Fabrication and Application of Ag, Black TiO2 and Nitrogen-Doped 3D Reduced Graphene Oxide (3D Black TiO2/Ag/N@rGO) Evaporator for Efficient Steam Generation
Abstract
:1. Introduction
2. Results
2.1. Structural and Morphological Analyses
2.1.1. BET Surface Area and Pore Size Distribution of the 3D Solar Absorber
2.1.2. Fourier Transform Infrared (FTIR) Spectroscopy Analysis of the 3D Absorber
2.2. Solar Absorption Potential and Optical Absorption Property
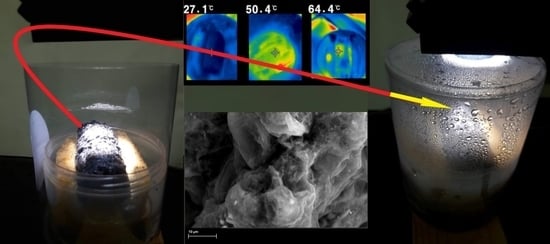
2.3. Photothermal Conversion and Solar-Driven Water Evaporation Potential
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Black TiO2, Ag, and Nitrogen-Doped 3D Reduced Graphene Oxide (3D Black TiO2/Ag/N@rGO)
4.2.1. Synthesis of GO
4.2.2. Synthesis of Black TiO2
4.2.3. Synthesis of 3D Black TiO2/Ag/N@rGO
4.3. Characterization
4.4. Solar Steam Generation/Desalination
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mekonnen, M.M.; Hoekstra, A.Y. Four billion people facing severe water scarcity. Sci. Adv. 2016, 2, e1500323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohiuddin, S.A.; Kaviti, A.K.; Rao, T.S.; Sakthivel, S. Effect of water depth in productivity enhancement of fouling-free non-contact nanostructure desalination system. Sustain. Energy Technol. Assess. 2022, 54, 102848. [Google Scholar] [CrossRef]
- Luo, X.; Shi, J.; Zhao, C.; Luo, Z.; Gu, X.; Bao, H. The energy efficiency of interfacial solar desalination. Appl. Energy 2021, 302, 117581. [Google Scholar] [CrossRef]
- Lu, Y.; Fan, D.; Wang, Y.; Xu, H.; Lu, C.; Yang, X. Surface patterning of two-dimensional nanostructure-embedded photothermal hydrogels for high-yield solar steam generation. ACS Nano 2021, 15, 10366–10376. [Google Scholar] [CrossRef]
- Wang, M.; Xu, G.; An, Z.; Xu, K.; Qi, C.; Das, R.; Zhao, H. Hierarchically structured bilayer Aerogel-based Salt-resistant solar interfacial evaporator for highly efficient seawater desalination. Sep. Purif. Technol. 2022, 287, 120534. [Google Scholar] [CrossRef]
- Tao, P.; Ni, G.; Song, C.; Shang, W.; Wu, J.; Zhu, J.; Chen, G.; Deng, T. Solar-driven interfacial evaporation. Nat. Energy 2018, 3, 1031–1041. [Google Scholar] [CrossRef]
- Peng, L.; Gu, X.; Yang, H.; Zheng, D.; Wang, P.; Cui, H. Ultra-high evaporation rate 3D evaporator with vertical sheets based on full use of convection flow. J. Clean. Prod. 2022, 345, 131172. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, C.; Wang, Y.; Cui, Y.; Wang, Q.; Liu, G.; Gao, S.; Yuan, Y. Plasmonic silver nanoparticles embedded in flexible three-dimensional carbonized melamine foam with enhanced solar-driven water evaporation. Desalination 2021, 507, 115038. [Google Scholar] [CrossRef]
- Ibrahim, I.; Seo, D.H.; McDonagh, A.M.; Shon, H.K.; Tijing, L. Semiconductor photothermal materials enabling efficient solar steam generation toward desalination and wastewater treatment. Desalination 2021, 500, 114853. [Google Scholar] [CrossRef]
- Zang, L.; Sun, L.; Zhang, S.; Finnerty, C.; Kim, A.; Ma, J.; Mi, B. Nanofibrous hydrogel-reduced graphene oxide membranes for effective solar-driven interfacial evaporation and desalination. Chem. Eng. J. 2021, 422, 129998. [Google Scholar] [CrossRef]
- Ali, A.; Liang, F.; Zhu, J.; Shen, P.K. The role of graphene in rechargeable lithium batteries: Synthesis, functionalisation, and perspectives. Nano Mater. Sci. 2022. [Google Scholar] [CrossRef]
- Caballero, Á.; Morales, J. Can the performance of graphene nanosheets for lithium storage in Li-ion batteries be predicted? Nanoscale 2012, 4, 2083–2092. [Google Scholar]
- Zhang, Z.; Cai, R.; Long, F.; Wang, J. Development and application of tetrabromobisphenol A imprinted electrochemical sensor based on graphene/carbon nanotubes three-dimensional nanocomposites modified carbon electrode. Talanta 2015, 134, 435–442. [Google Scholar] [CrossRef]
- Shangguan, Q.; Zhao, Y.; Song, Z.; Wang, J.; Yang, H.; Chen, J.; Liu, C.; Cheng, S.; Yang, W.; Yi, Z. High sensitivity active adjustable graphene absorber for refractive index sensing applications. Diam. Relat. Mater. 2022, 128, 109273. [Google Scholar] [CrossRef]
- Shangguan, Q.; Chen, Z.; Yang, H.; Cheng, S.; Yang, W.; Yi, Z.; Wu, X.; Wang, S.; Yi, Y.; Wu, P. Design of ultra-narrow band graphene refractive index sensor. Sensors 2022, 22, 6483. [Google Scholar] [CrossRef]
- Shi, L.; Wang, Y.; Zhang, L.; Wang, P. Rational design of a bi-layered reduced graphene oxide film on polystyrene foam for solar-driven interfacial water evaporation. J. Mater. Chem. A 2017, 5, 16212–16219. [Google Scholar] [CrossRef]
- Yin, H.; Xie, H.; Liu, J.; Zou, X.; Liu, J. Graphene tube shaped photothermal layer for efficient solar-driven interfacial evaporation. Desalination 2021, 511, 115116. [Google Scholar] [CrossRef]
- Yan, J.; Xiao, W.; Chen, L.; Wu, Z.; Gao, J.; Xue, H. Superhydrophilic carbon nanofiber membrane with a hierarchically macro/meso porous structure for high performance solar steam generators. Desalination 2021, 516, 115224. [Google Scholar] [CrossRef]
- Gao, M.; Zhu, L.; Peh, C.K.; Ho, G.W. Solar absorber material and system designs for photothermal water vaporization towards clean water and energy production. Energy Environ. Sci. 2019, 12, 841–864. [Google Scholar] [CrossRef]
- Liang, J.; Liu, H.; Yu, J.; Zhou, L.; Zhu, J. Plasmon-enhanced solar vapor generation. Nanophotonics 2019, 8, 771–786. [Google Scholar] [CrossRef]
- Jiang, H.; Tong, L.; Liu, H.; Xu, J.; Jin, S.; Wang, C.; Hu, X.; Ye, L.; Deng, H.; Cheng, G.J. Graphene-metal-metastructure monolith via laser shock-induced thermochemical stitching of MOF crystals. Matter 2020, 2, 1535–1549. [Google Scholar] [CrossRef] [Green Version]
- Andronic, L.; Enesca, A. Black TiO2 synthesis by chemical reduction methods for photocatalysis applications. Front. Chem. 2020, 8, 565489. [Google Scholar] [CrossRef] [PubMed]
- Naldoni, A.; Altomare, M.; Zoppellaro, G.; Liu, N.; Kment, S.; Zboril, R.; Schmuki, P. Photocatalysis with reduced TiO2: From black TiO2 to cocatalyst-free hydrogen production. ACS Catal. 2018, 9, 345–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Hou, B.; Wang, G.; Cui, Z.; Zhu, X.; Wang, X. Black titania/graphene oxide nanocomposite films with excellent photothermal property for solar steam generation. J. Mater. Res. 2018, 33, 674–684. [Google Scholar] [CrossRef]
- Sun, H.; Wang, Y.; Liu, S.; Ge, L.; Wang, L.; Zhu, Z.; Wang, S. Facile synthesis of nitrogen doped reduced graphene oxide as a superior metal-free catalyst for oxidation. Chem. Commun. 2013, 49, 9914–9916. [Google Scholar] [CrossRef]
- Bing, N.; Yang, J.; Zhang, Y.; Yu, W.; Wang, L.; Xie, H. 3D graphene nanofluids with high photothermal conversion and thermal transportation properties. Sustain. Energy Fuels 2020, 4, 1208–1215. [Google Scholar] [CrossRef]
- Kim, K.; Yu, S.; An, C.; Kim, S.W.; Jang, J.H. Mesoporous three-dimensional graphene networks for highly efficient solar desalination under 1 sun illumination. ACS Appl. Mater. Interfaces 2018, 10, 15602–15608. [Google Scholar] [CrossRef]
- Storer, D.P.; Phelps, J.L.; Wu, X.; Owens, G.; Khan, N.I.; Xu, H. Graphene and rice-straw-fiber-based 3D photothermal aerogels for highly efficient solar evaporation. ACS Appl. Mater. Interfaces 2020, 12, 15279–15287. [Google Scholar] [CrossRef]
- Finnerty, C.T.K.; Menon, A.K.; Conway, K.M.; Lee, D.; Nelson, M.; Urban, J.J.; Sedlak, D.; Mi, B. Interfacial Solar Evaporation by a 3D Graphene Oxide Stalk for Highly Concentrated Brine Treatment. Environ. Sci. Technol. 2021, 55, 15435–15445. [Google Scholar] [CrossRef]
- Gao, T.; Wu, X.; Owens, G.; Xu, H.L. A cobalt oxide@ polydopamine-reduced graphene oxide-based 3D photothermal evaporator for highly efficient solar steam generation. Tungsten 2020, 2, 423–432. [Google Scholar] [CrossRef]
- Xu, H.; Lu, Y.; Jiang, F.; Zhang, J.; Ge, Y.; Li, Z. 3D porous N-doped lignosulfonate/graphene oxide aerogel for efficient solar steam generation and desalination. Int. J. Biol. Macromol. 2023, 233, 123469. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Feng, H.P.; Niu, C.G.; Huang, D.W.; Guo, H.; Liang, C.; Liu, H.Y.; Chen, S.; Tang, N.; Li, L. Constructing a plasma-based Schottky heterojunction for near-infrared-driven photothermal synergistic water disinfection: Synergetic effects and antibacterial mechanisms. Chem. Eng. J. 2021, 426, 131902. [Google Scholar] [CrossRef]
- Adeel, M.; Bilal, M.; Rasheed, T.; Sharma, A.; Iqbal, H.M. Graphene and graphene oxide: Functionalization and nano-bio-catalytic system for enzyme immobilization and biotechnological perspective. Int. J. Biol. Macromol. 2018, 120, 1430–1440. [Google Scholar] [CrossRef]
- Gupta, B.; Kumar, N.; Panda, K.; Kanan, V.; Joshi, S.; Visoly-Fisher, I. Role of oxygen functional groups in reduced graphene oxide for lubrication. Sci. Rep. 2017, 7, 45030. [Google Scholar] [CrossRef] [Green Version]
- Pan, H.; Zhao, X.; Fu, Z.; Tu, W.; Fang, P.; Zhang, H. Visible-light induced photocatalysis of AgCl@Ag/titanate nanotubes/nitrogen-doped reduced graphite oxide composites. Appl. Surf. Sci. 2018, 442, 547–555. [Google Scholar] [CrossRef]
- Serafin, J.; Ouzzine, M.; Junior, O.F.C.; Sreńscek-Nazzal, J. Preparation of low-cost activated carbons from amazonian nutshells for CO2 storage. Biomass Bioenergy 2021, 144, 105925. [Google Scholar] [CrossRef]
- Yilmaz, M.S. Graphene oxide/hollow mesoporous silica composite for selective adsorption of methylene blue. Microporous Mesoporous Mater. 2022, 330, 111570. [Google Scholar] [CrossRef]
- Morsi, R.E.; Mohamed, R.S. Nanostructured mesoporous silica: Influence of the preparation conditions on the physical-surface properties for efficient organic dye uptake. R. Soc. Open Sci. 2018, 5, 172021. [Google Scholar] [CrossRef] [Green Version]
- Chun, S.E.; Whitacre, J.F. Formation of micro/mesopores during chemical activation in tailor-made nongraphitic carbons. Microporous Mesoporous Mater. 2017, 251, 34–41. [Google Scholar] [CrossRef]
- Chen, W.; Luo, M.; Yang, K.; Zhou, X. Microwave-assisted KOH activation from lignin into hierarchically porous carbon with super high specific surface area by utilizing the dual roles of inorganic salts: Microwave absorber and porogen. Microporous Mesoporous Mater. 2020, 300, 110178. [Google Scholar] [CrossRef]
- Tarannum, F.; Muthaiah, R.; Danayat, S.; Foley, K.; Annam, R.S.; Walters, K.B.; Garg, J. Chemically Edge-Carboxylated Graphene Enhances the Thermal Conductivity of Polyetherimide–Graphene Nanocomposites. ACS Appl. Mater. Interfaces 2022, 14, 14753–14763. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.; Wang, C.; Guo, R.; Lan, J.; Lin, S.; Jiang, S.; Lai, X.; Zhang, Y.; Xiao, H. Synthesis of carboxymethyl cellulose-reduced graphene oxide aerogel for efficient removal of organic liquids and dyes. J. Mater. Sci. 2019, 54, 1872–1883. [Google Scholar] [CrossRef]
- Xu, Y.; Fleischer, A.S.; Feng, G. Reinforcement and shape stabilization of phase-change material via graphene oxide aerogel. Carbon 2017, 114, 334–346. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, C.; Song, X.; Huang, M.; Megarajan, S.K.; Shaukat, S.F.; Jiang, H. Improved light-harvesting and thermal management for efficient solar-driven water evaporation using 3D photothermal cones. J. Mater. Chem. A 2018, 6, 9874–9881. [Google Scholar] [CrossRef]
- Cui, P.; Xue, Y. Tuning nonradiative recombination loss by selective oxidation patterns of epoxy groups bound to different sites of graphene quantum dots. Chem. Eng. J. 2022, 431, 134052. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Shnitov, V.V.; Dideikin, A.T.; Aleksenskii, A.E.; Vul’, S.P.; Baidakova, M.V.; Pronin, I.I.; Kirilenko, D.A.; Brunkov, P.N.; Weise, J.; et al. Nanoscale perforation of graphene oxide during photoreduction process in the argon atmosphere. J. Phys. Chem. C 2016, 120, 28261–28269. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, H.; Zhang, X.; Xu, W.; Li, Y.; Li, Q.; Wei, G.; Su, Z. Graphene film doped with silver nanoparticles: Self-assembly formation, structural characterizations, antibacterial ability, and biocompatibility. Biomater. Sci. 2015, 3, 852–860. [Google Scholar] [CrossRef]
- Bagheri, S.; Jamal, N.; Halilu, A.; TermehYousefi, A. Novel rGO-TC (n) Nanosheets developed via click chemistry as a lubricant anti-wear additive. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Huang, Y.; Min, C.; Chen, L.; Chen, L. Effect of γ-ray ra-diation on the polyacrylonitrile based carbon fibers. Radiat. Phys. Chem. 2010, 79, 839–843. [Google Scholar] [CrossRef]
- Yusoff, N.; Rameshkumar, P.; Shahid, M.M.; Huang, S.T.; Huang, N.M. Amperometric detection of nitric oxide using a glassy carbon electrode modified with gold nanoparticles incorporated into a nanohybrid composed of reduced graphene oxide and Nafion. Microchim. Acta 2017, 184, 3291–3299. [Google Scholar] [CrossRef]
- Li, Z.; Haidry, A.A.; Liu, Y.; Sun, L.; Xie, L.; Fatima, Q.; Yao, Z. Strongly coupled Ag/TiO2 heterojunction: From one-step facile synthesis to effective and stable ethanol sensing performances. J. Mater. Sci. Mater. Electron. 2018, 29, 19219–19227. [Google Scholar] [CrossRef]
- Dong, P.; Yang, F.; Cheng, X.; Huang, Z.; Nie, X.; Xiao, Y.; Zhang, X. Plasmon enhanced photocatalytic and antimicrobial activities of Ag-TiO2 nanocomposites under visible light irradiation prepared by DBD cold plasma treatment. Mater. Sci. Eng. C 2019, 96, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Makuła, P.; Pacia, M.; Macyk, W. How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV–Vis spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [Green Version]
- Xin, X.; Xu, T.; Wang, L.; Wang, C. Ti3+-self doped brookite TiO2 single-crystalline nanosheets with high solar absorption and excellent photocatalytic CO2 reduction. Sci. Rep. 2016, 6, 23684. [Google Scholar] [CrossRef] [Green Version]
- Shafiee, A.; Aibaghi, B.; Carrier, A.J.; Ehsan, M.F.; Nganou, C.; Zhang, X.; Oakes, K.D. Rapid photodegradation mechanism enabled by broad-spectrum absorbing black anatase and reduced graphene oxide nanocomposites. Appl. Surf. Sci. 2022, 575, 151718. [Google Scholar] [CrossRef]
- Zhu, G.; Xu, J.; Zhao, W.; Huang, F. Constructing black titania with unique nanocage structure for solar desalination. ACS Appl. Mater. Interfaces 2016, 8, 31716–31721. [Google Scholar] [CrossRef]
- Ye, M.; Jia, J.; Wu, Z.; Qian, C.; Chen, R.; O’Brien, P.G.; Sun, W.; Dong, Y.; Ozin, G.A. Synthesis of black TiOx nanoparticles by Mg reduction of TiO2 nanocrystals and their application for solar water evaporation. Adv. Energy Mater. 2017, 7, 1601811. [Google Scholar] [CrossRef]
- Han, X.; Besteiro, L.V.; Koh, C.S.L.; Lee, H.K.; Phang, I.Y.; Phan-Quang, G.C.; Ng, J.Y.; Sim, H.Y.F.; Lay, C.L.; Govorov, A.; et al. Intensifying heat using MOF-isolated graphene for solar-driven seawater desalination at 98% solar-to-thermal efficiency. Adv. Funct. Mater. 2021, 31, 2008904. [Google Scholar] [CrossRef]
- Mi, B. Graphene oxide membranes for ionic and molecular sieving. Science 2014, 343, 740–742. [Google Scholar] [CrossRef]
- Anand, A.; Unnikrishnan, B.; Mao, J.Y.; Lin, H.J.; Huang, C.C. Graphene-based nanofiltration membranes for improving salt rejection, water flux and antifouling—A review. Desalination 2018, 429, 119–133. [Google Scholar] [CrossRef]
- Liu, G.; Jin, W.; Xu, N. Graphene-based membranes. Chem. Soc. Rev. 2015, 44, 5016–5030. [Google Scholar] [CrossRef] [PubMed]
- Konatham, D.; Yu, J.; Ho, T.A.; Striolo, A. Simulation insights for graphene-based water desalination membranes. Langmuir 2013, 29, 11884–11897. [Google Scholar] [CrossRef] [PubMed]
- Rahiminejad, M.; Mortazavi, V.; Moosavi, A.; Nouri-Borujerdi, A. Transport of Water Contaminated with Various Ions Through Nanoporous Graphene: A Molecular Dynamics Simulation. Transp. Porous Media 2022, 146, 537–557. [Google Scholar] [CrossRef]
- Salahshoor, Z.; Shahbazi, A.; Koutahzadeh, N. Developing a novel nitrogen-doped hollow porous carbon sphere (N-HPCS) blended nanofiltration membrane with superior water permeance characteristic for high saline and colored wastewaters treatment. Chem. Eng. J. 2022, 431, 134068. [Google Scholar] [CrossRef]
- Ghasemi, H.; Ni, G.; Marconnet, A.M.; Loomis, J.; Yerci, S.; Miljkovic, N.; Chen, G. Solar steam generation by heat localization. Nat. Commun. 2014, 5, 4449. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://www.engineeringtoolbox.com/water-properties-d_1573.html (accessed on 1 December 2022).
- Ming, X.; Guo, A.; Zhang, Q.; Guo, Z.; Yu, F.; Hou, B.; Wang, Y.; Homewood, K.P.; Wang, X. 3D macroscopic graphene oxide/MXene architectures for multifunctional water purification. Carbon 2020, 167, 285–295. [Google Scholar] [CrossRef]
- Wei, C.; Zhang, X.; Ma, S.; Zhang, C.; Li, Y.; Chen, D.; Jiang, H.; Xu, Z.; Huang, X. Ultra-robust vertically aligned three-dimensional (3D) Janus hollow fiber membranes for interfacial solar-driven steam generation with salt-resistant and multi-media purification. Chem. Eng. J. 2021, 425, 130118. [Google Scholar] [CrossRef]
- Lei, Z.; Sun, X.; Zhu, S.; Dong, K.; Liu, X.; Wang, L.; Zhang, X.; Qu, L.; Zhang, X. Nature inspired MXene-decorated 3D honeycomb-fabric architectures toward efficient water desalination and salt harvesting. Nano-Micro Lett. 2022, 14, 10. [Google Scholar] [CrossRef]
- Hong, D.; Lyu, L.M.; Koga, K.; Shimoyama, Y.; Kon, Y. Plasmonic Ag@ TiO2 core–shell nanoparticles for enhanced CO2 photoconversion to CH4. ACS Sust Chem. Eng. 2019, 7, 18955–18964. [Google Scholar] [CrossRef]
- Li, X.; Zhu, X.; Zhu, Y.; Yuan, Z.; Si, L.; Qian, Y. Porous nitrogen-doped carbon vegetable-sponges with enhanced lithium storage performance. Carbon 2014, 69, 515–524. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, L.; Chen, S.; Wang, X.; Ma, Y.; Wu, Q.; Jiang, Y.; Qian, W.; Hu, Z. Can boron and nitrogen co-doping improve oxygen reduction reaction activity of carbon nanotubes? J. Am. Chem. Soc. 2013, 135, 1201–1204. [Google Scholar] [CrossRef]
- Habte, A.T.; Ayele, D.W. Synthesis and characterization of reduced graphene oxide (rGO) started from graphene oxide (GO) using the tour method with different parameters. Adv. Mater. Sci. Eng. 2019, 5058163, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Xing, Z.; Zou, J.; Li, Z.; Wu, X.; Zhang, Y.; Zhu, Q.; Yang, S.; Zhou, W. Black TiO2 nanobelts/g-C3N4 nanosheets laminated heterojunctions with efficient visible-light-driven photocatalytic performance. Sci. Rep. 2017, 7, 41978. [Google Scholar] [CrossRef] [Green Version]
- Haber, J.; Sokolov, K. Synthesis of stable citrate-capped silver nanoprisms. Langmuir 2017, 33, 10525–10530. [Google Scholar] [CrossRef]
- Le, G.T.; Manyam, J.; Opaprakasit, P.; Chanlek, N.; Grisdanurak, N.; Sreearunothai, P. Divergent mechanisms for thermal reduction of graphene oxide and their highly different ion affinities. Diam. Relat. Mater. 2018, 89, 246–256. [Google Scholar] [CrossRef]
- Wang, S.; Tristan, F.; Minami, D.; Fujimori, T.; Cruz-Silva, R.; Terrones, M.; Takeuchi, K.; Teshima, K.; Rodríguez-Reinoso, F.; Endo, M.; et al. Activation routes for high surface area graphene monoliths from graphene oxide colloids. Carbon 2014, 76, 220–231. [Google Scholar] [CrossRef] [Green Version]
- Ho, C.Y.; Wang, H.W. Characteristics of thermally reduced graphene oxide and applied for dye-sensitized solar cell counter electrode. Appl. Surf. Sci. 2015, 357, 147–154. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Q.; Ma, D.; Yuan, Y.; Yao, J.; Zhang, W.; Su, H.; Su, Y.; Gu, J.; Zhang, D. Simultaneously achieving thermal insulation and rapid water transport in sugarcane stems for efficient solar steam generation. J. Mater. Chem. A 2019, 7, 9034–9039. [Google Scholar] [CrossRef]












| Reference | Evaporation Flux (kg·m−2 h−1) |
|---|---|
| [16] | 1.31 under 1 sun |
| [17] | 1.44 under 1.2 suns |
| [18] | 1.36 under 1 sun |
| [27] | From 1.42 to 1.49 under 1 sun |
| [28] | 1.37, 1.85, and 2.25 under 1 sun |
| [21] | 1.28, 2.76, 4.01, and 5.43 kg under 1, 2, 3, and 4 suns, respectively |
| [30] | 1.6 to 3.71 under 1 sun |
| [31] | 1.57 under 1 sun |
| [67] | 1.27 kg/m2 h under 1 sun |
| [67] | 6.70 kg/m2 h under 5 suns |
| [68] | 1, 1.1, 1.3, 1.4, and up to 2.6 under 1 sun |
| [69] | 1.62 under 1 sun |
| Current study | 1.43 under 1 sun |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bezza, F.A.; Iwarere, S.A.; Tichapondwa, S.M.; Chirwa, E.M.N. Fabrication and Application of Ag, Black TiO2 and Nitrogen-Doped 3D Reduced Graphene Oxide (3D Black TiO2/Ag/N@rGO) Evaporator for Efficient Steam Generation. Catalysts 2023, 13, 514. https://doi.org/10.3390/catal13030514
Bezza FA, Iwarere SA, Tichapondwa SM, Chirwa EMN. Fabrication and Application of Ag, Black TiO2 and Nitrogen-Doped 3D Reduced Graphene Oxide (3D Black TiO2/Ag/N@rGO) Evaporator for Efficient Steam Generation. Catalysts. 2023; 13(3):514. https://doi.org/10.3390/catal13030514
Chicago/Turabian StyleBezza, Fisseha A., Samuel A. Iwarere, Shepherd M. Tichapondwa, and Evans M. N. Chirwa. 2023. "Fabrication and Application of Ag, Black TiO2 and Nitrogen-Doped 3D Reduced Graphene Oxide (3D Black TiO2/Ag/N@rGO) Evaporator for Efficient Steam Generation" Catalysts 13, no. 3: 514. https://doi.org/10.3390/catal13030514