Methane Dry Reforming over Ni/NiO Supported on Ce-, Zr-, and Al-Modified Y2O3 for Hydrogen Production
Abstract
:1. Introduction
2. Results
2.1. XRD and Raman Analysis of the Supports
2.2. Activity Evaluation
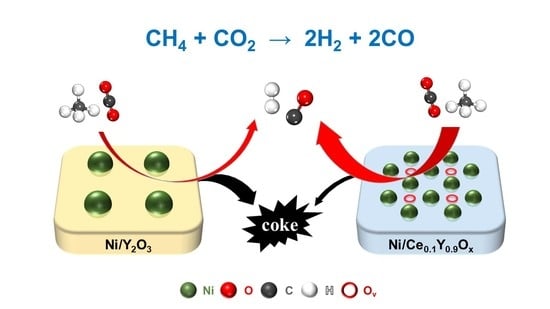
2.3. Carbon Deposition on the Spent Catalysts
2.4. XRD and N2-BET of the Freshly Calcined, Reduced, and Spent Catalysts
2.5. TEM Analysis of the Ni Distribution over the Fresh Ni/Y2O3 Catalysts
2.6. H2-TPR and XPS Studies of the Freshly Calcined Catalysts
2.7. H2 Adsorption–Desorption Analysis of the Freshly Reduced Catalysts
2.8. Surface Oxygen Properties of the Catalysts Investigated with XPS and O2-TPD
2.9. Identifying the Surface Oxygen Species with In Situ DRIFTS
2.10. CO2-TPD of the Reduced Catalysts
2.11. Probing the Reaction Intermediates with In Situ DRIFTS
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Activity Evaluation
3.3. Catalyst Characterization
4. Conclusions
- H2-TPR results have indicated that with the secondary metal doping, the Ni–support interaction is enhanced in comparison with the unmodified 5Ni/Y2O3 catalyst. As a result, the modified catalysts have decreased Ni crystallite sizes with improved Ni dispersion, as demonstrated by XRD, TEM, and H2 adsorption results, which is important to enhance the activity, stability, and anti-coking ability in the DRM reaction. In addition, all the modified catalysts have improved alkalinity, which is beneficial to activate CO2 and enhance the activity.
- O2-TPD and XPS O 1s analyses have testified that all the modified catalysts possess a richer amount of surface active oxygen species (O2δ− and O2−) than the unmodified 5Ni/Y2O3 catalyst, which obeys the order of 5Ni/Ce0.1Y0.9Ox > 5Ni/Zr0.1Y0.9Ox > 5Ni/Al0.1Y0.9Ox > 5Ni/Y2O3, and is well consistent with the coking-resistance sequence. This indicates that the surface active oxygen species is critical to eliminate carbon depositions.
- In situ DRIFTS results have confirmed that the addition of the secondary metals can improve the DRM activity of the Ni/Y2O3 catalyst by accelerating the conversion of formate intermediate species.
- Among all the catalysts, 5Ni/Al0.1Y0.9Ox owns the highest active Ni surface area, thus showing the best activity. In contrast, 5Ni/Ce0.1Y0.9Ox possesses the largest amount of surface alkaline sites and active oxygen species, hence displaying the highest stability and the best anti-coking ability.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Z.; Das, S.; Hongmanorom, P.; Dewangan, N.; Wai, M.H.; Kawi, S. Silica-based micro- and mesoporous catalysts for dry reforming of methane. Catal. Sci. Technol. 2018, 8, 2763–2778. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Abu-Dahrieh, J.K.; Atia, H.; Armbruster, U.; Ibrahim, A.A.; Khan, W.U.; Abasaeed, A.E.; Fakeeha, A.H. Effect of pre-treatment and calcination temperature on Al2O3-ZrO2 supported Ni-Co catalysts for dry reforming of methane. Int. J. Hydrog. Energy 2019, 44, 21546–21558. [Google Scholar] [CrossRef] [Green Version]
- Gopaul, S.G.; Dutta, A. Dry reforming of multiple biogas types for syngas production simulated using Aspen Plus: The use of partial oxidation and hydrogen combustion to achieve thermo-neutrality. Int. J. Hydrog. Energy 2015, 40, 6307–6318. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, J.; Xu, Y.; Sun, Y. A review of CH4-CO2 reforming to synthesis gas over Ni-based catalysts in recent years (2010–2017). Int. J. Hydrog. Energy 2018, 43, 15030–15054. [Google Scholar] [CrossRef]
- Motomura, A.; Nakaya, Y.; Sampson, C.; Higo, T.; Torimoto, M.; Tsuneki, H.; Furukawa, S.; Sekine, Y. Synergistic effects of Ni-Fe alloy catalysts on dry reforming of methane at low temperatures in an electric field. RSC Adv. 2022, 12, 28359–28363. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Deng, J.; Li, H.; Yan, T.; Zhang, J.; Zhang, D. Coking-resistant dry reforming of methane over Ni/gamma-Al2O3 catalysts by rationally steering metal-support interaction. Iscience 2021, 24, 102747. [Google Scholar] [CrossRef] [PubMed]
- Kuehl, S.; Duedder, H.; Girgsdies, F.; Kaehler, K.; Muhler, M.; Behrens, M. Perovskites as Precursors for Ni/La2O3 Catalysts in the Dry Reforming of Methane: Synthesis by Constant pH Co-Precipitation, Reduction Mechanism and Effect of Ru-Doping. Z. Anorg. Allg. Chem. 2017, 643, 1088–1095. [Google Scholar] [CrossRef]
- Xu, L.; Liu, W.; Zhang, X.; Tao, L.; Xia, L.; Xu, X.; Song, J.; Zhou, W.; Fang, X.; Wang, X. Ni/La2O3 Catalysts for Dry Reforming of Methane: Insights into the Factors Improving the Catalytic Performance. Chemcatchem 2019, 11, 2887–2899. [Google Scholar] [CrossRef]
- Liu, H.; Swirk, K.; Galvez, M.E.; Da Costa, P. Nickel Supported Modified Ceria Zirconia Lanthanum/Praseodymium/Yttrium Oxides Catalysts for Syngas Production through Dry Methane Reforming. In 10th International Conference on Processing and Manufacturing of Advanced Materials Processing, Fabrication, Properties, Applications (THERMEC); Cite Sci Paris: Paris, France, 2018; Volume 941, pp. 2214–2219. [Google Scholar]
- Sutthiumporn, K.; Kawi, S. Promotional effect of alkaline earth over Ni-La2O3 catalyst for CO2 reforming of CH4: Role of surface oxygen species on H2 production and carbon suppression. Int. J. Hydrog. Energy 2011, 36, 14435–14446. [Google Scholar] [CrossRef]
- Fujita, T.; Peng, X.B.; Yamaguchi, A.; Cho, Y.; Zhang, Y.Z.; Higuchi, K.; Yamamoto, Y.; Tokunaga, T.; Arai, S.; Miyauchi, M.; et al. Nanoporous Nickel Composite Catalyst for the Dry Reforming of Methane. Acs Omega 2018, 3, 16651–16657. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S. Promotional effect of Gd over Ni/Y2O3 catalyst used in dry reforming of CH4 for H2 production. Int. J. Hydrog. Energy 2017, 42, 18805–18816. [Google Scholar] [CrossRef]
- Bahari, M.B.; Setiabudi, H.D.; Nguyen, T.D.; Phuong, P.T.T.; Truong, Q.D.; Jalil, A.A.; Ainirazali, N.; Vo, D.-V.N. Insight into the influence of rare-earth promoter (CeO2, La2O3, Y2O3, and Sm2O3) addition toward methane dry reforming over Co/mesoporous alumina catalysts. Chem. Eng. Sci. 2020, 228, 115967. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Wang, Y.; Da Costa, P.; Hu, C. Highly Carbon-Resistant Y Doped NiO-ZrOm Catalysts for Dry Reforming of Methane. Catalysis 2019, 9, 1055. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhao, Z.-J.; Zeng, L.; Zhao, J.; Tian, H.; Chen, S.; Li, K.; Sang, S.; Gong, J. On the role of Ce in CO2 adsorption and activation over lanthanum species. Chem. Sci. 2018, 9, 3426–3437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, B.; Li, S.; Liu, Y.; Liang, X. Engineering metal-oxide interface by depositing ZrO2 overcoating on Ni/Al2O3 for dry reforming of methane. Chem. Eng. J. 2022, 436, 135195. [Google Scholar] [CrossRef]
- Diao, Y.A.; Zhang, X.; Liu, Y.; Chen, B.B.; Wu, G.H.; Shi, C. Plasma-assisted dry reforming of methane over Mo2C-Ni/Al2O3 catalysts: Effects of beta-Mo2C promoter. Appl. Catal. B 2022, 301, 120779. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, Q.; Ren, Y.; Peng, R.; Zhang, B.; Huang, W.; Zhao, Y.; Zhang, J. Experimental Investigations on Stability Tailoring of Ni-xFe/Mayenite for CO 2-CH4 Reforming. J. Combust. Sci. Technol. 2022, 28, 229–238. [Google Scholar]
- Liu, H.; He, D. Properties of Ni/Y2O3 and its catalytic performance in methane conversion to syngas. Int. J. Hydrog. Energy 2011, 36, 14447–14454. [Google Scholar] [CrossRef]
- Strnisa, F.; Sagar, V.T.; Djinovic, P.; Pintar, A.; Plazl, I. Ni-containing CeO2 rods for dry reforming of methane: Activity tests and a multiscale lattice Boltzmann model analysis in two model geometries. Chem. Eng. J. 2021, 413, 127498. [Google Scholar] [CrossRef]
- Wang, F.; Cai, W.; Provendier, H.; Schuurman, Y.; Descorme, C.; Mirodatos, C.; Shen, W. Hydrogen production from ethanol steam reforming over Ir/CeO2 catalysts: Enhanced stability by PrOx promotion. Int. J. Hydrog. Energy 2011, 36, 13566–13574. [Google Scholar] [CrossRef]
- Sharma, N.D.; Singh, J.; Vijay, A.; Samanta, K.; Pandey, S.D. Investigations of anharmonic effects via phonon mode variations in nanocrystalline Dy2O3, Gd2O3 and Y2O3. J. Raman Spectrosc. 2017, 48, 822–828. [Google Scholar] [CrossRef]
- Messaoudi, H.; Thomas, S.; Djaidja, A.; Slyemi, S.; Barama, A. Study of LaxNiOy and LaxNiOy/MgAl2O4 catalysts in dry reforming of methane. J. CO2 Util. 2017, 24, 40–49. [Google Scholar] [CrossRef]
- Fang, X.; Xia, L.; Li, S.; Hong, Z.; Yang, M.; Xu, X.; Xu, J.; Wang, X. Superior 3DOM Y2Zr2O7 supports for Ni to fabricate highly active and selective catalysts for CO2 methanation. Fuel 2021, 293, 120460. [Google Scholar] [CrossRef]
- Xia, L.; Fang, X.; Xu, X.; Liu, Q.; Yang, M.; Xu, J.; Gao, Z.; Wang, X. The promotional effects of plasma treating on Ni/Y2Ti2O7 for steam reforming of methane (SRM): Elucidating the NiO-support interaction and the states of the surface oxygen anions. Int. J. Hydrog. Energy 2020, 45, 4556–4569. [Google Scholar] [CrossRef]
- Silva, R.S.; Cunha, F.; Barrozo, P. Raman spectroscopy of the Al-doping induced structural phase transition in LaCrO3 perovskite. Solid State Commun. 2021, 333, 114346. [Google Scholar] [CrossRef]
- Triyono, D.; Hanifah, U.; Laysandra, H. Structural and optical properties of Mg-substituted LaFeO3 nanoparticles prepared by a sol-gel method. Results Phys. 2020, 16, 102995. [Google Scholar] [CrossRef]
- Xia, Y.; Xie, F.; Wang, L.; Peng, Y.; Ma, D.; Zhu, L.; Zhou, G.; Wang, X.; Zhang, G. Efficient dry reforming of methane with carbon dioxide reaction on Ni@Y2O3 nanofibers anti-carbon deposition catalyst prepared by electrospinning-hydrothermal method. Int. J. Hydrog. Energy 2020, 45, 31494–31506. [Google Scholar] [CrossRef]
- Sun, J.; Luo, D.; Xiao, P.; Jigang, L.; Yu, S. High yield hydrogen production from low CO selectivity ethanol steam reforming over modified Ni/Y2O3 catalysts at low temperature for fuel cell application. J. Power Sources 2008, 184, 385–391. [Google Scholar] [CrossRef]
- Fang, X.; Xia, L.; Peng, L.; Luo, Y.; Xu, J.; Xu, L.; Xu, X.; Liu, W.; Zheng, R.; Wang, X. Ln2Zr2O7 compounds (Ln = La, Pr, Sm, Y) with varied rare earth A sites for low temperature oxidative coupling of methane. Chin. Chem. Lett. 2019, 30, 1141–1146. [Google Scholar] [CrossRef]
- Pereñiguez, R.; Gonzalez-delaCruz, V.M.; Caballero, A.; Holgado, J.P. LaNiO3 as a precursor of Ni/La2O3 for CO2 reforming of CH4: Effect of the presence of an amorphous NiO phase. Appl. Catal. B 2012, 123–124, 324–332. [Google Scholar] [CrossRef]
- Sun, G.B.; Hidajat, K.; Wu, X.S.; Kawi, S. A crucial role of surface oxygen mobility on nanocrystalline Y2O3 support for oxidative steam reforming of ethanol to hydrogen over Ni/Y2O3 catalysts. Appl. Catal. B 2008, 81, 303–312. [Google Scholar] [CrossRef]
- Nurk, G.; Kooser, K.; Urpelainen, S.; Käämbre, T.; Joost, U.; Kodu, M.; Kivi, I.; Kanarbik, R.; Kukk, E.; Lust, E. Near ambient pressure X-ray photoelectron-and impedance spectroscopy study of NiO-Ce0.9Gd0.1O2-δ anode reduction using a novel dual-chamber spectroelectrochemical cell. J. Power Sources 2018, 378, 589–596. [Google Scholar] [CrossRef]
- Oemar, U.; Hidajat, K.; Kawi, S. High catalytic stability of Pd-Ni/Y2O3 formed by interfacial Cl for oxy-CO2 reforming of CH4. Catal. Today 2017, 281, 276–294. [Google Scholar] [CrossRef]
- Singha, R.K.; Yadav, A.; Agrawal, A.; Shukla, A.; Adak, S.; Sasaki, T.; Bal, R. Synthesis of highly coke resistant Ni nanoparticles supported MgO/ZnO catalyst for reforming of methane with carbon dioxide. Appl. Catal. B 2016, 191, 165–178. [Google Scholar] [CrossRef]
- Singha, R.K.; Shukla, A.; Yadav, A.; Konathala, L.N.S.; Bal, R. Effect of metal-support interaction on activity and stability of Ni-CeO2 catalyst for partial oxidation of methane. Appl. Catal. B 2016, 202, 473–488. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, J.; Zhou, Z.; Chen, S.; Zhang, T.; Song, F.; Zhang, Q.; Tsubaki, N.; Tan, Y.; Han, Y. Effects of the surface adsorbed oxygen species tuned by rare-earth metal doping on dry reforming of methane over Ni/ZrO2 catalyst. Appl. Catal. B 2019, 264, 118522. [Google Scholar] [CrossRef]
- Fan, Y.; Miao, C.; Yue, Y.; Hua, W.; Gao, Z. Oxidative coupling of methane over Y2O3 and Sr–Y2O3 nanorods. React. Kinet. Mech. Catal. 2021, 134, 711–725. [Google Scholar] [CrossRef]
- Zeng, R.; Jin, G.; He, D.; Zhang, L.; Chen, D.; Zhang, Y.; Zhu, L.; Mei, Y.; Wu, W.; Luo, Y. Oxygen vacancy promoted CO2 activation over acidic-treated LaCoO3 for dry reforming of propane. Mater. Today 2022, 19, 100162. [Google Scholar] [CrossRef]
- Yang, J.; Hu, S.; Fang, Y.; Hoang, S.; Li, L.; Yang, W.; Liang, Z.; Wu, J.; Hu, J.; Xiao, W.; et al. Oxygen Vacancy Promoted O2 Activation over Perovskite Oxide for Low-Temperature CO Oxidation. ACS Catal. 2019, 11, 9757–9763. [Google Scholar] [CrossRef]
- Fei, Z.; He, S.; Li, L.; Ji, W.; Au, C.-T. Morphology-directed synthesis of Co3O4 nanotubes based on modified Kirkendall effect and its application in CH4 combustion. Chem. Commun. 2012, 48, 853–855. [Google Scholar] [CrossRef] [Green Version]
- Long, R.Q.; Wan, H.L. In situ confocal microprobe Ramanspectroscopy study of CeO2/BaF2 catalyst for theoxidative coupling of methane. J. Chem. Soc. Faraday Trans. 1997, 93, 355–358. [Google Scholar] [CrossRef]
- Xu, J.; Xi, R.; Zhang, Z.; Zhang, Y.; Xu, X.; Fang, X.; Wang, X. Promoting the surface active sites of defect BaSnO3 perovskite with BaBr2 for the oxidative coupling of methane. Catal. Today 2020, 374, 29–37. [Google Scholar] [CrossRef]
- Abd Ghani, N.A.; Azapour, A.; Syed Muhammad, A.F.; Abdullah, B. Dry reforming of methane for hydrogen production over NiCo catalysts: Effect of NbZr promoters. Int. J. Hydrog. Energy 2018, 44, 20881–20888. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Y.; Zou, H.; Guo, X.; Wang, Z.-j. Ni catalysts supported on nanosheet and nanoplate γ-Al2O3 for carbon dioxide methanation. J. Energy Chem. 2017, 29, 3–7. [Google Scholar] [CrossRef] [Green Version]
- Solis-Garcia, A.; Louvier-Hernandez, J.F.; Almendarez-Camarillo, A.; Fierro-Gonzalez, J.C. Participation of surface bicarbonate, formate and methoxy species in the carbon dioxide methanation catalyzed by ZrO2-supported Ni. Appl. Catal. B 2017, 218, 611–620. [Google Scholar] [CrossRef]
- Zhang, S.; Ying, M.; Yu, J.; Zhan, W.; Wang, L.; Guo, Y.; Guo, Y. NixAl1O2-δ mesoporous catalysts for dry reforming of methane: The special role of NiAl2O4 spinel phase and its reaction mechanism. Appl. Catal. B 2021, 291, 120047. [Google Scholar] [CrossRef]
- Cao, T.; You, R.; Zhang, X.; Chen, S.; Li, D.; Zhang, Z.; Huang, W. An in situ DRIFTS mechanistic study of CeO2-catalyzed acetylene semihydrogenation reaction. Phys. Chem. Chem. Phys. 2018, 20, 9659–9670. [Google Scholar] [CrossRef]
- Lavoie, J.-M. Review on dry reforming of methane, a potentially more environmentally-friendly approach to the increasing natural gas exploitation. Front. Chem. 2014, 2, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.; Lu, Y.; Wang, S.; Zhao, Y.; Ma, X. Insight into the reaction mechanism of CO2 activation for CH4 reforming over NiO-MgO: A combination of DRIFTS and DFT study. Appl. Surf. Sci. 2017, 416, 59–68. [Google Scholar] [CrossRef]
- Munera, J.; Irusta, S.; Cornaglia, L.; Lombardo, E.; Vargascesar, D.; Schmal, M. Kinetics and reaction pathway of the CO2 reforming of methane on Rh supported on lanthanum-based solid. J. Catal. 2007, 245, 25–34. [Google Scholar] [CrossRef]











| Catalysts | Rw 450 °C (10−3 mmol·g−1·s−1) [a] | Rs 450 °C (10−4 mmol·m−2·s−1) [b] | TOF 450 °C (10−2·s−1) | Ea (KJ·mol−1) |
|---|---|---|---|---|
| 5Ni/Y2O3 | 3.3 | 0.8 | 9.6 | 102.7 |
| 5Ni/Ce0.1Y0.9Ox | 5.5 | 1.2 | 12.7 | 93.4 |
| 5Ni/Zr0.1Y0.9Ox | 5.2 | 1.0 | 11.3 | 96.0 |
| 5Ni/Al0.1Y0.9Ox | 6.4 | 1.3 | 13.6 | 92.8 |
| Catalysts | Weight Loss of Carbon Deposition (%) | Coking Rate (mg·gcat−1·h−1) [a] | IG/ID |
|---|---|---|---|
| 5Ni/Y2O3 | 11.3 | 2.3 | 1.10 |
| 5Ni/Ce0.1Y0.9Ox | 3.5 | 0.7 | 0.67 |
| 5Ni/Zr0.1Y0.9Ox | 4.8 | 1.0 | 0.97 |
| 5Ni/Al0.1Y0.9Ox | 7.4 | 1.5 | 1.07 |
| Catalysts | Fresh Catalysts | Reduced Catalysts | Used Catalysts | |||
|---|---|---|---|---|---|---|
| Surface Area (m2·g−1) [b] | NiO Crystallite Size (nm) [a] | Surface Area (m2·g−1) | Ni Crystallite Size (nm) | Surface Area (m2·g−1) | Ni Crystallite Size (nm) | |
| 5Ni/Y2O3 | 39.7 | 11.5 | 37.9 | 12.6 | 33.5 | 22.5 |
| 5Ni/Ce0.1Y0.9Ox | 43.4 | 10.3 | 41.1 | 11.0 | 39.5 | 14.5 |
| 5Ni/Zr0.1Y0.9Ox | 43.8 | 10.0 | 42.0 | 10.9 | 39.8 | 15.7 |
| 5Ni/Al0.1Y0.9Ox | 53.1 | 9.2 | 51.3 | 10.2 | 48.3 | 17.3 |
| Catalysts | H2 Consumption (mmol·g−1) | |||
|---|---|---|---|---|
| α Peak | β Peak | Total | H/Ni | |
| 5Ni/Y2O3 | 0.37 | 0.49 | 0.86 | 2.0 |
| 5Ni/Ce0.1Y0.9Ox | 0.16 | 0.71 | 0.87 | 2.0 |
| 5Ni/Zr0.1Y0.9Ox | 0.30 | 0.59 | 0.89 | 2.1 |
| 5Ni/Al0.1Y0.9Ox | 0.27 | 0.60 | 0.87 | 2.0 |
| Catalysts | Ni Content (wt.%) [a] | H2 Desorption (µmol·g−1) | Ni Surface Area (m2·gcat−1) | Ni Surface Area (m2·gNi−1) [b] | Ni Dispersion (%) |
|---|---|---|---|---|---|
| 5Ni/Y2O3 | 4.98 | 31.2 | 1.5 | 30.9 | 3.6 |
| 5Ni/Ce0.1Y0.9Ox | 4.97 | 42.9 | 2.0 | 42.3 | 5.0 |
| 5Ni/Zr0.1Y0.9Ox | 4.97 | 43.2 | 2.1 | 42.8 | 5.1 |
| 5Ni/Al0.1Y0.9Ox | 4.98 | 46.8 | 2.3 | 46.4 | 5.5 |
| Catalysts | O 1s, B.E./FWHM (eV) | Oads/(Oads + Olatt + Ocarb) (%) | O2 Desorption Amount (µmol·g−1) | ||||
|---|---|---|---|---|---|---|---|
| Oads | Ocarb | Olatt | α Peak | β Peak | Total | ||
| 5Ni/Y2O3 | 532.6/1.8 | 530.9/2.0 | 528.6/1.9 | 6.6 | 10.0 | 10.4 | 20.4 |
| 5Ni/Ce0.1Y0.9Ox | 532.7/2.0 | 530.9/2.1 | 528.7/1.8 | 12.0 | 7.3 | 20.0 | 27.3 |
| 5Ni/Zr0.1Y0.9Ox | 532.8/2.0 | 531.0/2.0 | 528.6/1.8 | 10.1 | 10.7 | 14.2 | 24.9 |
| 5Ni/Al0.1Y0.9Ox | 532.8/1.9 | 531.1/2.0 | 528.8/1.8 | 7.7 | 10.3 | 11.8 | 22.1 |
| Catalysts | CO2 Desorption (µmol·g−1) | |||
|---|---|---|---|---|
| Weak | Moderate | Strong | Total | |
| 5Ni/Y2O3 | 17.1 | 14.8 | 5.0 | 36.9 |
| 5Ni/Ce0.1Y0.9Ox | 16.2 | 18.7 | 4.8 | 39.7 |
| 5Ni/Zr0.1Y0.9Ox | 16.0 | 15.1 | 4.6 | 35.7 |
| 5Ni/Al0.1Y0.9Ox | 15.1 | 15.0 | 4.8 | 34.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Mao, L.; Fang, X.; Xu, X.; Xu, J.; Wang, X. Methane Dry Reforming over Ni/NiO Supported on Ce-, Zr-, and Al-Modified Y2O3 for Hydrogen Production. Catalysts 2023, 13, 430. https://doi.org/10.3390/catal13020430
Chen Z, Mao L, Fang X, Xu X, Xu J, Wang X. Methane Dry Reforming over Ni/NiO Supported on Ce-, Zr-, and Al-Modified Y2O3 for Hydrogen Production. Catalysts. 2023; 13(2):430. https://doi.org/10.3390/catal13020430
Chicago/Turabian StyleChen, Zijian, Lei Mao, Xiuzhong Fang, Xianglan Xu, Junwei Xu, and Xiang Wang. 2023. "Methane Dry Reforming over Ni/NiO Supported on Ce-, Zr-, and Al-Modified Y2O3 for Hydrogen Production" Catalysts 13, no. 2: 430. https://doi.org/10.3390/catal13020430