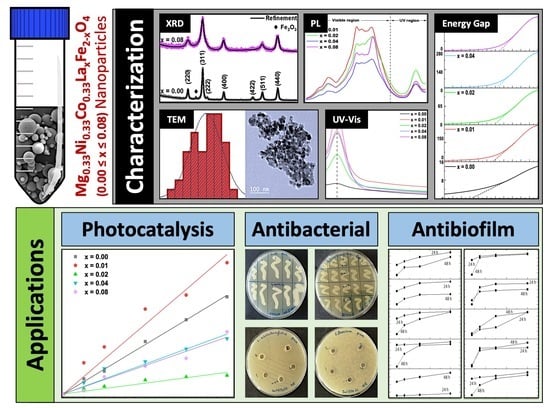
1. Introduction
The most fundamental component for the survival of living things is water. A healthier life is facilitated by the usage of clean and safe water. However, large amounts of dye-containing effluents are discharged untreated into the water, which, in turn, causes water pollution [
1]. Dyes, characterized by their cheap production and stability, are widely used in textiles, paper, plastics, leather, printing, cosmetics and coatings. Dye-containing water affects human and animal health and is visible in water even at very low concentrations (
1 ppm) [
2]. Furthermore, microbiological water pollution is recognized as one of the major problems across the world [
3]. Consequently, removing pollutants such as bacteria and dyes from wastewater is highly desirable.
Magnetic NPs are characterized by their unique properties that are not observed in bulk materials. These characteristics can be attributed to the finite size effects, high surface-to-volume ratio and distinct crystal structures. Numerous physical, chemical and biological techniques can be employed to synthesize these NPs. However, choosing the preparation technique depends on the targeted morphology, size, properties and application of the NPs [
4]. The co-precipitation method is recognized for its simplicity, affordability and wide size distribution. Furthermore, it gives rise to NPs that can be functionalized in several applications, including catalysis, gas sensing and antibacterial applications [
5,
6]. Owing to their size, structures and unique optical and magnetic properties, NPs have been used in a variety of fields, including the electrical and electronic industries and the biomedicine field [
7]. Various types and structures have been investigated, including the hexagonal and spinel ferrites. Studies on ferrites are fast-moving, owing to their exponentially growing usage in magnetic biosensors, magnetic shielding, information storage, magnetic recording devices, electronic devices, mobile communication, pollution control, medical devices, transformers and catalysis [
8].
Concerning the role of nano-ferrites as a pollutant control, they stand out among the most efficient advanced materials in terms of their potential to remove pollutants [
9]. This is due to the economic profile and efficiency of nano-ferrites, which permit their usage in the removal of various pollutants such as pesticides, dyes, medicines and hazardous chemicals. In addition, the improvement of the properties of ferrites can be done by modifying the synthesis parameters and the doping or formation of nano-composites [
10]. The doping of rare earth metals, such as Samarium (Sm) and Lanthanum (La), improves the surface-to-volume ratio and enhances the optical, electrical and magnetic properties of host materials [
11]. For example, doping CoFe
2O
4 with La improved the magnetic properties, reduced the bandgap energy and increased the surface area [
12]. The improved properties permit their usage in environmental applications. Furthermore, La-doped BiFeO
3 NPs exhibited superior catalytic performance in the photodegradation of Rhodamine B dye compared to that of pure BiFeO
3 NPs [
13]. As for the antibacterial activity, magnetic NPs were shown to exhibit a significant antibacterial activity, depending on their shape, size and density of oxygen vacancies [
14]. In addition, rare earth metals were shown to exert unique antibacterial advantages, especially against
Escherichia coli and
Staphylococcus aureus. They act by penetrating the bacterial cells and changing the cytoplasmic composition, thus leading to the destruction of the bacterial cells [
15]. Regarding water treatment, some studies reported that La-doped NPs can remove pollutants and microbes, such as
Klebsiella pneumonia and other pathogens, during the treatment process. This activity is inversely proportional to the size, as the antibacterial activity can be enhanced by smaller-sized NPs [
16].
Among several spinel ferrites, MgFe
2O
4, NiFe
2O
4 and CoFe
2O
4 NPs have attracted the attention of scientists and are useful due to their unique characteristics. It is known that MgFe
2O
4 is a soft magnetic semiconducting material with a normal spinel structure [
17]. NiFe
2O
4, having an inverse spinel structure, is a soft magnetic semiconducting material [
18]. Whereas CoFe
2O
4, characterized by its inverse spinel structure, is classified as a semi-hard material [
19]. Thus, it is interesting to investigate the properties of Mg
0.33Ni
0.33Co
0.33Fe
2O
4 NPs. As mentioned before, doping ferrites with rare earth metals improves their properties. In this work, La-doped Mg
0.33Ni
0.33Co
0.33La
xFe
2−xO
4 spinel ferrites where 0.00 ≤ x ≤ 0.08 were synthesized using the co-precipitation technique. In addition, the prepared NPs were characterized by XRD, TEM, UV and PL techniques. Finally, La-doped Mg
0.33Ni
0.33Co
0.33La
xFe
2−xO
4 NPs were used to disinfect water from methylene blue (MB) dye and bacteria.
3. Materials and Methods
3.1. Synthesis of the Mg0.33Ni0.33Co0.33LaxFe2−xO4 NPs Where 0.00 ≤ x ≤ 0.08
The La-doped magnesium-nickel-cobalt ferrite NPs were prepared by the co-precipitation method. The required masses of magnesium chloride hexahydrate (MgCl2.6H2O), nickel (II) chloride hexahydrate (NiCl2.6H2O), cobalt (II) chloride hydrate (CoCl2.H2O), iron (III) chloride hexahydrate (FeCl3.6H2O) and lanthanum (III) chloride hexahydrate (LaCl3.6H2O) were dissolved in deionized water and stirred for 30 min. The mixture was titrated by careful addition of 3 M sodium hydroxide (NaOH) solution until the pH reached 12. Then, the obtained solution was heated at a temperature of 80 °C, with constant magnetic stirring for 2 h. After that, the solution was kept at room temperature for 10 min to cool down. This was followed by filtration and washing of the obtained precipitate with a solution of 75% deionized water and 25% ethanol until the pH dropped to 7. The obtained precipitates were then dried at 100 °C for 18 h and finally annealed at 550 °C for 4 h.
3.2. Characterization Techniques
XRD patterns were recorded in the range of 20° ≤ 2θ ≤ 80° using Cu-kα radiation source (= 1.54056 Å) by X ray diffractometer (D8 Advance, Bruker, Billerica, United states). The morphology and size of the prepared samples were evaluated by TEM using JEM-1400 Plus (JEOL, Tokyo, Japan). To perform the UV test, 0.01 g of each of the prepared NPs was dissolved in 50 mL of 1 M HCl solution. Afterward, the mixtures were sonicated for 5 min. Subsequently, the optical properties of the samples were estimated by ultraviolet-visible (UV–Vis) spectroscopic examinations that were performed at room temperature in the range of 300–700 nm using UV-Vis spectrophotometer (V-670, JASCO, Tokyo, Japan). The PL spectra were recorded at room temperature between 1.8 and 3.9 eV at an excitation wavelength of 330 nm via a fluorescence spectrometer (FP8300, JASCO, Tokyo, Japan).
3.3. Photocatalytic Activity of the Mg0.33Ni0.33Co0.33LaxFe2−xO4 NPs
The photocatalytic activity of the Mg0.33Ni0.33Co0.33LaxFe2−xO4 NPs, where 0.00 ≤ x ≤ 0.08, was examined by mixing 0.06 g of each catalyst with 100 mL of 3 ppm methylene blue (MB) solution. The obtained solutions were then stirred for 30 min in the darkness to achieve the adsorption-desorption equilibrium. Then, the mixture was placed under direct sunlight between 11 am and 2 pm. The photodegradation reactions were performed in August. The effect of the catalyst dose, temperature, pH and graphene (Gr) addition was investigated. To study the effect of pH on the photodegradation reaction, a few drops of 0.5 M HCl and NaOH were added to adjust the pH of MB solution between 2 and 11. Furthermore, to prepare nanocomposites with 5, 10, 15 and 20 wt.% Gr, 0.003, 0.006, 0.009 and 0.012 g of Gr, respectively, were mixed with 0.06 g of the most efficient catalyst using 10 mL ethanol solution. Afterward, the mixture was sonicated for 20 min and placed in the oven at 70 °C to remove the ethanol. 3 mL of the MB solution was taken at different time intervals and analyzed using a UV-Vis spectrophotometer (SPECORD 200, Analytik Jena, Thuringia, Germany).
3.4. Antibacterial Activity of the Mg0.33Ni0.33Co0.33LaxFe2−xO4 NPs
3.4.1. Bacterial Isolation and Identification
Bacteria were isolated from wastewater samples. 100 µL of the isolated bacteria were spread on agar plates containing different selective media. The plates were then incubated at 37 °C for 24 h. Then, the bacterial isolates were Gram stained for differentiating between Gram-positive and Gram-negative bacteria prior to VITEK (VITEK 2 Automated Systems, bioMérieux Inc., Massachusetts, United states) for further identification of the bacterial species [
62].
3.4.2. Determination of the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) by the Microdilution Method
The MICs of Mg
0.33Ni
0.33Co
0.33La
xFe
2−xO
4 NPs where 0.00 ≤ x ≤ 0.08 were determined by the micro-well dilution assay. The assay was done by dispensing 90 µL of nutrient broth (NB) into the wells of sterile 96-well microplates. Following that, 10 µL of previously prepared bacterial suspensions adjusted to 0.5 McFarland were added to the wells. Then, 100 µL of each NP of different concentrations (0.375–3 mg/mL) were added to the wells. The microplates were then incubated for 24 h at 37 °C. After incubation, the optical density (O.D.) was measured at 595 nm by an ELISA microtiter plate reader (SN 357-912359, Thermo Fisher Scientific, Shanghai, China). The MIC was recorded as the lowest concentration of the NPs that inhibited visible growth of the bacteria. Following the MIC recording, 10 µL of the solutions in the clear wells were spread on plates containing Muller Hinton agar (MHA) and incubated for 24 h at 37 °C for detecting the MBC [
63]. All experiments were repeated at least three times.
3.4.3. Determination of the Zones of Inhibition (ZOI) by the Agar Well Diffusion Assay
The agar well diffusion assay was performed by evenly spreading 100 µL of the isolated bacterial suspensions (0.5 McFarland) over the surface of the MHA plates. The plates were then punched with a 6 mm cork-borer to create wells. Then, 100 μL of increasing concentrations of each NP (0.375–3 mg/mL) were added to the wells. The plates were then incubated for 24 h at 37 °C. After incubation, the diameter of the ZOI was measured, in which ZOI > 7 mm was considered effective [
64,
65]. All experiments were repeated at least three times.
3.4.4. Antibiofilm Activity of the Mg0.33Ni0.33Co0.33LaxFe2−xO4 NPs
The biofilm inhibition assay was done to determine the potential of Mg
0.33Ni
0.33Co
0.33La
xFe
2−xO
4 NPs where 0.00 ≤ x ≤ 0.08 to prevent the formation of biofilms. The assay was done by adding 100 µL of bacterial cultures into 96-well microtiter plates and incubating for 4 h at 37 °C to allow the attachment/formation of the biofilms. Then, 100 µL of increasing concentrations of each NP (0.375–3 mg/mL) were added to the wells. A culture medium without any inoculum was used as a negative control. The plates were incubated at 37 °C for 24 and 48 h. The biomass was quantified by the crystal violet (CV) staining technique. Briefly, following the incubation period, the plates were washed with sterile distilled water 5 times, then air-dried and oven-dried for 15 min at 60 °C. Then, 100 µL of 1% CV was added to the wells and incubated for 15 min at room temperature. To remove the unabsorbed stain, the plates were washed with sterile distilled water 5 times and the biofilms were observed in the form of purple rings. To de-stain the wells, 100 µL of 95% ethanol was then added. Finally, the O.D. was measured at 595 nm using an ELISA microplate reader (SN 357-912359, Thermo Fisher Scientific, Shanghai, China) [
66]. All experiments were repeated at least three times.
The percentage of inhibition of the formation of the bacterial biofilms was determined using the following equation:
The potential of the NPs to eradicate the pre-formed bacterial biofilms was tested by adding 100 µL of the standard cultures of the bacterial isolates into 96-well microtiter plates and incubating for 30 h at 37 °C to form the biofilms. After incubation, 100 µL of increasing concentrations of each NP (0.375–3 mg/mL) were added into the wells and the plates were incubated at 37 °C for 24 and 48 h. A culture medium without any inoculum was considered the negative control. The biomass of each biofilm was detected by CV staining, and the percentage of eradication of the pre-formed bacterial biofilms was determined as mentioned above in the inhibition of the formation of biofilms [
66]. All experiments were repeated at least three times.
3.4.5. Statistical Analyses
The statistical tests were done using Excel software. The graphs were drawn using Origin software. The statistical significance was determined by t-Test.
4. Conclusions
Mg0.33Ni0.33Co0.33LaxFe2−xO4 NPs where 0.00 ≤ x ≤ 0.08 were successively synthesized by the co-precipitation method. As x increases from 0.00 to 0.08, Eg increases from 2.92 to 3.25 eV, whereas DTEM decreases from 28.72 to 20.20 nm. Doping Mg0.33Ni0.33Co0.33LaxFe2−xO4 NPs with La enhanced their photocatalytic performance. Among the prepared NPs, enhanced photocatalytic activity was exhibited by NPs where x = 0.01. This was attributed to the slow recombination rate of the electron-hole pair, as revealed by PL analysis. In addition, the optimal catalyst amount was 0.06 g. Furthermore, the incorporation of 20 wt.% of graphene (Gr) improved the photocatalytic activity of Mg0.33Ni0.33Co0.33La0.01Fe1.99O4 NPs. Among the tested NPs, the Mg0.33Ni0.33Co0.33La0.00Fe2O4 NPs had antibacterial activity. An antibiofilm action was observed for some of the doped NPs, especially those where x = 0.01, 0.02 and 0.04, mainly against biofilms of Gram-positive bacteria. Due to their high antibiofilm activity, the Mg0.33Ni0.33Co0.33La0.00Fe2−xO4 NPs are applicable in the biomedical field, especially against bacterial pathogens.