J. 2014, 1, 1–17. [Google Scholar] [CrossRef]
- Yuan, L.; Geng, Z.; Zhang, S.; Xu, J.; Guo, F.; Kumar Kundu, B.; Han, C. Efficient all-in-one removal of total chromium over nonconjugated polymer-inorganic ZnIn2S4 semiconductor hybrid. J. Colloid Interface Sci. 2022, 628, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Md Rosli, N.I.; Lam, S.-M.; Sin, J.-C.; Satoshi, I.; Mohamed, A.R. Photocatalytic Performance of ZnO/g-C 3 N 4 for Removal of Phenol under Simulated Sunlight Irradiation. J. Environ. Eng. 2018, 144, 04017091. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, Y.; Dong, F. Graphitic carbon nitride based nanocomposites: A review. Nanoscale 2015, 7, 15–37. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Chai, S.-P.; Yong, S.-T. Graphene oxide as a structure-directing agent for the two-dimensional interface engineering of sandwich-like graphene–gC3N4 hybrid nanostructures with enhanced visible-light photoreduction of CO2 to methane. Chem. Commun. 2015, 51, 858–861. [Google Scholar] [CrossRef]
- Yang, C.; Xue, Z.; Qin, J.; Sawangphruk, M.; Rajendran, S.; Zhang, X.; Liu, R. Visible light-driven photocatalytic H2 generation and mechanism insights into Bi2O2CO3/G-C3N4 Z-scheme photocatalyst. J. Phys. Chem. C 2019, 123, 4795–4804. [Google Scholar] [CrossRef]
- Farooq, N.; Luque, R.; Hessien, M.M.; Qureshi, A.M.; Sahiba, F.; Nazir, M.A.; ur Rehman, A. A Comparative Study of Cerium- and Ytterbium-Based GO/g-C3N4/Fe2O3 Composites for Electrochemical and Photocatalytic Applications. Appl. Sci. 2021, 11, 9000. [Google Scholar] [CrossRef]
- Qin, J.; Zhang, M.; Rajendran, S.; Zhang, X.; Liu, R. Two-dimensional porous sheet-like carbon-doped ZnO/g-C3N4 nanocomposite with high visible-light photocatalytic performance. Mater. Lett. 2017, 189, 156–159. [Google Scholar] [CrossRef]
- Kumar, O.P.; Shahzad, K.; Nazir, M.A.; Farooq, N.; Malik, M.; Ahmad Shah, S.S.; Rehman, A.u. Photo-Fenton activated C3N4x/AgOy@Co1-xBi0.1-yO7 dual s-scheme heterojunction towards degradation of organic pollutants. Opt. Mater. 2022, 126, 112199. [Google Scholar] [CrossRef]
- Yang, C.; Qin, J.; Rajendran, S.; Zhang, X.; Liu, R. WS2 and C-TiO2 nanorods acting as effective charge separators on g-C3N4 to boost visible-light activated hydrogen production from seawater. ChemSusChem 2018, 11, 4077–4085. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Zhu, B.; Jiang, C.; Cheng, B.; Yu, J. Mechanistic insight into the enhanced photocatalytic activity of single-atom Pt, Pd or Au-embedded g-C3N4. Appl. Surf. Sci. 2018, 433, 1175–1183. [Google Scholar] [CrossRef]
- Liu, S.; Li, D.; Sun, H.; Ang, H.M.; Tadé, M.O.; Wang, S. Oxygen functional groups in graphitic carbon nitride for enhanced photocatalysis. J. Colloid Interface Sci. 2016, 468, 176–182. [Google Scholar] [CrossRef]
- Kheirabadi, M.; Samadi, M.; Asadian, E.; Zhou, Y.; Dong, C.; Zhang, J.; Moshfegh, A.Z. Well-designed Ag/ZnO/3D graphene structure for dye removal: Adsorption, photocatalysis and physical separation capabilities. J. Colloid Interface Sci. 2019, 537, 66–78. [Google Scholar] [CrossRef]
- Fu, X.; Hu, X.; Yan, Z.; Lei, K.; Li, F.; Cheng, F.; Chen, J. Template-free synthesis of porous graphitic carbon nitride/carbon composite spheres for electrocatalytic oxygen reduction reaction. Chem. Commun. 2016, 52, 1725–1728. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Su, F.; Mathew, S.C.; Lipner, G.; Fu, X.; Antonietti, M.; Blechert, S.; Wang, X. mpg-C3N4-catalyzed selective oxidation of alcohols using O2 and visible light. J. Am. Chem. Soc. 2010, 132, 16299–16301. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Wu, G.; Chen, W. Porous graphitic carbon nitride synthesized via direct polymerization of urea for efficient sunlight-driven photocatalytic hydrogen production. Nanoscale 2012, 4, 5300–5303. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Shao, C.; Yang, S.; Li, X.; Guo, X.; Wang, X.; Li, X.; Liu, Y. Heterojunction of g-C3N4/BiOI immobilized on flexible electrospun polyacrylonitrile nanofibers: Facile preparation and enhanced visible photocatalytic activity for floating photocatalysis. Acs Sustain. Chem. Eng. 2018, 6, 2316–2323. [Google Scholar] [CrossRef]
- Kumar, O.P.; Ahmad, M.; Nazir, M.A.; Anum, A.; Jamshaid, M.; Shah, S.S.A.; Rehman, A. Strategic combination of metal–organic frameworks and C3N4 for expeditious photocatalytic degradation of dye pollutants. Environ. Sci. Pollut. Res. 2022, 29, 35300–35313. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Yu, S.; Dong, F.; Zhang, Q.; Zhou, Y. Efficient C3N4/graphene oxide macroscopic aerogel visible-light photocatalyst. J. Mater. Chem. A 2016, 4, 7823–7829. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, M.; Zhang, Q.; Dong, F.; Zhou, Y. Multifunctional g-C3N4/graphene oxide wrapped sponge monoliths as highly efficient adsorbent and photocatalyst. Appl. Catal. B Environ. 2018, 235, 17–25. [Google Scholar] [CrossRef]
- Li, R.; Cai, M.; Xie, Z.; Zhang, Q.; Zeng, Y.; Liu, H.; Liu, G.; Lv, W. Construction of heterostructured CuFe2O4/g-C3N4 nanocomposite as an efficient visible light photocatalyst with peroxydisulfate for the organic oxidation. Appl. Catal. B Environ. 2019, 244, 974–982. [Google Scholar] [CrossRef]
- Liang, M.; Borjigin, T.; Zhang, Y.; Liu, B.; Liu, H.; Guo, H. Controlled assemble of hollow heterostructured g-C3N4@ CeO2 with rich oxygen vacancies for enhanced photocatalytic CO2 reduction. Appl. Catal. B Environ. 2019, 243, 566–575. [Google Scholar] [CrossRef]
- Xiao, T.; Tang, Z.; Yang, Y.; Tang, L.; Zhou, Y.; Zou, L. In situ construction of hierarchical WO3/g-C3N4 composite hollow microspheres as a Z-scheme photocatalyst for the degradation of antibiotics. . Appl. Catal. B Environ. 2018, 220, 417–428. [Google Scholar] [CrossRef]
- Di, T.; Zhu, B.; Cheng, B.; Yu, J.; Xu, J. A direct Z-scheme g-C3N4/SnS2 photocatalyst with superior visible-light CO2 reduction performance. J. Catal. 2017, 352, 532–541. [Google Scholar] [CrossRef]
- Huang, Z.; Zeng, X.; Li, K.; Gao, S.; Wang, Q.; Lu, J. Z-scheme NiTiO3/g-C3N4 heterojunctions with enhanced photoelectrochemical and photocatalytic performances under visible LED light irradiation. ACS Appl. Mater. Interfaces 2017, 9, 41120–41125. [Google Scholar] [CrossRef] [PubMed]
- Kundu, B.K.; Han, G.; Sun, Y. Derivatized benzothiazoles as two-photon-absorbing organic photosensitizers active under near infrared light irradiation. J. Am. Chem. Soc. 2023, 145, 3535–3542. [Google Scholar] [CrossRef]
- Wu, Z.; Kundu, B.K.; Kang, W.; Mao, L.; Zhang, S.; Yuan, L.; Guo, F.; Han, C. Self-adaptive bulk/surface engineering of BixOyBrz towards enhanced photocatalysis: Current status and future challenges. J. Energy Chem. 2023, in press.
- Gomez, J.L.; Tigli, O. Zinc oxide nanostructures: From growth to application. J. Mater. Sci. 2013, 48, 612–624. [Google Scholar] [CrossRef]
- Sakthivel, S.; Neppolian, B.; Shankar, M.; Arabindoo, B.; Palanichamy, M.; Murugesan, V. Solar photocatalytic degradation of azo dye: Comparison of photocatalytic efficiency of ZnO and TiO2. Sol. Energy Mater. Sol. Cells 2003, 77, 65–82. [Google Scholar] [CrossRef]
- Li, Y.; Xie, W.; Hu, X.; Shen, G.; Zhou, X.; Xiang, Y.; Zhao, X.; Fang, P. Comparison of dye photodegradation and its coupling with light-to-electricity conversion over TiO2 and ZnO. Langmuir 2010, 26, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Dindar, B.; Içli, S. Unusual photoreactivity of zinc oxide irradiated by concentrated sunlight. J. Photochem. Photobiol. A Chem. 2001, 140, 263–268. [Google Scholar] [CrossRef]
- Gouvea, C.A.; Wypych, F.; Moraes, S.G.; Duran, N.; Nagata, N.; Peralta-Zamora, P. Semiconductor-assisted photocatalytic degradation of reactive dyes in aqueous solution. Chemosphere 2000, 40, 433–440. [Google Scholar] [CrossRef]
- Laouedj, E.; Ahmed, B. ZnOAssisted Photocatalytic Degradation of Congo Red and Benzopurpurine 4B in Aqueous Solution. J. Chem. Eng. Process Technol. 2011, 2, 1–9. [Google Scholar]
- Zhu, L.; Liu, Z.; Xia, P.; Li, H.; Xie, Y. Synthesis of hierarchical ZnO&Graphene composites with enhanced photocatalytic activity. Ceram. Int. 2018, 44, 849–856. [Google Scholar]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. Review on the improvement of the photocatalytic and antibacterial activities of ZnO. J. Alloys Compd. 2017, 727, 792–820. [Google Scholar] [CrossRef]
- Kumar, S.; Dhiman, A.; Sudhagar, P.; Krishnan, V. ZnO-graphene quantum dots heterojunctions for natural sunlight-driven photocatalytic environmental remediation. Appl. Surf. Sci. 2018, 447, 802–815. [Google Scholar] [CrossRef]
- Luu Thi, L.A.; Neto, M.M.; Van, T.P.; Nguyen Ngoc, T.; Nguyen Thi, T.M.; Nguyen, X.S.; Nguyen, C.T. In Situ g-C3N4@ Zno Nanocomposite: One-Pot Hydrothermal Synthesis and Photocatalytic Performance under Visible Light Irradiation. Adv. Mater. Sci. Eng. 2021, 2021, 6651633. [Google Scholar] [CrossRef]
- Iqbal, J.; Liu, X.; Zhu, H.; Wu, Z.; Zhang, Y.; Yu, D.; Yu, R. Raman and highly ultraviolet red-shifted near band-edge properties of LaCe-co-doped ZnO nanoparticles. Acta Mater. 2009, 57, 4790–4796. [Google Scholar] [CrossRef]
- Ngom, B.; Mpahane, T.; Manyala, N.; Nemraoui, O.; Buttner, U.; Kana, J.; Fasasi, A.; Maaza, M.; Beye, A. Structural and optical properties of nano-structured tungsten-doped ZnO thin films grown by pulsed laser deposition. Appl. Surf. Sci. 2009, 255, 4153–4158. [Google Scholar] [CrossRef]
- Ahmad, K.; Nazir, M.A.; Qureshi, A.K.; Hussain, E.; Najam, T.; Javed, M.S.; Shah, S.S.A.; Tufail, M.K.; Hussain, S.; Khan, N.A.; et al. Engineering of Zirconium based metal-organic frameworks (Zr-MOFs) as efficient adsorbents. Mater. Sci. Eng. B 2020, 262, 114766. [Google Scholar] [CrossRef]
- Shahzad, K.; Nazir, M.A.; Jamshaid, M.; Kumar, O.P.; Najam, T.; Shah, S.S.A.; Rehman, A. Synthesis of nanoadsorbent entailed mesoporous organosilica for decontamination of methylene blue and methyl orange from water. Int. J. Environ. Anal. Chem. 2021, 1–14. [Google Scholar] [CrossRef]
- Malik, M.; Len, T.; Luque, R.; Osman, S.M.; Paone, E.; Khan, M.I.; Wattoo, M.A.; Jamshaid, M.; Anum, A.; ur Rehman, A. Investigation on synthesis of hybrid g-C3N4/ZnO–W/M nanocomposites integrated heterojunction II as efficient photocatalyst for environmental applications. Environ. Res. 2023, 217, 114621. [Google Scholar] [CrossRef]
- Paul, D.R.; Sharma, R.; Panchal, P.; Nehra, S.; Gupta, A.; Sharma, A. Synthesis, characterization and application of silver doped graphitic carbon nitride as photocatalyst towards visible light photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2020, 45, 23937–23946. [Google Scholar] [CrossRef]
- Tian, H.; Fan, H.; Ma, J.; Liu, Z.; Ma, L.; Lei, S.; Fang, J.; Long, C. Pt-decorated zinc oxide nanorod arrays with graphitic carbon nitride nanosheets for highly efficient dual-functional gas sensing. J. Hazard. Mater. 2018, 341, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hong, M.; Zhang, F.; Zhuang, Z.; Yu, Y. Recyclable nanoscale zero valent iron doped g-C3N4/MoS2 for efficient photocatalysis of RhB and Cr (VI) driven by visible light. ACS Sustain. Chem. Eng. 2016, 4, 4055–4063. [Google Scholar] [CrossRef]
- Tahir, N.; Zahid, M.; Bhatti, I.A.; Jamil, Y. Fabrication of Visible Light Active Mn-Doped Bi2WO6-GO/MoS2 Heterostructure for Enhanced Photocatalytic Degradation of Methylene Blue. Environ. Sci. Pollut. Res. 2021, 29, 6552–6567. [Google Scholar] [CrossRef]
- Nyquist, R.A.; Kagel, R.O. Handbook of Infrared and Raman Spectra of Inorganic Compounds and Organic Salts: Infrared Spectra of Inorganic Compounds; Academic Press: Cambridge, MA, USA, 2012; Volume 4. [Google Scholar]
- Al-Hajry, A.; Umar, A.; Hahn, Y.; Kim, D. Growth, properties and dye-sensitized solar cells–applications of ZnO nanorods grown by low-temperature solution process. Superlattices Microstruct. 2009, 45, 529–534. [Google Scholar] [CrossRef]
- Sher, M.; Javed, M.; Shahid, S.; Iqbal, S.; Qamar, M.A.; Bahadur, A.; Qayyum, M.A. The controlled synthesis of gC3N4/Cd-doped ZnO nanocomposites as potential photocatalysts for the disinfection and degradation of organic pollutants under visible light irradiation. RSC Adv. 2021, 11, 2025–2039. [Google Scholar] [CrossRef]
- Le, S.; Jiang, T.; Li, Y.; Zhao, Q.; Li, Y.; Fang, W.; Gong, M. Highly efficient visible-light-driven mesoporous graphitic carbon nitride/ZnO nanocomposite photocatalysts. Appl. Catal. B Environ. 2017, 200, 601–610. [Google Scholar] [CrossRef]
- Kumaresan, S.; Vallalperuman, K.; Sathishkumar, S. A Novel one-step synthesis of Ag-doped ZnO nanoparticles for high performance photo-catalytic applications. J. Mater. Sci. Mater. Electron. 2017, 28, 5872–5879. [Google Scholar] [CrossRef]
- Boonprakob, N.; Wetchakun, N.; Phanichphant, S.; Waxler, D.; Sherrell, P.; Nattestad, A.; Chen, J.; Inceesungvorn, B. Enhanced visible-light photocatalytic activity of g-C3N4/TiO2 films. J. Colloid Interface Sci. 2014, 417, 402–409. [Google Scholar] [CrossRef]
- Ravichandran, K.; Seelan, K.S.; Ibrahim, M.M.; Kavitha, P. Improved solar light responsive photocatalytic activity of ZnO: W films: Effect of W loading level. Mater. Today Proc. 2020, 48, 216–228. [Google Scholar] [CrossRef]
- Vafaee, M.; Olya, M.; Drean, J.-Y.; Hekmati, A. Synthesize, characterization and application of ZnO/W/Ag as a new nanophotocatalyst for dye removal of textile wastewater; kinetic and economic studies. J. Taiwan Inst. Chem. Eng. 2017, 80, 379–390. [Google Scholar] [CrossRef]
- Alosaimi, E.H.; Azeem, N.; Tahir, N.; Jilani, A.; Zahid, M.; Alharthi, S.; Iqbal, J.; Yaseen, M.; Ahmad Rehan, Z.; Shahid, I. Investigation of Fe-Doped Graphitic Carbon Nitride-Silver Tungstate as a Hybrid Visible Light Active Photocatalyst. J. Chem. 2021, 2021, 1–18. [Google Scholar]
- Wojtyła, S.; Śpiewak, K.; Baran, T. Doped graphitic carbon nitride: Insights from spectroscopy and electrochemistry. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3418–3428. [Google Scholar] [CrossRef]
- Hezam, A.; Namratha, K.; Ponnamma, D.; Drmosh, Q.; Saeed, A.M.N.; Cheng, C.; Byrappa, K. Direct Z-scheme Cs2O–Bi2O3–ZnO heterostructures as efficient sunlight-driven photocatalysts. ACS Omega 2018, 3, 12260–12269. [Google Scholar] [CrossRef]
- Rahman, M.U.; Qazi, U.Y.; Hussain, T.; Nadeem, N.; Zahid, M.; Bhatti, H.N.; Shahid, I. Solar driven photocatalytic degradation potential of novel graphitic carbon nitride based nano zero-valent iron doped bismuth ferrite hybrid composite. Opt. Mater. 2021, 120, 111408. [Google Scholar] [CrossRef]
- Neena, D.; Humayun, M.; Zuo, W.; Liu, C.; Gao, W.; Fu, D.J. Hierarchical hetero-architectures of in-situ g-C3N4-coupled Fe-doped ZnO micro-flowers with enhanced visible-light photocatalytic activities. Appl. Surf. Sci. 2020, 506, 145017. [Google Scholar] [CrossRef]
- Praus, P. On electronegativity of graphitic carbon nitride. Carbon 2021, 172, 729–732. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, J.; Yu, S.; Alcocer, E.J.; Shahid, M.; Wang, Z.; Pan, W. Synergistic effect of N-decorated and Mn2+ doped ZnO nanofibers with enhanced photocatalytic activity. Sci. Rep. 2016, 6, 32711. [Google Scholar] [CrossRef] [PubMed]
- Mohite, S.; Rajpure, K. Oxidative degradation of salicylic acid by sprayed WO3 photocatalyst. Mater. Sci. Eng. B 2015, 200, 78–83. [Google Scholar] [CrossRef]
- Munawar, T.; Yasmeen, S.; Hussain, F.; Mahmood, K.; Hussain, A.; Asghar, M.; Iqbal, F. Synthesis of novel heterostructured ZnO-CdO-CuO nanocomposite: Characterization and enhanced sunlight driven photocatalytic activity. Mater. Chem. Phys. 2020, 249, 122983. [Google Scholar] [CrossRef]
- Chen, S.; Hu, Y.; Meng, S.; Fu, X. Study on the separation mechanisms of photogenerated electrons and holes for composite photocatalysts g-C3N4-WO3. Appl. Catal. B Environ. 2014, 150, 564–573. [Google Scholar] [CrossRef]
- Fageria, P.; Nazir, R.; Gangopadhyay, S.; Barshilia, H.C.; Pande, S. Graphitic-carbon nitride support for the synthesis of shape-dependent ZnO and their application in visible light photocatalysts. RSC Adv. 2015, 5, 80397–80409. [Google Scholar] [CrossRef]
- Jamshaid, M.; Nazir, M.A.; Najam, T.; Shah, S.S.A.; Khan, H.M.; Rehman, A.u. Facile synthesis of Yb3+-Zn2+ substituted M type hexaferrites: Structural, electric and photocatalytic properties under visible light for methylene blue removal. Chem. Phys. Lett. 2022, 805, 139939. [Google Scholar] [CrossRef]
- Shahzad, K.; Najam, T.; Bashir, M.S.; Nazir, M.A.; ur Rehman, A.; Bashir, M.A.; Shah, S.S.A. Fabrication of Periodic Mesoporous Organo Silicate (PMOS) composites of Ag and ZnO: Photo-catalytic degradation of methylene blue and methyl orange. Inorg. Chem. Commun. 2021, 123, 108357. [Google Scholar] [CrossRef]
- Mohammad, A.; Ahmad, K.; Qureshi, A.; Tauqeer, M.; Mobin, S.M. Zinc oxide-graphitic carbon nitride nanohybrid as an efficient electrochemical sensor and photocatalyst. Sens. Actuators B Chem. 2018, 277, 467–476. [Google Scholar] [CrossRef]
- Ge, L.; Han, C.; Liu, J. In situ synthesis and enhanced visible light photocatalytic activities of novel PANI-g-C3N4 composite photocatalysts. J. Mater. Chem. 2012, 22, 11843–11850. [Google Scholar] [CrossRef]
- Meng, Y.; Shen, J.; Chen, D.; Xin, G. Photodegradation performance of methylene blue aqueous solution on Ag/g-C3N4 catalyst. Rare Met. 2011, 30, 276–279. [Google Scholar] [CrossRef]
- Lv, T.; Pan, L.; Liu, X.; Lu, T.; Zhu, G.; Sun, Z. Enhanced photocatalytic degradation of methylene blue by ZnO-reduced graphene oxide composite synthesized via microwave-assisted reaction. J. Alloys Compd. 2011, 509, 10086–10091. [Google Scholar] [CrossRef]
- Nazir, M.A.; Khan, N.A.; Cheng, C.; Shah, S.S.A.; Najam, T.; Arshad, M.; Sharif, A.; Akhtar, S.; ur RehmanRehman, A. Surface induced growth of ZIF-67 at Co-layered double hydroxide: Removal of methylene blue and methyl orange from water. Appl. Clay Sci. 2020, 190, 105564. [Google Scholar] [CrossRef]
- Jamshaid, M.; ur Rehman, A.; Kumar, O.P.; Iqbal, S.; Nazir, M.A.; Anum, A.; Khan, H.M. Design of dielectric and photocatalytic properties of Dy–Ni substituted Ca0.5Pb0.5−xFe12−yO19 M-type hexaferrites. J. Mater. Sci. Mater. Electron. 2021, 32, 16255–16268. [Google Scholar] [CrossRef]










| Sample | a (Å) | c (Å) | Volume (Å)3 | Crystallite Size (nm) | Strain ε (nm−2) | Dislocation Density | Lattice Spacing |
|---|---|---|---|---|---|---|---|
| g-C3N4/ZnO-W/Co(0.001) | 3.432 | 6.029 | 61.523 | 15.183 | 2.424 × 10−6 | 4.337 × 10−6 | 2.731 |
| g-C3N4/ZnO-W/Co(0.003) | 3.265 | 5.748 | 53.092 | 15.811 | 2.353 × 10−6 | 3.999 × 10−6 | 2.742 |
| g-C3N4/ZnO-W/Co(0.005) | 3.392 | 5.964 | 59.454 | 15.823 | 2.351 × 10−6 | 3.994 × 10−6 | 2.715 |
| g-C3N4/ZnO-W/Co(0.007) | 3.306 | 5.820 | 55.113 | 15.840 | 2.349 × 10−6 | 3.985 × 10−6 | 2.794 |
| g-C3N4/ZnO-W/Co(0.009) | 3.312 | 5.829 | 55.412 | 15.786 | 2.356 × 10−6 | 4.012 × 10−6 | 2.793 |
| g-C3N4/ZnO-W/Co(0.010) | 3.231 | 5.689 | 51.440 | 15.826 | 2.351 × 10−6 | 3.992 × 10−6 | 2.721 |
| Sample | Dye | Degradation Efficiency % | Rate Constant (k) (min−1) | R2 | Band Gap (±0.1 eV) |
|---|---|---|---|---|---|
| g-C3N4/ZnO-W/Co(0.001) | MB | 75 | 0.024 | 0.989 | 3.22 |
| g-C3N4/ZnO-W/Co(0.003) | MB | 78 | 0.025 | 0.985 | 3.10 |
| g-C3N4/ZnO-W/Co(0.005) | MB | 82 | 0.027 | 0.996 | 2.83 |
| g-C3N4/ZnO-W/Co(0.007) | MB | 85 | 0.029 | 0.988 | 2.65 |
| g-C3N4/ZnO-W/Co(0.009) | MB | 87 | 0.031 | 0.988 | 2.32 |
| g-C3N4/ZnO-W/Co(0.010) | MB | 90 | 0.037 | 0.993 | 2.28 |
| Catalyst | Catalyst (g/L) | Irradiation Time | Irradiation Source | % Degradation | Reference |
|---|---|---|---|---|---|
| Ca0.5Pb0.5−xYbxZnyFe12−yO19 | 0.5 g/L | 90 min | Visible light | 96.1% | [68] |
| ZnO/PMOS | 10 mg/30 mL | 60 min | Visible light | 48% | [69] |
| Urea and [Zn(hmp-H)2(H2O)(μ-Cl)Zn (μ-Cl)(Cl)3] | 0.02 g/L | 150 min | Visible light | 84% | [70] |
| g-C3N4-PANI | 100 mg/L | 160 min | Visible light | 92% | [71] |
| Ag/g-C3N4 hybrid catalyst | 1 g/L | 300 min | 300 W Xe lamp | 58% | [72] |
| g-C3N4/TiO2 films | NM | 180 min | 50 W Halogen Lamp | 68% | [54] |
| ZnO/rGO | 1.5 g/L | 250 min | Hg lamp, 500 W | 88% | [73] |
| g-C3N4/ZnO-W/Co(0.010) | 0.05 mg/L | 90 min | Visible light | 90% | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malik, M.; Ibrahim, S.M.; Nazir, M.A.; Tahir, A.A.; Tufail, M.K.; Shah, S.S.A.; Anum, A.; Wattoo, M.A.; Rehman, A.u. Engineering of a Hybrid g-C3N4/ZnO-W/Cox Heterojunction Photocatalyst for the Removal of Methylene Blue Dye. Catalysts 2023, 13, 813. https://doi.org/10.3390/catal13050813
Malik M, Ibrahim SM, Nazir MA, Tahir AA, Tufail MK, Shah SSA, Anum A, Wattoo MA, Rehman Au. Engineering of a Hybrid g-C3N4/ZnO-W/Cox Heterojunction Photocatalyst for the Removal of Methylene Blue Dye. Catalysts. 2023; 13(5):813. https://doi.org/10.3390/catal13050813
Chicago/Turabian StyleMalik, Misbah, Sobhy M. Ibrahim, Muhammad Altaf Nazir, Asif A. Tahir, Muhammad Khurram Tufail, Syed Shoaib Ahmad Shah, Aqsa Anum, Muhammad Ahmad Wattoo, and Aziz ur Rehman. 2023. "Engineering of a Hybrid g-C3N4/ZnO-W/Cox Heterojunction Photocatalyst for the Removal of Methylene Blue Dye" Catalysts 13, no. 5: 813. https://doi.org/10.3390/catal13050813