Interaction of Jania rubens Polyphenolic Extract as an Antidiabetic Agent with α-Amylase, Lipase, and Trypsin: In Vitro Evaluations and In Silico Studies
Abstract
:1. Introduction
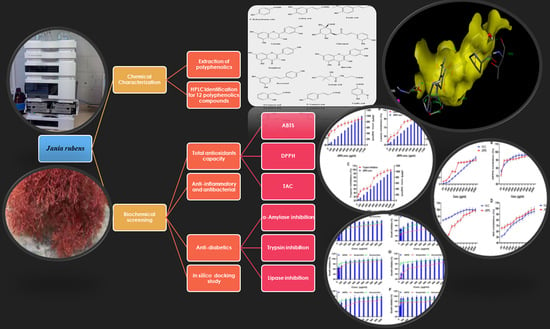
2. Results
2.1. The HPLC Analysis of JRPE
2.2. The Antioxidant Properties of the JRPE
2.3. The Anti-Inflammatory Activity Using Nitric Oxides Assay
2.4. The Antibacterial Activity of the JRPE
2.5. Inhibitory Effects of JRPE toward α-Amylase, Pepsin, Trypsin, and Lipase
2.6. Docking Studies of Polyphenolics in JRPE against Amylase, Lipase, and Trypsin
2.7. Interactions Assessment between the Twelve Polyphenolic Compounds and α-Amylase
2.8. Interactions between the Twelve Polyphenolic Compounds and Lipase
2.9. Interactions between the Twelve Polyphenolic Compounds and Trypsin
2.10. Pharmacodynamics and Pharmacokinetics of Polyphenolics Composition in JRPE
3. Discussion
4. Materials and Methods
4.1. Samples Collection
4.2. Extraction and Preparation of Jania rubens Polyphenolic Extract (JRPE)
4.3. High-Performance Liquid Chromatography (HPLC) Analysis of JRPE
4.4. Antioxidant Activity of JRPE
4.5. Anti-Inflammatory Activity of JRPE
4.6. Antibacterial Assessments of JRPE
4.7. Anti-Diabetics and Anti-Obesity of JRPE Using Inhibition of Digestive Enzymes
4.8. Molecular Docking Studies
4.9. In Silico Pharmacodynamics and Pharmacokinetics
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Forouhi, N.G.; Wareham, N.J. Epidemiology of diabetes. Medicine 2014, 42, 698–702. [Google Scholar] [CrossRef] [Green Version]
- Federation, I.D. IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019; Available online: https://www.diabetesatlas.org (accessed on 20 November 2022).
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, P.; Ande, C.; Vankar, Y.D. Synthesis of (5,6 & 6,6)-oxa-oxa annulated sugars as glycosidase inhibitors from 2-formyl galactal using iodocyclization as a key step. ARKIVOC 2022, 2022, 5–23. [Google Scholar]
- Yang, L.-F.; Shimadate, Y.; Kato, A.; Li, Y.-X.; Jia, Y.-M.; Fleet, G.W.J.; Yu, C.-Y. Synthesis and glycosidase inhibition of N-substituted derivatives of 1,4-dideoxy-1,4-imino-d-mannitol (DIM). Org. Biomol. Chem. 2020, 18, 999–1011. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Jacobo-Herrera, N.; Altemimi, A.; Lakhssassi, N. A Comprehensive Review on Medicinal Plants as Antimicrobial Therapeutics: Potential Avenues of Biocompatible Drug Discovery. Metabolites 2019, 9, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shreadah, M.A.; El Moneam, N.; El-Assar, S.A.; Nabil-Adam, A. Metabolomics and pharmacological screening of aspergillus versicolor isolated from Hyrtios erectus Red Sea sponge; Egypt. Curr. Bioact. Compd. 2020, 16, 1083–1102. [Google Scholar] [CrossRef]
- Nabil-Adam, A.; Shreadah, A.M.; Abd El-Moneam, M.N.; El-Assar, A.S. Marine Algae of the Genus Gracilaria as Multi Products Source for Different Biotechnological and Medical Applications. Recent Pat. Biotechnol. 2020, 14, 203–228. [Google Scholar] [CrossRef] [PubMed]
- AbdelMonein, N.M.; Yacout, G.A.; Aboul-Ela, H.M.; Shreadah, M.A. Hepatoprotective Activity of Chitosan Nanocarriers Loaded with the Ethyl Acetate Extract of a Stenotrophomonas sp. Bacteria Associated with the Red Sea Sponge Amphimedon ochracea in CCl4 Induced Hepatotoxicty in Rats. Adv. Biosci. Biotechnol. 2017, 8, 27–50. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Moneam, N.M.; Shreadah, M.A.; El-Assar, S.A.; Nabil-Adam, A. Protective role of antioxidants capacity of Hyrtios aff. Erectus sponge extract against mixture of persistent organic pollutants (POPs)-induced hepatic toxicity in mice liver: Biomarkers and ultrastructural study. Environ. Sci. Pollut. Res. 2017, 24, 22061–22072. [Google Scholar] [CrossRef]
- Abd El-Moneam, N.M.; El-Assar, S.A.; Shreadah, M.A.; Nabil-Adam, A. Isolation, identification and molecular screening of Pseudomonas sp. metabolic pathways NRPs and PKS associated with the Red Sea sponge, Hyrtios aff. Erectus, Egypt. J. Pure Appl. Microbiol. 2017, 11, 1299–1311. [Google Scholar] [CrossRef]
- Wang, C.C.L.; Hess, C.N.; Hiatt, W.R.; Goldfine, A.B. Clinical Update: Cardiovascular Disease in Diabetes Mellitus. Circulation 2016, 133, 2459–2502. [Google Scholar] [CrossRef]
- Chaudhury, A.; Duvoor, C.; Reddy Dendi, V.S.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, N.S.; Montales, M.T.; Kuriakose, K.; et al. Clinical Review of Antidiabetic Drugs: Implications for Type 2 Diabetes Mellitus Management. Front. Endocrinol. 2017, 8, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen, J.A.M.J.L. Hyperinsulinemia and Its Pivotal Role in Aging, Obesity, Type 2 Diabetes, Cardiovascular Disease and Cancer. Int. J. Mol. Sci. 2021, 22, 7797. [Google Scholar] [CrossRef]
- Gong, L.; Feng, D.; Wang, T.; Ren, Y.; Liu, Y.; Wang, J. Inhibitors of α-amylase and α-glucosidase: Potential linkage for whole cereal foods on prevention of hyperglycemia. Food Sci. Nutr. 2020, 8, 6320–6337. [Google Scholar] [CrossRef] [PubMed]
- Arbi, B.; Bouchentouf, S.; El-Shazly, M. Investigation Of The Potential Antidiabetic Effect Of Zygophyllum Sp. By Studying The Interaction Of Its Chemical Compounds With Alpha-Amylase And Dpp-4 Enzymes Using A Molecular Docking Approach. Curr. Enzym. Inhib. 2023, 19. [Google Scholar] [CrossRef]
- Abol-Fotouh, D.; AlHagar, O.E.A.; Hassan, M.A. Optimization, purification, and biochemical characterization of thermoalkaliphilic lipase from a novel Geobacillus stearothermophilus FMR12 for detergent formulations. Int. J. Biol. Macromol. 2021, 181, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Zhang, B.; Wu, B.; Xiao, H.; Li, Z.; Li, R.; Xu, X.; Li, T. Signaling pathways in obesity: Mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2022, 7, 298. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Park, Y.H.; Lee, J.S.; Jeong, H.I.; Lee, K.W.; Kang, T.H. Anti-Obesity Effect of DKB-117 through the Inhibition of Pancreatic Lipase and α-Amylase Activity. Nutrients 2020, 12, 3053. [Google Scholar] [CrossRef] [PubMed]
- Leandro, A.; Pereira, L.; Gonçalves, A.M.M. Diverse Applications of Marine Macroalgae. Mar. Drugs 2020, 18, 17. [Google Scholar] [CrossRef] [Green Version]
- Daniotti, S.; Re, I. Marine Biotechnology: Challenges and Development Market Trends for the Enhancement of Biotic Resources in Industrial Pharmaceutical and Food Applications. A Statistical Analysis of Scientific Literature and Business Models. Mar. Drugs 2021, 19, 61. [Google Scholar] [CrossRef]
- Sudhakar, K.; Mamat, R.; Samykano, M.; Azmi, W.H.; Ishak, W.F.W.; Yusaf, T. An overview of marine macroalgae as bioresource. Renew. Sustain. Energy Rev. 2018, 91, 165–179. [Google Scholar] [CrossRef]
- Carpena, M.; Garcia-Perez, P.; Garcia-Oliveira, P.; Chamorro, F.; Otero, P.; Lourenço-Lopes, C.; Cao, H.; Simal-Gandara, J.; Prieto, M.A. Biological properties and potential of compounds extracted from red seaweeds. Phytochem. Rev. 2022, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Lakey-Beitia, J.; Burillo, A.M.; La Penna, G.; Hegde, M.L.; Rao, K.S. Polyphenols as Potential Metal Chelation Compounds Against Alzheimer’s Disease. J. Alzheimer’s Dis. 2021, 82, S335–S357. [Google Scholar] [CrossRef]
- Shreadah, M.; Abdel-El Moneam, N.; Al-Assar, S.; Nabil-Adam, A. The ameliorative role of a marine sponge extract against mixture of persistent organic pollutants induced changes in hematological parameters in mice. Expert Opin. Env. Biol. 2017, 6, 2. [Google Scholar] [CrossRef]
- Abdel Moneam, N.; Al-Assar, S.; Shreadah, M.; Nabil-Adam, A. The hepatoprotective effect of Hyrtios aff. Erectus sponge isolated from the Red sea extract against the toxicity of Persistent organic pollutants (POPs) from Sediments of Lake Mariout. J. Biotechnol. Biotechnol. Equip. 2017, 32, 734–743. [Google Scholar] [CrossRef] [Green Version]
- Shreadah, M.A.; El Moneam, N.M.A.; Al-Assar, S.A.; Nabil-Adam, A. Phytochemical and pharmacological screening of Sargassium vulgare from Suez Canal, Egypt. Food Sci. Biotechnol. 2018, 27, 963–979. [Google Scholar] [CrossRef]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Gonçalves, A.M.M.; da Silva, G.J.; Pereira, L. Seaweed Phenolics: From Extraction to Applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef]
- Lomartire, S.; Cotas, J.; Pacheco, D.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Environmental Impact on Seaweed Phenolic Production and Activity: An Important Step for Compound Exploitation. Mar. Drugs 2021, 19, 245. [Google Scholar] [CrossRef]
- Abd El Moneam, N.M.; Shreadah, M.A.; El-Assar, S.A.; De Voogd, N.J.; Nabil-Adam, A. Hepatoprotective effect of Red Sea sponge extract against the toxicity of a real-life mixture of persistent organic pollutants. Biotechnol. Biotechnol. Equip. 2018, 32, 734–743. [Google Scholar] [CrossRef] [Green Version]
- Sęczyk, Ł.; Świeca, M.; Kapusta, I.; Gawlik-Dziki, U. Protein–Phenolic Interactions as a Factor Affecting the Physicochemical Properties of White Bean Proteins. Molecules 2019, 24, 408. [Google Scholar] [CrossRef] [Green Version]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of In Vitro Digestion on Composition, Bioaccessibility and Antioxidant Activity of Food Polyphenols—A Non-Systematic Review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef]
- Susmitha, A.; Bajaj, H.; Madhavan Nampoothiri, K. The divergent roles of sortase in the biology of Gram-positive bacteria. Cell Surf. 2021, 7, 100055. [Google Scholar] [CrossRef]
- Nguyen, T.L.A.; Bhattacharya, D. Antimicrobial Activity of Quercetin: An Approach to Its Mechanistic Principle. Molecules 2022, 27, 2494. [Google Scholar] [CrossRef] [PubMed]
- Makarewicz, M.; Drożdż, I.; Tarko, T.; Duda-Chodak, A. The Interactions between Polyphenols and Microorganisms, Especially Gut Microbiota. Antioxidants 2021, 10, 188. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Rao, S.; Sun, J.; Ma, C.; Li, J. p-Coumaric acid kills bacteria through dual damage mechanisms. Food Control 2012, 25, 550–554. [Google Scholar] [CrossRef]
- Wang, H.; Chu, W.; Ye, C.; Gaeta, B.; Tao, H.; Wang, M.; Qiu, Z. Chlorogenic acid attenuates virulence factors and pathogenicity of Pseudomonas aeruginosa by regulating quorum sensing. Appl. Microbiol. Biotechnol. 2019, 103, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Liu, F.; Luo, Z.; Wu, H.; Zhang, X.; Wang, D.; Zhu, Y.; Sun, Z.; Xu, W.; Miao, Y. The Antibacterial Activity and Mechanism of Chlorogenic Acid Against Foodborne Pathogen Pseudomonas aeruginosa. Foodborne Pathog. Dis. 2019, 16, 823–830. [Google Scholar] [CrossRef]
- Mu, Y.; Zeng, H.; Chen, W. Quercetin Inhibits Biofilm Formation by Decreasing the Production of EPS and Altering the Composition of EPS in Staphylococcus epidermidis. Front. Microbiol. 2021, 12, 631058. [Google Scholar] [CrossRef]
- Vipin, C.; Saptami, K.; Fida, F.; Mujeeburahiman, M.; Rao, S.S.; Athmika; Arun, A.B.; Rekha, P.D. Potential synergistic activity of quercetin with antibiotics against multidrug-resistant clinical strains of Pseudomonas aeruginosa. PloS ONE 2020, 15, e0241304. [Google Scholar] [CrossRef]
- Al Azzaz, J.; Al Tarraf, A.; Heumann, A.; Da Silva Barreira, D.; Laurent, J.; Assifaoui, A.; Rieu, A.; Guzzo, J.; Lapaquette, P. Resveratrol Favors Adhesion and Biofilm Formation of Lacticaseibacillus paracasei subsp. paracasei Strain ATCC334. Int. J. Mol. Sci. 2020, 21, 5423. [Google Scholar] [CrossRef]
- Kato, E.; Tsuruma, A.; Amishima, A.; Satoh, H. Proteinous pancreatic lipase inhibitor is responsible for the antiobesity effect of young barley (Hordeum vulgare L.) leaf extract. Biosci. Biotechnol. Biochem. 2021, 85, 1885–1889. [Google Scholar] [CrossRef] [PubMed]
- Oluwagunwa, O.A.; Alashi, A.M.; Aluko, R.E. Inhibition of the in vitro Activities of α-Amylase and Pancreatic Lipase by Aqueous Extracts of Amaranthus viridis, Solanum macrocarpon and Telfairia occidentalis Leaves. Front. Nutr. 2021, 8, 772903. [Google Scholar] [CrossRef]
- Oboh, G.; Ademosun, A.O.; Ayeni, P.O.; Omojokun, O.S.; Bello, F. Comparative effect of quercetin and rutin on α-amylase, α-glucosidase, and some pro-oxidant-induced lipid peroxidation in rat pancreas. Comp. Clin. Pathol. 2015, 24, 1103–1110. [Google Scholar] [CrossRef]
- Zhu, W.; Jia, Y.; Peng, J.; Li, C.-M. Inhibitory Effect of Persimmon Tannin on Pancreatic Lipase and the Underlying Mechanism in Vitro. J. Agric. Food Chem. 2018, 66, 6013–6021. [Google Scholar] [CrossRef]
- Swilam, N.; Nawwar, M.A.M.; Radwan, R.A.; Mostafa, E.S. Antidiabetic Activity and In Silico Molecular Docking of Polyphenols from Ammannia baccifera L. subsp. Aegyptiaca (Willd.) Koehne Waste: Structure Elucidation of Undescribed Acylated Flavonol Diglucoside. Plants 2022, 11, 452. [Google Scholar] [CrossRef]
- Baruah, I.; Kashyap, C.; Guha, A.K.; Borgohain, G. Insights into the Interaction between Polyphenols and β-Lactoglobulin through Molecular Docking, MD Simulation, and QM/MM Approaches. ACS Omega 2022, 7, 23083–23095. [Google Scholar] [CrossRef]
- Zhang, R.; Wei, Y.; Yang, T.; Huang, X.; Zhou, J.; Yang, C.; Zhou, J.; Liu, Y.; Shi, S. Inhibitory effects of quercetin and its major metabolite quercetin-3-O-β-D-glucoside on human UDP-glucuronosyltransferase 1A isoforms by liquid chromatography-tandem mass spectrometry. Exp. Ther. Med. 2021, 22, 842. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Cosme, P.; Rodríguez, A.B.; Espino, J.; Garrido, M. Plant Phenolics: Bioavailability as a Key Determinant of Their Potential Health-Promoting Applications. Antioxidants 2020, 9, 1263. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, K.; Paulraj, R. Sesquiterpenoids with free-radical-scavenging properties from marine macroalga Ulva fasciata Delile. Food Chem. 2010, 122, 31–41. [Google Scholar] [CrossRef]
- Tamer, T.M.; Sabet, M.M.; Alhalili, Z.A.H.; Ismail, A.M.; Mohy-Eldin, M.S.; Hassan, M.A. Influence of Cedar Essential Oil on Physical and Biological Properties of Hemostatic, Antibacterial, and Antioxidant Polyvinyl Alcohol/Cedar Oil/Kaolin Composite Hydrogels. Pharmaceutics 2022, 14, 2649. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Garratt, D.C. The Quantitative Analysis of Drugs. In The Quantitative Analysis of Drugs; Garratt, D.C., Ed.; Springer US: Boston, MA, USA, 1964; pp. 1–669. [Google Scholar]
- El-Samad, L.M.; Hassan, M.A.; Basha, A.A.; El-Ashram, S.; Radwan, E.H.; Abdul Aziz, K.K.; Tamer, T.M.; Augustyniak, M.; El Wakil, A. Carboxymethyl cellulose/sericin-based hydrogels with intrinsic antibacterial, antioxidant, and anti-inflammatory properties promote re-epithelization of diabetic wounds in rats. Int. J. Pharm. 2022, 629, 122328. [Google Scholar] [CrossRef]
- Hassan, M.A.; Abd El-Aziz, S.; Elbadry, H.M.; El-Aassar, S.A.; Tamer, T.M. Prevalence, antimicrobial resistance profile, and characterization of multi-drug resistant bacteria from various infected wounds in North Egypt. Saudi J. Biol. Sci. 2022, 29, 2978–2988. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Choi, S.-J.; Hwang, J.-M.; Kim, S.-I. A colorimetric microplate assay method for high throughput analysis of lipase activity. BMB Rep. 2003, 36, 417–420. [Google Scholar] [CrossRef] [PubMed]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminformatics 2011, 3, 33. [Google Scholar] [CrossRef] [Green Version]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [Green Version]
- Mbarik, M.; Poirier, S.J.; Doiron, J.; Selka, A.; Barnett, D.A.; Cormier, M.; Touaibia, M.; Surette, M.E. Phenolic acid phenethylesters and their corresponding ketones: Inhibition of 5-lipoxygenase and stability in human blood and HepaRG cells. Pharmacol. Res. Perspect. 2019, 7, e00524. [Google Scholar] [CrossRef]







| Polyphenolic Compounds | R. T/min | Con. (mg/kg) |
|---|---|---|
| p-Hydroxybenzoic acid | 7.618 | 14.61616 |
| Caffeic acid | 9.954 | 6.93052 |
| Catechin | 9.124 | 3.99015 |
| Chlorogenic | 9.408 | 17.91833 |
| Ferulic acid | 15.715 | 25.81511 |
| Kaempferol | 24.757 | 140.68073 |
| o-Coumaric acid | 17.874 | 8.01992 |
| p-Coumaric acid | 13.526 | 1.71484 |
| Quercetin | 21.666 | 67.48636 |
| Resveratrol | 19.470 | 96.88487 |
| Syringic acid | 10.705 | 49.60852 |
| Vanillic acid | 15.40824 | 7.13708 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nabil-Adam, A.; Ashour, M.L.; Tamer, T.M.; Shreadah, M.A.; Hassan, M.A. Interaction of Jania rubens Polyphenolic Extract as an Antidiabetic Agent with α-Amylase, Lipase, and Trypsin: In Vitro Evaluations and In Silico Studies. Catalysts 2023, 13, 443. https://doi.org/10.3390/catal13020443
Nabil-Adam A, Ashour ML, Tamer TM, Shreadah MA, Hassan MA. Interaction of Jania rubens Polyphenolic Extract as an Antidiabetic Agent with α-Amylase, Lipase, and Trypsin: In Vitro Evaluations and In Silico Studies. Catalysts. 2023; 13(2):443. https://doi.org/10.3390/catal13020443
Chicago/Turabian StyleNabil-Adam, Asmaa, Mohamed L. Ashour, Tamer M. Tamer, Mohamed A. Shreadah, and Mohamed A. Hassan. 2023. "Interaction of Jania rubens Polyphenolic Extract as an Antidiabetic Agent with α-Amylase, Lipase, and Trypsin: In Vitro Evaluations and In Silico Studies" Catalysts 13, no. 2: 443. https://doi.org/10.3390/catal13020443