1. Introduction
Industrial development provided mankind with a great deal of convenience. As the industrialized civilization continues to develop, our society has relied heavily on fossil-fuel-based energy resources. Most industrial advancements have been accompanied by fossil fuel use. However, technological improvements based on the use of fossil fuel have contributed to increasing greenhouse gas emissions [
1]. Climate change, which causes a rise in sea level, heat waves and desertification, is largely accepted as a result of greenhouse gas emissions from the use of fossil fuel energy [
2].
To mitigate global climate change caused by greenhouse gases, sustainable and eco-friendly energy systems should be explored and implanted to replace fossil-fuel-based energy [
3]. Biogas, composed mainly of methane and carbon dioxide, can be produced by the anaerobic digestion of organic wastes such as sewage sludge, food waste, and livestock night soil [
4,
5,
6]. This gas can be used to generate heat and power generation and can be purified and upgraded to biomethane for further energy applications [
7,
8]. In addition, biomethane can be converted to syngas (CO/H
2) through catalytic reforming processes and CO
2 separation processes such as steam methane reforming (SMR) and pressure swing adsorption (PSA) [
9].
It is well known that the CH
4-to-CO
2 ratio in biogas is generally in the range from 7:3 to 5.5:4.5, which is highly dependent on the feedstock of biogas generation [
10]. The methane content in landfill gas (LFG) is about 40–60%, while the methane content of the gas generated by the anaerobic digestion of sewage sludge is about 40–50% On the other hand, the methane content in the biogas produced by the livestock manure of food waste treatment is 50–70%, which is relatively high [
11]. Furthermore, the methane-rich biogas can directly be converted to produce syngas (CO/H
2) and green hydrogen through the dry-reforming reaction of methane (DRM) with a small makeup of CO
2 followed by a water shift reaction (WGS) [
12]. Since CH
4 and CO
2 in purified biogas can be directly utilized without any expensive PSA separation units, there has been increased attention recently focused on biogas-linked DRM reactions for green hydrogen production [
13].
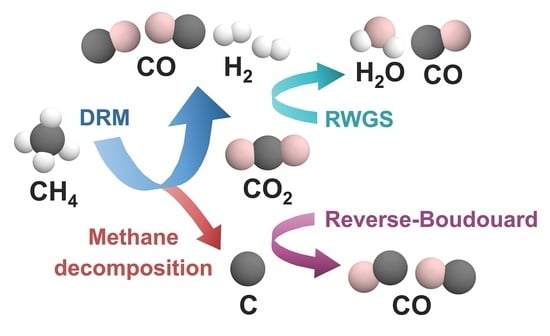
It is widely acknowledged that the dry reforming of methane (DRM) is an effective method for producing syngas from methane and carbon dioxide—two greenhouse gases—without additional carbon dioxide emissions [
14]. Additionally, syngas can be converted into valuable chemicals and used for hydrogen production [
15]. When biogas —which has highly variable methane content—is applied directly to the DRM process, the H
2/CO ratio of the resultant syngas can be adjusted. However, this can lead to an increased tendency for carbon deposition [
16]. Therefore, it is necessary to evaluate how catalytic activity is affected when the feed composition is changed, as well as which operation parameters are essential for reaction performance. To maximize efficiency, the DRM reaction unit and catalysts must be tailored to the target biogas stream.
Although numerous catalysts have been suggested in fundamental research, Ni-based catalysts are commonly used in both lab-scale and practical processes in the methane reformation processes due to high catalytic activity, relatively low cost and wide availability [
17,
18,
19]. Generally, it is accepted that Ni-based catalysts are the most effective ones on reforming reactions. So far, most studies on reforming catalysts are mainly focused on Ni-Al
2O
3-based catalysts and the one for DRM reaction is no exception [
19,
20]. The dry-reforming process, which is a highly endothermic reaction (Equation (1)), is usually carried out at high reaction temperatures (700–850 °C). These high-temperature conditions often induce the agglomeration of nickel particles (also known as thermal sintering), leading to the loss of the number of active sites and deactivation of the catalyst [
21,
22]. In addition, the aggregated Ni particles can accelerate the formation of carbon (coking), resulting in an overall performance drop of the DRM unit [
23].
Many researchers have sought to understand the relationships between the catalytic chemistry of Ni-based catalysts and reaction conditions in methane dry-reforming environments. Song et al. proposed a locking mechanism wherein the suitable particle size of active metal remains a crucial factor in achieving sintering resistance, even after extended testing. They demonstrated that metal particulates move on high-energy step edges of the support during activation to form stable particles. In addition, they exhibited that carbon deposition (coking) easily occurs on Ni catalysts when operating a dry-reforming reaction at low temperatures [
24]. Charisiou et al. reported that carbon deposition and defects in the carbon increased as the reaction temperature decreased from 800 to 600 °C [
25]. Research groups have studied catalyst deactivation by varying reaction temperatures to understand the activity and durability of Ni-based catalysts [
17,
26]. However, there is not enough research that comprehensively compares the effect of operating parameters in the dry-reforming process, focusing on the feed gas composition and side reactions. It is still difficult to systematically investigate the catalytic activity, coke formation and reaction behavior according to operating conditions such as temperature, WHSV and gas compositions for the syngas–hydrogen production via practical biogas-linked DRM reactors. The optimal conditions for maximizing syngas yield and minimizing coking must be also figured out, since purified biogas can intrinsically contain CH
4-rich gas. Furthermore, it is critical to identify the optimal operating conditions of the dry-reforming reaction on each situational case for designing practical biogas-linked DRM processes which can be applied to practical organic waste treatment sites. This is because the composition of feed biogas can be varied. By controlling the operating parameters, it is possible to reduce carbon deposition and increase hydrogen yield while suppressing undesirable side reactions.
In this study, we synthesized a conventional Ni/Al2O3 catalyst which is well known as a highly active catalyst in methane conversion reactions. The characteristics of the catalyst were investigated by BET, XRD, TEM and H2-TPR. We applied the catalyst to the dry-reforming reaction of CO2/CH4 using the model purified-biogas stream. We investigated the effects of operating conditions such as temperature, WHSV and the CO2-to-CH4 ratio on the DRM reaction, and both the reaction activity and deactivation behavior of the Ni/Al2O3 catalyst are systemically studied. In addition, we also investigated the relationship between operation conditions and the tendency of side reactions. The spent catalyst with a different feed ratio was also analyzed with TGA, TEM and Raman spectroscopy. In this paper, we discussed our systemically experimental results and suggested the optimal operation window of the DRM unit which could be potentially used for designing practical biogas-linked DRM processes.
2. Results and Discussion
Figure 1 shows the physical and chemical properties of alumina support and Ni/Al
2O
3 catalysts.
Figure 1a illustrates the N
2 adsorption–desorption isotherms and the corresponding pore size distribution of the mother Al
2O
3 support and the calcined Ni/Al
2O
3 catalyst. The commercial alumina support exhibited a type-IV isotherm, which is associated with capillary condensation in mesopores [
27]. It exhibited precipitous adsorption at the relative pressure of approximately 0.7–0.8, suggesting that the original Al
2O
3 sample had a considerably uniform mesopore with well-developed pore connectivity [
28]. Furthermore, Ni/Al
2O
3 showed a similar isotherm pattern, although its adsorbed volume was slightly lower than the original Al
2O
3 support. This suggests that the mesoporous structure of the Al
2O
3 support is well preserved even after Ni loading and calcination. This is further confirmed by the results of the pore size distribution (PSD) (see
Figure 1a inset). Both Al
2O
3 support and Ni/Al
2O
3 catalysts have similar PSD patterns which show a sharp distribution peak in the range of 5–10 nm with the maximum point at ca. 7.4 nm. The calculated surface area and pore volumes of Ni/Al
2O
3 catalyst (153.7 m
2/g and 0.38 cm
3/g) are slightly smaller than those of original Al
2O
3 support (213.9 m
2/g and 0.52 cm
3/g), respectively. This indicates that some nickel oxide nanoparticles may have grown inside the pore during the nickel impregnation that followed the calcination step, which results in the pore blockage of alumina support.
Figure 1b presents X-ray diffraction (XRD) patterns of the alumina support, calcined Ni/Al
2O
3 catalyst and the Ni/Al
2O
3 catalyst after reduction at 800 °C. The alumina support exhibits clear peaks at 2θ = 37.8, 45.7 and 66.8°, indicating a typical γ-Al
2O
3 (ICDD PDF 00-029-0063) phase. The peaks related to the γ-Al
2O
3 phase are well maintained even after Ni loading, followed by calcination and reduction in all samples. After calcination, the Ni/Al
2O
3 catalyst shows an increment of peak intensity around 2θ = 37°, which indicates the existence of the NiAl
2O
4 (ICDD PDF 00-010-0339) phase. The calcined Ni/Al
2O
3 catalyst had a weak shoulder peak at 2θ = 43°, which indicates the presence of NiO (ICDD PDF 00-044-1159) nanoparticles highly dispersed on the support. For the reduced Ni/Al
2O
3 catalyst, the peak related to NiO disappeared and new diffraction peaks appeared. The reduced Ni/Al
2O
3 showed obvious diffraction peaks at 44.3, 51.7 and 76.2° which ascribe to (111), (200) and (220) planes of metallic Ni. The average crystallite size of the Ni nanoparticle of the reduced Ni/Al
2O
3 was estimated using the Scherrer equation based on the (200) peak and was found to be approximately 11 nm.
We investigated the overall morphology and metal dispersion of the reduced Ni/Al
2O
3 catalyst by TEM analysis. As shown in
Figure 1c, it can be easily observed that alumina support has a porous structure, and the nickel nanoparticles are well dispersed both on the surface and inside the pore of alumina support. The observed size of the supported Ni nanoparticles was ca. 11 nm. This is consistent with the results of the XRD analysis.
The hydrogen temperature-programmed reduction (H
2-TPR) was conducted to examine the reduction characteristics of the Ni/Al
2O
3 catalyst (
Figure 1d). As shown in the TPR results, the reduction starts at ca. 520 °C, and then the maximum peak is observed at ca. 786 °C. The hydrogen is continuously consumed until 970 °C. The Ni/Al
2O
3 catalyst exhibits different reduction patterns according to the interaction property between the metal and support material. It is known that the peaks corresponding to NiO species which have a weak metal–support interaction, nickel oxide species which have a strong interaction with alumina support, and nickel aluminate spinel appear in order of temperature increment [
29]. When a Ni ion is incorporated into the alumina structure, stable nickel aluminate (NiAl
2O
4)-like species can be formed [
19]. The Ni species in NiAl
2O
4 can be reduced to even higher temperature ranges [
30]. Thus, it could be concluded that our Ni/Al
2O
3 have negligible Ni species of weakly interacted NiO-Al
2O
3, and mainly consist of strongly interacted NiO-Al
2O
3 and NiAl
2O
4.
Figure 2 exhibits the effects of the reaction temperature varying from 700 to 850 °C on CH
4 conversion, CO
2 conversion, H
2/CO ratio and H
2 selectivity, which were estimated by Equations (2)–(5).
As the reaction temperature increased, the conversions of both CO
2 and CH
4 rose, which is in line with the endothermic characteristics of a dry-reforming reaction. As the CO
2/CH
4 feed ratio increased, CH
4 conversion also increased; however, CO
2 conversion decreased except CO
2/CH
4 = 0.8 (
Figure 2a,b). DRM is a reaction that consumes an equal amount of CO
2 and CH
4. As the ratio of CO
2/CH
4 rose, the amount of CO
2 that does not participate in DRM reaction increased. Therefore, CO
2 conversion decreased in order of CO
2/CH
4 = 1.0 > 1.25 > 1.5 > 2.0. Furthermore, the CH
4-rich condition led to a significant carbon deposition, which resulted in a reduction in the Ni active site; consequently, the opportunity of the CO
2 conversion decreased. Therefore, CO
2 conversion at CO
2/CH
4 = 0.8 was lower than CO
2/CH
4 = 1.0. As a result, CO
2 conversion at CO
2/CH
4 = 1.0 was the highest compared to other feed compositions. Chein et al. claimed that CO
2 takes a similar role to an oxidation agent in the combustion reaction. Thus, the more CO
2 in the feed gas, the more advantageous the reaction environment, resulting in the CH
4 conversion increasing in dry reforming [
31]. Das et al. suggested the reaction mechanisms for the dry-reforming reaction, which involve the dissociative adsorption of CH
4 to form H
2 and CH
x intermediates, followed by the dissociation of adsorbed CO
2 on the Ni surface to form CO and Ni-O species. Subsequently, Ni-O and Ni-C react to form CO [
32]. However, an insufficient amount of CO
2 (e.g., CO
2/CH
4 = 0.8) can lead to a deficiency of Ni-O, which is necessary for the reaction with Ni-C to produce CO. In terms of CO
2 conversion, when the CO
2/CH
4 ratio is greater than 1, it is an excess amount, and CO
2 conversion seems to decrease, since in the study, it was sufficiently used in the drying reforming reaction and surplus CO
2 remained. The highest CO
2 conversion at all temperatures was observed when CO
2/CH
4 = 1 is used. As the amount of CO
2 exceeds CH
4, the H
2/CO ratio decreases due to the reverse water gas shift (RWGS) reaction (Equation (6)), which consumes hydrogen gas produced from DRM.
In addition, RWGS easily occurs when the flow rate of CO
2 is greater than that of CH
4. As a product of the RWGS reaction, we can visually confirm that water is produced and condensed at the end of the reactor. Serrano et al. experimentally showed that the increased CO
2/CH
4 ratio drove up the proportion of H
2O and reduced the H
2/CO ratio in the product gas [
33]. When either CO
2/CH
4 = 1 or CO
2/CH
4 = 0.8 is used, the H
2/CO ratio and H
2 selectivity are significantly higher than those of other runs. This suggests that the dry-reforming reaction preferentially occurs in these cases rather than other side reactions, such as RWGS. With the exception of CO
2/CH
4 = 2.0, the H
2/CO ratio and H
2 selectivity increased as temperature increased, which is ascribed to the increment of hydrogen production attributed to the dry reforming of methane. However, when CO
2/CH
4 = 2.0, the H
2/CO ratio and H
2 selectivity decrease with the increasing temperature from 700 to 850 °C, suggesting that hydrogen consumption via the RWGS reaction is greater than hydrogen production from DRM as the reaction temperature rises.
Figure 3 shows the effects of space velocity varying from 60 to 240 L/g·h
−1 on CH
4 conversion, CO
2 conversion, H
2/CO ratio, and H
2 selectivity. As the WHSV increased, the conversion of CO
2 and CH
4 significantly decreased. The higher WHSV means a shorter residence time of reactants with a catalyst bed, resulting in the conversion of CO
2 and CH
4 being decreased. For the case of CO
2/CH
4 = 0.8, conversion values dramatically decreased when WHSV is increased from 120 to 240 L/g·h
−1, compared to other cases (
Figure 3a,b). This indicates that a significant deactivation occurred due to a significant carbon deposition, as discussed later. The CH
4 conversion and CO
2 conversion for the CO
2/CH
4 feed ratio are a similar trend of the effect of a reaction temperature change, as shown in
Figure 2. As shown in
Figure 3a,b, the H
2/CO ratio and H
2 selectivity slightly decreased as WHSV increased, except for the CO
2/CH
4 = 0.8 run. For the case of CO
2/CH
4 = 0.8, the significant deactivation from 120 to 240 L/g·h
−1 was observed due to a significant carbon deposition, as discussed later. Based on the carbon balance, we attempted to calculate the theoretical amount of carbon formation by employing Equations (7)–(9). The tendency of the carbon deposition amount relative to the feed ratio was consistent with the theoretical carbon formation.
To estimate the catalytic activity of the Ni/Al
2O
3 catalyst in this work, we carried out stability tests at WHSV = 60 L/g·h
−1, CH
4:CO
2:N
2 = 3:3:4 and T = 850 °C. For 38 h of continuous DRM reactions, the CH
4 conversion (ca. 93%) and CO
2 conversion (ca. 96%) were well maintained; this should be expected to retain the catalytic activity for a longer period (
Figure S2). We also operated a cyclic test for the Ni/Al
2O
3 catalyst at different WHSV of 60, 120, 180 L/g·h
−1, CH
4:CO
2:N
2 = 3:3:4 and T = 800 °C. The CH
4 conversion at each space velocity was 84%, 70% and 65%, respectively. The ratio of H
2/CO was 0.93, 0.87 and 0.82 in order of increased space velocity. In addition, this indicates that the CH
4 conversion, CO
2 conversion and ratio of H
2/CO were almost recovered with minor changes. This means that the stability and durability of Ni/Al
2O
3 catalyst is well maintained during severe reaction condition changes (
Figure S3). Compared to the other previous research that applied the Ni/Al
2O
3 catalyst for DRM, we obtained reliable experimental results, and the Ni/Al
2O
3 catalyst in this study showed a reasonably stable catalytic performance (
Table S1).
To evaluate the stability and durability of Ni catalysts in the DRM system, we conducted long-term activity tests at 850 °C for 600 min with different feed ratios (
Figure 4). As shown in
Figure 4a, for CO
2/CH
4 = 1.0 and 1.2, there was no significant deactivation until 10 h and the CH
4 conversions of 96 and 98% were maintained, respectively. In the case of CO
2/CH
4 = 0.8, the catalytic activity severely decreased until 120 min from the beginning of the dry-reforming reaction, and CH
4 conversion dropped from 80 to 73% at 30 min and 120 min, respectively (
Figure 4a). After 120 min, the catalytic activity became quite stable, which can be ascribed to a reverse Boudouard reaction (Equation (10)). Based on the calculation of carbon balance and H
2/CO ratio, it should be assumed that a significant amount of carbon was formed at the beginning of the reaction due to the decomposition reaction of excess carbon (Excess CH
4 → C + 2H
2). As the carbon deposition led to deactivation, the conversion of CH
4 and CO
2 dropped drastically within 60 min. Although the carbon deposited on the Ni surface can hamper the DRM activity, it could be also used as a reactant on the reverse Boudouard reaction (Equation (10)), which made the aspect of deactivation quite stable [
17]. As both the DRM reaction and reverse Boudouard reaction subsequently occurred, the catalytic activity can be quite elongated and become stable compared to that of the beginning. To understand the effect of side reactions, the amount of CO that can be produced by DRM was estimated by Equation (11) and was compared with the actual CO production, which were denoted as CO
DRM and CO
out, respectively (
Figure S1b). In case of R0.8 conditions (CO
2/CH
4 = 0.8), CO
out was larger than CO
DRM, which indicates the occurrence of side reactions which can generate additional CO. The side reactions that produce CO should be the reverse water gas shift (RWGS) reaction and the reverse Boudouard reaction. Since the RWGS reaction consumes hydrogen produced from DRM, the H
2 selectivity can be significantly decreased if it is the main side reaction. When we compared hydrogen selectivity at R0.8 (CO
2/CH
4 = 0.8) and R1.0 (CO
2/CH
4 = 1.0), both cases showed similar selectivity ca. 98% (
Figure S1a). It should be concluded that the catalyst drastically deactivated at the beginning due to a significant carbon formation and deposition; subsequently, the reverse Boudouard reaction decelerated the degradation in the CH
4-rich conditions. Regarding CO
2/CH
4 = 1.2, the H
2/CO ratio was relatively lower than CO
2/CH
4 = 1.0 and 0.8 (
Figure 4b). The H
2/CO ratios of CO
2/CH
4 = 1.2, 1.0 and 0.8 were 0.90, 0.97 and 0.96, respectively. Excessive CO
2 is attributed to H
2 consumption due to the reverse water gas shift reaction (RWGS). Based on the above results, it is clear that insufficient CO
2 (CO
2/CH
4 = 0.8) induces significant coking, resulting in low performance in terms of both conversion and H
2 production. While the CO
2-rich condition (CO
2/CH
4 = 1.2) is slightly better in terms of conversion, it induces a significant RWGS reaction, resulting in a low H
2/CO ratio and H
2 selectivity. Therefore, we concluded that stoichiometric ratio (CO
2/CH
4 = 1.0) conditions are most beneficial for the DRM reaction.
The Ni/Al
2O
3 catalysts after the DRM reaction were also characterized. As shown in
Figure 5a, the Ni/Al
2O
3 catalyst showed well-maintained Ni peaks at 2θ = 44.3, 51.7 and 76.2°, and no NiO peak was observed after the dry-reforming reaction for 10 h. This indicates that nickel particles did not oxidize to NiO during the dry-reforming reaction. In addition, the Ni/Al
2O
3 catalyst after the DRM reaction showed a significant carbon (ICDD PDF 00-026-1076) peak at 2θ = 25.8° compared to the reduced catalyst before the reaction. We also evaluated the particle size of Ni after the DRM reaction. As shown in the XRD results, the Ni/Al
2O
3 catalyst after DRM showed a sharper Ni peak than the one before the reaction. The Ni particle size, which was calculated by the Scherrer formula, was changed from 10 nm to 16 nm after the DRM reaction. This implies the aggregation of Ni particles due to exposure to high temperatures during the DRM process [
24], and can be also confirmed by TEM analysis. As shown in
Figure 5b, it can be easily seen that a Ni particle is sintered to a large particle (ca. ~17 nm) after the DRM reaction.
Figure 6 shows TGA profiles of the used catalysts after 10 h of the DRM reaction with different feed ratios (CO
2/CH
4 = 0.8, 1.0 and 1.2). The used catalysts were denoted as R0.8, R1.0 and R1.2, respectively, according to their feed ratio. It is widely acknowledged that weight loss at temperatures between 20 and 300 °C is a result of moisture being removed from the samples [
34,
35]. The amount of weight loss follows this order in this range: (R0.8 ≤ R1.0 ≤ R1.2). The moisture on the catalyst could correspond to water generated from the RWGS reaction during DRM. The consumption of hydrogen from DRM increased as the CO
2/CH
4 ratio became larger, which is consistent with the TGA results. The weight increase in the range of 300–400 °C is related to the oxidation of Ni to NiO on the surface of the catalyst [
36]. All the catalysts showed weight increases due to Ni oxidation in this range, even though the increasing degree is slightly different. Finally, the significant weight loss is observed above 400 °C, which is ascribed to the oxidation of the coke on the catalyst surface [
36,
37]. Among the three catalysts, R0.8 exhibited a dramatic weight loss between 400 and 650 °C, and then a slight weight increase until 800 °C due to Ni oxidation after completely burning out the coke. The R1.0 sample exhibited a sharp decrease from 500 to 650 °C and a slight weight increase after 650 °C (
Figure 6 inset), which is ascribed to the oxidation of Ni after the removal of carbon that has covered the surface of Ni during the DRM reaction. The R1.2 sample also showed similar behavior to R1.0. The weight loss from burning coke followed in order of R1.2(0.5%) < R1.0(2.6%) < R0.8(28.1%). A significant amount of filamentous carbon on the surface of the catalyst was observed in the TEM images of R0.8 (
Figure 6b).
We also analyzed the structures of carbon deposited on the catalysts.
Figure 7 displays the Raman spectra of two catalysts (R0.8 and R1.0) which exhibited obvious TGA weight loss above 500 °C after a 10 h DRM reaction. Corresponding to the TGA analysis, there was no significant peak in the Raman spectra of R1.2 due to the negligible amount of carbon deposited on R1.2. Both R0.8 and R1.0 showed two obvious vibration bands: the D-band (1350 cm
−1) and the G-band (1580 cm
−1), which are typical characteristics of carbon materials. The Raman spectrum intensity of R0.8 was higher than that of R1.0, indicating that R0.8 has a large number of carbon species on the surface compared to R1.0. It is known that the D band is related to the presence of defects or a disordered carbon structure, and the G band is attributed to the C = C stretching vibration of sp
2 carbon [
38,
39]. Generally, the intensity ratio of the D band and G band (I
D/I
G) is an indicator of the graphitic degree of carbon; the lower the value, the more graphitic the structure [
40,
41]. For R0.8 and R1.0, the respective I
D/I
G values are 0.919 and 0.751, indicating that the graphitic degree becomes lower as the R-value increases. Therefore, the carbon deposited on R0.8 has as less graphitic properties as that on R1.0 [
42]. Although the absolute amount of carbon deposited on R0.8 is much larger than that of R1.0, as a result of the more defective structure of the coke deposited on R0.8 than R1.0, the carbon on R1.0 oxidized at a higher temperature than R0.8. This is consistent with the TGA results—weight loss by the carbon oxidation of R0.8 started at 450 °C, while weight loss by coke burning of R1.0 started at 550 °C. Charisiou et al. reported that the combustion temperature varies depending on the species of carbon [
25]. The temperature increases in the order of amorphous carbon, carbon allotropes with defects in the graphitic lattice and highly graphitized carbon nanotubes. Guo et al. similarly observed that two types of carbon, amorphous and graphitic-like carbon, deposited on the Ni catalyst after methane decomposition. Graphitic-like carbon fully oxidized at higher temperatures than amorphous carbon [
43].