Cu-Based Catalysts with Enhanced Thermal Stability for Cyclohexyl Acetate Hydrogenation: Studies on Cu+ and Cu0 Sites at Different Reduction Temperatures
Abstract
:1. Introduction
2. Results and Discussion
2.1. Different Cu-Based Catalysts
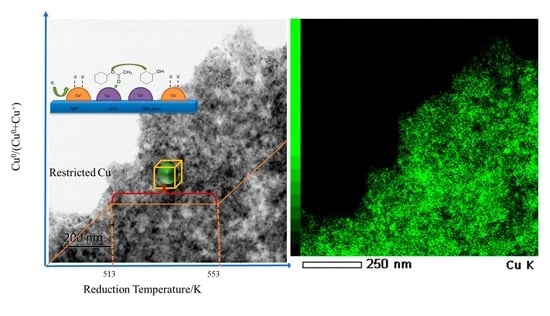
2.2. Thermal Stability
2.3. Induced Activation Process
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Activity Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nagaraja, B.M.; Siva Kumar, V.; Shashikala, V.; Padmasri, A.H.; Sreevardhan Reddy, S.; David Raju, B.; Rama Rao, K.S. Effect of method of preparation of copper—Magnesium oxide catalyst on the dehydrogenation of cyclohexanol. J. Mol. Catal. A Chem. 2004, 223, 339–345. [Google Scholar] [CrossRef]
- Høiset, S.; Hjertager, B.H.; Solberg, T.; Malo, K.A. Flixborough revisited—An explosion simulation approach. J. Hazard. Mater. 2000, 77, 1–9. [Google Scholar] [CrossRef]
- Ishida, H. Liquid-phase hydration process of cyclohexene with zeolites. Catal. Surv. Asia 1997, 1, 241–246. [Google Scholar] [CrossRef]
- Meng, F.; Wang, Y.; Wang, S.; Wang, S. Hydration of cyclohexene over zeolite ZSM-5: Improved catalyst performance by alkali treatment. React. Kinet. Mech. Catal. 2016, 119, 671–683. [Google Scholar] [CrossRef]
- Steyer, F.; Freund, H.; Sundmacher, K. A novel reactive distillation process for the indirect hydration of cyclohexene to cyclohexanol using a reactive entrainer. Ind. Eng. Chem. Res. 2008, 47, 9581–9587. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, T.; Han, B.; Liang, S.; Zhou, Y. Selective phenol hydrogenation to cyclohexanone over a dual supported Pd–lewis acid catalyst. Science 2009, 326, 1250–1252. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, L.; Wen, L.; Zong, B.; Wang, H.; Qiao, M. Cyclohexene esterification–hydrogenation for efficient production of cyclohexanol. Green Chem. 2021, 23, 1185–1192. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Y.; Zhao, Y.; Lv, J.; Wang, S.; Ma, X. Insight into the Balancing Effect of Active Cu Species for Hydrogenation of Carbon–Oxygen Bonds. ACS Catal. 2015, 5, 6200–6208. [Google Scholar] [CrossRef]
- Zheng, J.; Huang, L.; Cui, C.-H.; Chen, Z.-C.; Liu, X.-F.; Duan, X.; Cao, X.-Y.; Yang, T.-Z.; Zhu, H.; Shi, K.; et al. Ambient-pressure synthesis of ethylene glycol catalyzed by C60-buffered Cu/SiO2. Science 2022, 376, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, J.; Lv, J.; Zhao, Y.; Ma, X. Hydrogenation of dimethyl oxalate over copper-based catalysts: Acid–base properties and reaction paths. Ind. Eng. Chem. Res. 2015, 54, 9699–9707. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Wang, X.; Tan, Y.; Yang, W.; Zhu, H.; Chen, X.; Lu, W.; Ding, Y. Hydrogenation of dimethyl oxalate to ethanol over Mo-doped Cu/SiO2 catalyst. Chem. Eng. J. 2023, 454, 140001. [Google Scholar] [CrossRef]
- Ye, R.; Zhang, C.; Zhang, P.; Lin, L.; Huang, L.; Huang, Y.; Li, T.; Zhou, Z.; Zhang, R.; Feng, G.; et al. Facile preparation of efficient Cu-SiO2 catalysts using a polyhydroxy molecular template to regulate surface copper species for dimethyl oxalate hydrogenation. Catal. Commun. 2023, 174, 106586. [Google Scholar] [CrossRef]
- Bjelić, A.; Grilc, M.; Huš, M.; Likozar, B. Hydrogenation and hydrodeoxygenation of aromatic lignin monomers over Cu/C, Ni/C, Pd/C, Pt/C, Rh/C and Ru/C catalysts: Mechanisms, reaction micro-kinetic modelling and quantitative structure-activity relationships. Chem. Eng. J. 2019, 359, 305–320. [Google Scholar] [CrossRef]
- Bjelić, A.; Grilc, M.; Likozar, B. Bifunctional metallic-acidic mechanisms of hydrodeoxygenation of eugenol as lignin model compound over supported Cu, Ni, Pd, Pt, Rh and Ru catalyst materials. Chem. Eng. J. 2020, 394, 124914. [Google Scholar] [CrossRef]
- Bjelić, A.; Likozar, B.; Grilc, M. Scaling of lignin monomer hydrogenation, hydrodeoxygenation and hydrocracking reaction micro-kinetics over solid metal/acid catalysts to aromatic oligomers. Chem. Eng. J. 2020, 399, 125712. [Google Scholar] [CrossRef]
- Van Den Berg, R.; Parmentier, T.E.; Elkjær, C.F.; Gommes, C.J.; Sehested, J.; Helveg, S.; De Jongh, P.E.; De Jong, K.P. Support functionalization to retard ostwald ripening in copper methanol synthesis catalysts. ACS Catal. 2015, 5, 4439–4448. [Google Scholar] [CrossRef]
- Sun, J.; Yu, J.; Ma, Q.; Meng, F.; Wei, X.; Sun, Y.; Tsubaki, N. Freezing copper as a noble metal–like catalyst for preliminary hydrogenation. Sci. Adv. 2018, 4, eaau3275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Yang, M.; Zhang, J.; Ge, Q.; Zimina, A.; Pruessmann, T.; Zheng, L.; Grunwaldt, J.-D.; Sun, J. Stabilizing Cu+ in Cu/SiO2 catalysts with a shattuckite-like structure boosts CO2 hydrogenation into methanol. ACS Catal. 2020, 10, 14694–14706. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, J.; Zhang, J.; Ge, Q.; Xu, H.; Dallmann, F.; Dittmeyer, R.; Sun, J. Tailored metastable Ce–Zr oxides with highly distorted lattice oxygen for accelerating redox cycles. Chem. Sci. 2018, 9, 3386–3394. [Google Scholar] [CrossRef] [Green Version]
- Chinchen, G.C.; Denny, P.J.; Parker, D.G.; Spencer, M.S.; Whan, D.A. Mechanism of methanol synthesis from CO2/CO/H2 mixtures over copper/zinc oxide/alumina catalysts: Use of 14C-labelled reactants. Appl. Catal. 1987, 30, 333–338. [Google Scholar] [CrossRef]
- Amann, P.; Klötzer, B.; Degerman, D.; Köpfle, N.; Götsch, T.; Lömker, P.; Rameshan, C.; Ploner, K.; Bikaljevic, D.; Wang, H.-Y.; et al. The state of zinc in methanol synthesis over a Zn/ZnO/Cu(211) model catalyst. Science 2022, 376, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Behrens, M.; Studt, F.; Kasatkin, I.; Kühl, S.; Hävecker, M.; Abild-Pedersen, F.; Zander, S.; Girgsdies, F.; Kurr, P.; Kniep, B.-L.; et al. The active site of methanol synthesis over Cu/ZnO/Al2O3 industrial catalysts. Science 2012, 336, 893–897. [Google Scholar] [CrossRef]
- Kattel, S.; Ramírez, P.J.; Chen, J.G.; Rodriguez, J.A.; Liu, P. Active sites for CO2 hydrogenation to methanol on Cu/ZnO catalysts. Science 2017, 355, 1296–1299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klier, K. Methanol Synthesis. In Advances in Catalysis; Eley, D.D., Pines, H., Weisz, P.B., Eds.; Academic Press: Berlin, Germany, 1982; Volume 31, pp. 243–313. [Google Scholar]
- Kuld, S.; Thorhauge, M.; Falsig, H.; Elkjær, C.F.; Helveg, S.; Chorkendorff, I.; Sehested, J. Quantifying the promotion of Cu catalysts by ZnO for methanol synthesis. Science 2016, 352, 969–974. [Google Scholar] [CrossRef] [Green Version]
- Pandit, L.; Boubnov, A.; Behrendt, G.; Mockenhaupt, B.; Chowdhury, C.; Jelic, J.; Hansen, A.-L.; Saraçi, E.; Ras, E.-J.; Behrens, M.; et al. Unravelling the Zn-Cu interaction during activation of a Zn-promoted Cu/MgO model methanol catalyst. ChemCatChem 2021, 13, 4120–4132. [Google Scholar] [CrossRef]
- Van Den Berg, R.; Prieto, G.; Korpershoek, G.; van der Wal, L.I.; van Bunningen, A.J.; Lægsgaard-Jørgensen, S.; De Jongh, P.E.; De Jong, K.P. Structure sensitivity of Cu and CuZn catalysts relevant to industrial methanol synthesis. Nat. Commun. 2016, 7, 13057. [Google Scholar] [CrossRef]
- Song, T.; Chen, W.; Qi, Y.; Lu, J.; Wu, P.; Li, X. Efficient synthesis of methanol and ethylene glycol via the hydrogenation of CO2-derived ethylene carbonate on Cu/SiO2 catalysts with balanced Cu+–Cu0 sites. Catal. Sci. Technol. 2020, 10, 5149–5162. [Google Scholar] [CrossRef]
- Wen, C.; Cui, Y.; Dai, W.-L.; Xie, S.; Fan, K. Solvent feedstock effect: The insights into the deactivation mechanism of Cu/SiO2 catalysts for hydrogenation of dimethyl oxalate to ethylene glycol. Chem. Commun. 2013, 49, 5195–5197. [Google Scholar] [CrossRef] [PubMed]
- Yin, A.; Guo, X.; Dai, W.-L.; Fan, K. The Nature of Active Copper Species in Cu-HMS Catalyst for Hydrogenation of Dimethyl Oxalate to Ethylene Glycol: New Insights on the Synergetic Effect between Cu0 and Cu+. J. Phys. Chem. C 2009, 113, 11003–11013. [Google Scholar] [CrossRef]
- Zheng, X.; Lin, H.; Zheng, J.; Duan, X.; Yuan, Y. Lanthanum oxide-modified Cu/SiO2 as a high-performance catalyst for chemoselective hydrogenation of dimethyl oxalate to ethylene glycol. ACS Catal. 2013, 3, 2738–2749. [Google Scholar] [CrossRef]
- Xia, S.; Nie, R.; Lu, X.; Wang, L.; Chen, P.; Hou, Z. Hydrogenolysis of glycerol over Cu0.4/Zn5.6−xMgxAl2O8.6 catalysts: The role of basicity and hydrogen spillover. J. Catal. 2012, 296, 1–11. [Google Scholar] [CrossRef]
- Zhang, S.; Fan, G.; Li, F. Lewis-base-promoted copper-based catalyst for highly efficient hydrogenation of dimethyl 1,4-cyclohexane dicarboxylate. Green Chem. 2013, 15, 2389–2393. [Google Scholar] [CrossRef]
- Qi, Y.; Song, T.; Li, K.; Wu, P.; Zhu, Z.; Li, X. Synthesis of cyclohexanol and ethanol via the hydrogenation of cyclohexyl acetate with Cu2Znx/Al2O3 catalysts. Catal. Sci. Technol. 2021, 11, 7035–7046. [Google Scholar] [CrossRef]
- Shi, J.; He, Y.; Ma, K.; Tang, S.; Liu, C.; Yue, H.; Liang, B. Cu active sites confined in MgAl layered double hydroxide for hydrogenation of dimethyl oxalate to ethanol. Catal. Today 2021, 365, 318–326. [Google Scholar] [CrossRef]
- Ren, Y.; Yang, Y.; Chen, L.; Wang, L.; Shi, Y.; Yin, P.; Wang, W.; Shao, M.; Zhang, X.; Wei, M. Synergetic effect of Cu0−Cu+ derived from layered double hydroxides toward catalytic transfer hydrogenation reaction. Appl. Catal. B Environ. 2022, 314, 121515. [Google Scholar] [CrossRef]
- Schwenk, C.F.; Rode, B.M. The influence of the Jahn–Teller effect and of heteroligands on the reactivity of Cu2+. Chem. Commun. 2003, 11, 1286–1287. [Google Scholar] [CrossRef]
- Gong, J.; Yue, H.; Zhao, Y.; Zhao, S.; Zhao, L.; Lv, J.; Wang, S.; Ma, X. Synthesis of Ethanol via Syngas on Cu/SiO2 Catalysts with Balanced Cu0–Cu+ Sites. J. Am. Chem. Soc. 2012, 134, 13922–13925. [Google Scholar] [CrossRef]
- Yao, Y.; Wu, X.; Gutiérrez, O.Y.; Ji, J.; Jin, P.; Wang, S.; Xu, Y.; Zhao, Y.; Wang, S.; Ma, X.; et al. Roles of Cu+ and Cu0 sites in liquid-phase hydrogenation of esters on core-shell CuZnx@C catalysts. Appl. Catal. B Environ. 2020, 267, 118698. [Google Scholar] [CrossRef]
- Dai, W.-L.; Sun, Q.; Deng, J.-F.; Wu, D.; Sun, Y.-H. XPS studies of Cu/ZnO/Al2O3 ultra-fine catalysts derived by a novel gel oxalate co-precipitation for methanol synthesis by CO2 + H2. Appl. Surf. Sci. 2001, 177, 172–179. [Google Scholar] [CrossRef]
- Hu, J.; Li, Y.; Zhen, Y.; Chen, M.; Wan, H. In situ FTIR and ex situ XPS/HS-LEIS study of supported Cu/Al2O3 and Cu/ZnO catalysts for CO2 hydrogenation. Chin. J. Catal. 2021, 42, 367–375. [Google Scholar] [CrossRef]
- Li, D.; Xu, F.; Tang, X.; Dai, S.; Pu, T.; Liu, X.; Tian, P.; Xuan, F.; Xu, Z.; Wachs, I.E.; et al. Induced activation of the commercial Cu/ZnO/Al2O3 catalyst for the steam reforming of methanol. Nat. Catal. 2022, 5, 99–108. [Google Scholar] [CrossRef]
- Cui, G.; Meng, X.; Zhang, X.; Wang, W.; Xu, S.; Ye, Y.; Tang, K.; Wang, W.; Zhu, J.; Wei, M.; et al. Low-temperature hydrogenation of dimethyl oxalate to ethylene glycol via ternary synergistic catalysis of Cu and acid−base sites. Appl. Catal. B Environ. 2019, 248, 394–404. [Google Scholar] [CrossRef]
- Gong, X.; Wang, M.; Fang, H.; Qian, X.; Ye, L.; Duan, X.; Yuan, Y. Copper nanoparticles socketed in situ into copper phyllosilicate nanotubes with enhanced performance for chemoselective hydrogenation of esters. Chem. Commun. 2017, 53, 6933–6936. [Google Scholar] [CrossRef]
- Dandekar, A.; Vannice, M.A. Determination of the Dispersion and Surface Oxidation States of Supported Cu Catalysts. J. Catal. 1998, 178, 621–639. [Google Scholar] [CrossRef]
- Sakakini, B.; Tabatabaei, J.; Watson, M.J.; Waugh, K.C. Structural changes of the Cu surface of a Cu/ZnO/Al2O3 catalyst, resulting from oxidation and reduction, probed by CO infrared spectroscopy. J. Mol. Catal. A-Chem. 2000, 162, 297–306. [Google Scholar] [CrossRef]
- Lv, M.; Liu, H. Photocatalytic property and structural stability of CuAl-based layered double hydroxides. J. Solid State Chem. 2015, 227, 232–238. [Google Scholar] [CrossRef]
- Chen, Y.; Hong, H.; Cai, J.; Li, Z. Highly Efficient CO2 to CO Transformation over Cu-Based Catalyst Derived from a CuMgAl-Layered Double Hydroxide (LDH). ChemCatChem 2021, 13, 656–663. [Google Scholar] [CrossRef]
- Batyrev, E.D.; van den Heuvel, J.C.; Beckers, J.; Jansen, W.P.A.; Castricum, H.L. The effect of the reduction temperature on the structure of Cu/ZnO/SiO2 catalysts for methanol synthesis. J. Catal. 2005, 229, 136–143. [Google Scholar] [CrossRef] [Green Version]










| Catalysts | Elemental Compositions 1 (wt %) | SBET 2 (m2/g) | Vpore 2 (cm3/g) | Dpore 2 (nm) | DM 3 (%) | ||
|---|---|---|---|---|---|---|---|
| CuO | MgO or ZnO | Al2O3 | |||||
| CMA-L | 51.0 | 20.6 (MgO) | 26.6 | 154.8 | 0.55 | 14.2 | 40 |
| CMA-A | 34.3 | 17.2 (MgO) | 45.6 | 133.2 | 0.46 | 14.0 | 34 |
| CZA-C | 27.5 | 39.0 (ZnO) | 31.4 | 144.7 | 0.25 | 6.9 | 38 |
| Catalyst | STY | |
|---|---|---|
| Fresh | Spent | |
| CMA-L | 0.91 | 0.82 |
| CMA-A | 0.67 | 0.28 |
| CZA-C | 0.92 | 0.50 |
| Catalyst | Reduced Temperature/K | TOF/h−1 | Conversion/% | Selectivity/% |
|---|---|---|---|---|
| CMA-L | 453 | 2.4 | 56.8 | 98.6 |
| 513 | 3.3 | 78.7 | 98.0 | |
| 533 | 3.3 | 78.9 | 99.1 | |
| 553 | 3.2 | 76.4 | 98.8 | |
| 593 | 2.3 | 56.4 | 97.9 | |
| CZA-C | 533 | 2.6 | 57.2 | 95.7 |
| CMA-A | 533 | 2.5 | 38.4 | 97.2 |
| Reduction Temperature/K | Kinetic Energy (eV) | Ratio/% | Cu0/ (Cu0 + Cu+) | ||||
|---|---|---|---|---|---|---|---|
| Cu2+ | Cu0 | Cu+ | Cu2+ | Cu0 | Cu+ | ||
| 453 | 916.2 | 918.5 | 913.0 | 47.0 | 32.2 | 20.8 | 0.61 |
| 493 | 916.2 | 918.5 | 913.1 | 41.0 | 40.0 | 19.0 | 0.68 |
| 533 | 916.2 | 918.5 | 913.4 | 40.5 | 40.4 | 19.1 | 0.68 |
| 553 | 916.2 | 918.5 | 913.0 | 37.3 | 43.8 | 19.0 | 0.70 |
| 593 | 916.2 | 918.5 | 913.5 | 27.3 | 55.7 | 17.1 | 0.77 |
| Catalysts with Reduction Temperature/K | CO Frequency/cm−1 | Ratio/% | Cu0/ (Cu0 + Cu+) | ||
|---|---|---|---|---|---|
| Cu+ | Cu0 | Cu+ | Cu0 | ||
| 453 | 2108 | 2093 | 47.64 | 33.74 | 0.41 |
| 493 | 2111 | 2094 | 48.86 | 44.84 | 0.48 |
| 533 | 2107 | 2080 | 28.21 | 71.79 | 0.72 |
| 553 | 2108 | 2080 | 32.01 | 67.99 | 0.68 |
| 593 | 2110 | 2084 | 40.30 | 59.70 | 0.60 |
| Catalysts with Reduction Temperature/K | Cu LMM | In Situ FTIR of Adsorbed CO Method |
|---|---|---|
| 453 | 0.61 | 0.41 |
| 493 | 0.68 | 0.48 |
| 533 | 0.68 | 0.72 |
| 553 | 0.70 | 0.68 |
| 593 | 0.77 | 0.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Xin, M.; Yuan, H.; Gao, L.; Zheng, A.; Xing, E.; Zhang, X.; Zhang, C.; Zong, B. Cu-Based Catalysts with Enhanced Thermal Stability for Cyclohexyl Acetate Hydrogenation: Studies on Cu+ and Cu0 Sites at Different Reduction Temperatures. Catalysts 2023, 13, 737. https://doi.org/10.3390/catal13040737
Yu X, Xin M, Yuan H, Gao L, Zheng A, Xing E, Zhang X, Zhang C, Zong B. Cu-Based Catalysts with Enhanced Thermal Stability for Cyclohexyl Acetate Hydrogenation: Studies on Cu+ and Cu0 Sites at Different Reduction Temperatures. Catalysts. 2023; 13(4):737. https://doi.org/10.3390/catal13040737
Chicago/Turabian StyleYu, Xinyao, Mudi Xin, Hui Yuan, Liang Gao, Aiguo Zheng, Enhui Xing, Xiaoxin Zhang, Chengxi Zhang, and Baoning Zong. 2023. "Cu-Based Catalysts with Enhanced Thermal Stability for Cyclohexyl Acetate Hydrogenation: Studies on Cu+ and Cu0 Sites at Different Reduction Temperatures" Catalysts 13, no. 4: 737. https://doi.org/10.3390/catal13040737