Recent Progress in Surface-Defect Engineering Strategies for Electrocatalysts toward Electrochemical CO2 Reduction: A Review
Abstract
:1. Introduction
2. Electrocatalytic CO2 Reduction Reaction (CO2-RR) Mechanism on Electrode Surface
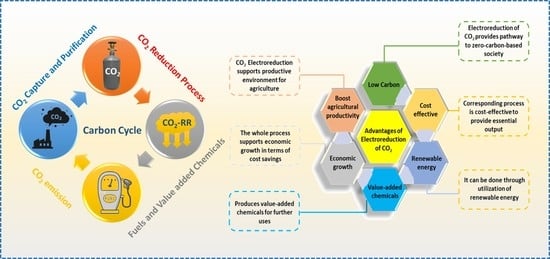
2.1. Advantages and Economic Benefits of Electrochemical CO2 Conversion
2.2. Challenges of CO2 Electroreduction
2.3. Factors Affecting CO2 Electroreduction
3. Effect of Surface Defect Sites on CO2-RR Electrocatalysts
3.1. Single/Bimetallic Materials
| Single and Bimetallic Materials for CO2-RR | |||||
|---|---|---|---|---|---|
| Catalyst | Type of Defect | Electrolyte/E vs. RHE | Product | * FE (%) | Ref. |
| BiOx@C | O2 vacancy | 0.1 M KHCO3/−0.52 V | HCOO− | 89.3 | [116] |
| Bi-few layers | Exfoliation | 0.5 M KHCO3/−0.7 V | HCOO− | 85 | [117] |
| Exfoliated Bi nanosheets | Increased edge sites/defects | 0.5 M KHCO3/−1.1 V | HCOO− | 86 | [118] |
| Bulk Bi nanosheets | 64.9 | ||||
| Sn foil | High native O2 content | 0.1 M KHCO3/−1.36 V | HCOO− | 49.1 | [119] |
| Heat-treated Sn dendrites | 71.6 | ||||
| Few-layer Sb nanosheets | Exfoliation/active edge sites | 0.5 M NaHCO3/−1.07 V | HCOO− | 84 | [120] |
| Cu dendrite | Oxide-derived surface active sites | 1.0 M Na2SO4/−0.8 V | C2H4 | 55 | [121] |
| C2H6 | |||||
| Cu (100) | Crystal orientations/ defects | 0.1 M KHCO3/−5 mA/cm2 | C2H4 | 40.4 | [122] |
| C2H5OH | 9.7 | ||||
| CO | 0.9 | ||||
| CH4 | 30.4 | ||||
| Cu mesocrystals | Facets/edge defects | 0.1 M KHCO3/−0.99 V | C2H4 | 27.2 | [123] |
| HCOO− | 4.3 | ||||
| CO | 0.55 | ||||
| CH4 | 1.47 | ||||
| Bi/Cu | Surface oxide layer | 0.1 M KHCO3/−1.69 V | HCOO− | 91.3 | [124] |
| Pd-Sn | Alloying/tuning surface electronic structures | 0.5 M KHCO3/−0.26 V | HCOO− | 99 | [125] |
| Sn56.3-Pb43.7/ Carbon cloth | Alloying | 0.5 M KHCO3/−2.19 V | HCOO− | 79.8 | [126] |
| Core–shell Ag-Sn NPs | O2 vacant sites | 0.5 M NaHCO3/−0.81 V | HCOO− | 87.2 | [127] |
| Cu/Au | Core–shell structures | 0.5 M KHCO3/−0.65 V | CO | 30 | [128] |
| Pd85Cu15 | Metal doping/alloying | 0.1 M KHCO3/−0.89 V | CO | 86 | [129] |
| Cu38Cd62 | Alloying | 0.05 M KHCO3/−1.12 V | HCOO− | 10 | [130] |
| CO | 43 | ||||
| Cu-In | Alloying/edge sites | 0.1 M KHCO3/−0.7 V | CO | 95 | [131] |
| Ordered Cu-Pd | Alloying/tuned geometric effects | 1.0 M KOH/−0.55 V | CO | 80 | [132] |
| Disordered Cu-Pd | 1.0 M KOH/−0.60 V | CO | 60 | ||
| CH4 | 1.0 | ||||
| C2H4 | 4.0 | ||||
| C2H5OH | 2.0 | ||||
| Phase-separated Cu-Pd | 1.0 M KOH/−0.71 V | CO | 17 | ||
| C2H4 | 48 | ||||
| C2H5OH | 15 | ||||
3.2. Metal Alloys
3.3. Metal Oxides
3.4. Metal Sulfides
3.5. Carbon-Based Materials
3.6. Metal Nitrides
4. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining Theory and Experiment in Electrocatalysis: Insights into Materials Design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef]
- Whipple, D.T.; Kenis, P.J.A. Prospects of CO2 Utilization via Direct Heterogeneous Electrochemical Reduction. J. Phys. Chem. Lett. 2010, 1, 3451–3458. [Google Scholar] [CrossRef]
- Jouny, M.; Luc, W.; Jiao, F. General Techno-Economic Analysis of CO2 Electrolysis Systems. Ind. Eng. Chem. Res. 2018, 57, 2165–2177. [Google Scholar] [CrossRef]
- Wu, B.; Chen, J.; Qian, L. Recent Advances in Heterogeneous Electroreduction of CO2 on Copper-Based Catalysts. Catalysts 2022, 12, 860. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, S.-X.; Gandionco, K.A.; Bond, A.M.; Zhang, J. Electrocatalytic Carbon Dioxide Reduction: From Fundamental Principles to Catalyst Design. Mater. Today Adv. 2020, 7, 100074. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, Y.; Ma, L.; Zhu, G.; Wang, Y.; Xue, X.; Chen, R.; Yang, S.; Jin, Z. Progress and Perspective of Electrocatalytic CO2 Reduction for Renewable Carbonaceous Fuels and Chemicals. Adv. Sci. 2018, 5, 1700275. [Google Scholar] [CrossRef]
- Jin, S.; Hao, Z.; Zhang, K.; Yan, Z.; Chen, J. Advances and Challenges for the Electrochemical Reduction of CO2 to CO: From Fundamentals to Industrialization. Angew. Chem. Int. Ed. 2021, 60, 20627–20648. [Google Scholar] [CrossRef]
- Hoang, V.C.; Gomes, V.G.; Kornienko, N. Metal-Based Nanomaterials for Efficient CO2 Electroreduction: Recent Advances in Mechanism, Material Design and Selectivity. Nano Energy 2020, 78, 105311. [Google Scholar] [CrossRef]
- Jones, J.-P.; Prakash, G.K.S.; Olah, G.A. Electrochemical CO2 Reduction: Recent Advances and Current Trends. Isr. J. Chem. 2014, 54, 1451–1466. [Google Scholar] [CrossRef]
- Tomboc, G.M.; Choi, S.; Kwon, T.; Hwang, Y.J.; Lee, K. Potential Link between Cu Surface and Selective CO2 Electroreduction: Perspective on Future Electrocatalyst Designs. Adv. Mater. 2020, 32, 1908398. [Google Scholar] [CrossRef]
- Han, N.; Ding, P.; He, L.; Li, Y.; Li, Y. Promises of Main Group Metal–Based Nanostructured Materials for Electrochemical CO2 Reduction to Formate. Adv. Energy Mater. 2020, 10, 1902338. [Google Scholar] [CrossRef]
- Hori, Y.; Wakebe, H.; Tsukamoto, T.; Koga, O. Electrocatalytic Process of CO Selectivity in Electrochemical Reduction of CO2 at Metal Electrodes in Aqueous Media. Electrochim. Acta 1994, 39, 1833–1839. [Google Scholar] [CrossRef]
- Feaster, J.T.; Shi, C.; Cave, E.R.; Hatsukade, T.; Abram, D.N.; Kuhl, K.P.; Hahn, C.; Nørskov, J.K.; Jaramillo, T.F. Understanding Selectivity for the Electrochemical Reduction of Carbon Dioxide to Formic Acid and Carbon Monoxide on Metal Electrodes. ACS Catal. 2017, 7, 4822–4827. [Google Scholar] [CrossRef]
- Hori, Y.; Takahashi, R.; Yoshinami, Y.; Murata, A. Electrochemical Reduction of CO at a Copper Electrode. J. Phys. Chem. B 1997, 101, 7075–7081. [Google Scholar] [CrossRef]
- Goodpaster, J.D.; Bell, A.T.; Head-Gordon, M. Identification of Possible Pathways for C–C Bond Formation during Electrochemical Reduction of CO2: New Theoretical Insights from an Improved Electrochemical Model. J. Phys. Chem. Lett. 2016, 7, 1471–1477. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Vasileff, A.; Zhou, X.; Jiao, Y.; Jaroniec, M.; Qiao, S.-Z. Understanding the Roadmap for Electrochemical Reduction of CO2 to Multi-Carbon Oxygenates and Hydrocarbons on Copper-Based Catalysts. J. Am. Chem. Soc. 2019, 141, 7646–7659. [Google Scholar] [CrossRef]
- Garza, A.J.; Bell, A.T.; Head-Gordon, M. Mechanism of CO2 Reduction at Copper Surfaces: Pathways to C2 Products. ACS Catal. 2018, 8, 1490–1499. [Google Scholar] [CrossRef]
- Schouten, K.J.P.; Kwon, Y.; van der Ham, C.J.M.; Qin, Z.; Koper, M.T.M. A New Mechanism for the Selectivity to C1 and C2 Species in the Electrochemical Reduction of Carbon Dioxide on Copper Electrodes. Chem. Sci. 2011, 2, 1902–1909. [Google Scholar] [CrossRef]
- Varandili, S.B.; Stoian, D.; Vavra, J.; Rossi, K.; Pankhurst, J.R.; Guntern, Y.T.; López, N.; Buonsanti, R. Elucidating the Structure-Dependent Selectivity of CuZn towards Methane and Ethanol in CO2 Electroreduction Using Tailored Cu/ZnO Precatalysts. Chem. Sci. 2021, 12, 14484–14493. [Google Scholar] [CrossRef]
- Iyengar, P.; Kolb, M.J.; Pankhurst, J.R.; Calle-Vallejo, F.; Buonsanti, R. Elucidating the Facet-Dependent Selectivity for CO2 Electroreduction to Ethanol of Cu–Ag Tandem Catalysts. ACS Catal. 2021, 11, 4456–4463. [Google Scholar] [CrossRef]
- Pei, Y.; Zhong, H.; Jin, F. A Brief Review of Electrocatalytic Reduction of CO2—Materials, Reaction Conditions, and Devices. Energy Sci. Eng. 2021, 9, 1012–1032. [Google Scholar] [CrossRef]
- Yi, J.; Li, Q.; Chi, S.; Huang, Y.; Cao, R. Boron-Doped Covalent Triazine Framework for Efficient CO2 Electroreduction. Chem. Res. Chin. Univ. 2022, 38, 141–146. [Google Scholar] [CrossRef]
- Costamagna, J.A.; Isaacs, M.; Aguirre, M.J.; Ramírez, G.; Azocar, I. Electroreduction of CO2 Catalyzed by Metallomacrocyclics BT-N4-Macrocyclic Metal Complexes; Zagal, J.H., Bedioui, F., Dodelet, J.-P., Eds.; Springer New York: New York, NY, USA, 2006; pp. 191–254. ISBN 978-0-387-28430-9. [Google Scholar]
- Bevilacqua, M.; Filippi, J.; Miller, H.A.; Vizza, F. Recent Technological Progress in CO2 Electroreduction to Fuels and Energy Carriers in Aqueous Environments. Energy Technol. 2015, 3, 197–210. [Google Scholar] [CrossRef]
- Ye, W.; Guo, X.; Ma, T. A Review on Electrochemical Synthesized Copper-Based Catalysts for Electrochemical Reduction of CO2 to C2+ Products. Chem. Eng. J. 2021, 414, 128825. [Google Scholar] [CrossRef]
- Bao, K.; Shi, J.; Liao, F.; Huang, H.; Liu, Y.; Kang, Z. The Advance and Critical Functions of Energetic Carbon Dots in Carbon Dioxide Photo/Electroreduction Reactions. Small Methods 2022, 6, 2200914. [Google Scholar] [CrossRef]
- Al-Tamreh, S.A.; Ibrahim, M.H.; El-Naas, M.H.; Vaes, J.; Pant, D.; Benamor, A.; Amhamed, A. Electroreduction of Carbon Dioxide into Formate: A Comprehensive Review. ChemElectroChem 2021, 8, 3207–3220. [Google Scholar] [CrossRef]
- Feng, D.-M.; Zhu, Y.-P.; Chen, P.; Ma, T.-Y. Recent Advances in Transition-Metal-Mediated Electrocatalytic CO2 Reduction: From Homogeneous to Heterogeneous Systems. Catalysts 2017, 7, 373. [Google Scholar] [CrossRef]
- Hou, M.; xia Shi, Y.; jun Li, J.; Gao, Z.; Zhang, Z. Cu-Based Organic-Inorganic Composite Materials for Electrochemical CO2 Reduction. Chem. Asian J. 2022, 17, e202200624. [Google Scholar] [CrossRef] [PubMed]
- Lü, F.; Bao, H.; Mi, Y.; Liu, Y.; Sun, J.; Peng, X.; Qiu, Y.; Zhuo, L.; Liu, X.; Luo, J. Electrochemical CO2 Reduction: From Nanoclusters to Single Atom Catalysts. Sustain. Energy Fuels 2020, 4, 1012–1028. [Google Scholar] [CrossRef]
- Wang, G.; Chen, J.; Ding, Y.; Cai, P.; Yi, L.; Li, Y.; Tu, C.; Hou, Y.; Wen, Z.; Dai, L. Electrocatalysis for CO2 Conversion: From Fundamentals to Value-Added Products. Chem. Soc. Rev. 2021, 50, 4993–5061. [Google Scholar] [CrossRef]
- Larrazábal, G.O.; Strøm-Hansen, P.; Heli, J.P.; Zeiter, K.; Therkildsen, K.T.; Chorkendorff, I.; Seger, B. Analysis of Mass Flows and Membrane Cross-over in CO2 Reduction at High Current Densities in an MEA-Type Electrolyzer. ACS Appl. Mater. Interfaces 2019, 11, 41281–41288. [Google Scholar] [CrossRef]
- Gabardo, C.M.; O’Brien, C.P.; Edwards, J.P.; McCallum, C.; Xu, Y.; Dinh, C.-T.; Li, J.; Sargent, E.H.; Sinton, D. Continuous Carbon Dioxide Electroreduction to Concentrated Multi-Carbon Products Using a Membrane Electrode Assembly. Joule 2019, 3, 2777–2791. [Google Scholar] [CrossRef]
- Gawel, A.; Jaster, T.; Siegmund, D.; Holzmann, J.; Lohmann, H.; Klemm, E.; Apfel, U.-P. Electrochemical CO2 Reduction—The Macroscopic World of Electrode Design, Reactor Concepts & Economic Aspects. IScience 2022, 25, 104011. [Google Scholar] [CrossRef]
- Jeanty, P.; Scherer, C.; Magori, E.; Wiesner-Fleischer, K.; Hinrichsen, O.; Fleischer, M. Upscaling and Continuous Operation of Electrochemical CO2 to CO Conversion in Aqueous Solutions on Silver Gas Diffusion Electrodes. J. CO2 Util. 2018, 24, 454–462. [Google Scholar] [CrossRef]
- Verma, S.; Hamasaki, Y.; Kim, C.; Huang, W.; Lu, S.; Jhong, H.-R.M.; Gewirth, A.A.; Fujigaya, T.; Nakashima, N.; Kenis, P.J.A. Insights into the Low Overpotential Electroreduction of CO2 to CO on a Supported Gold Catalyst in an Alkaline Flow Electrolyzer. ACS Energy Lett. 2018, 3, 193–198. [Google Scholar] [CrossRef]
- Oloman, C.; Li, H. Electrochemical Processing of Carbon Dioxide. ChemSusChem 2008, 1, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, K.; Asokan, K.; Jeevarathinam, D.; Chandrasekaran, M. Electrochemical Membrane Reactor for the Reduction of Carbondioxide to Formate. J. Appl. Electrochem. 2007, 37, 255–260. [Google Scholar] [CrossRef]
- Ai, L.; Ng, S.-F.; Ong, W.-J. A Prospective Life Cycle Assessment of Electrochemical CO2 Reduction to Selective Formic Acid and Ethylene. ChemSusChem 2022, 15, e202200857. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.H.; Halim, I.; Handoko, A.D. LCA of Electrochemical Reduction of CO2 to Ethylene. J. CO2 Util. 2020, 41, 101229. [Google Scholar] [CrossRef]
- Yang, H.; Han, N.; Deng, J.; Wu, J.; Wang, Y.; Hu, Y.; Ding, P.; Li, Y.; Li, Y.; Lu, J. Selective CO2 Reduction on 2D Mesoporous Bi Nanosheets. Adv. Energy Mater. 2018, 8, 1801536. [Google Scholar] [CrossRef]
- Gong, Q.; Ding, P.; Xu, M.; Zhu, X.; Wang, M.; Deng, J.; Ma, Q.; Han, N.; Zhu, Y.; Lu, J.; et al. Structural Defects on Converted Bismuth Oxide Nanotubes Enable Highly Active Electrocatalysis of Carbon Dioxide Reduction. Nat. Commun. 2019, 10, 2807. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Wang, Y.; Yang, H.; Deng, J.; Wu, J.; Li, Y.; Li, Y. Ultrathin Bismuth Nanosheets from in Situ Topotactic Transformation for Selective Electrocatalytic CO2 Reduction to Formate. Nat. Commun. 2018, 9, 1320. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Wang, Y.; Ma, L.; Wen, J.; Li, J.; Zheng, H.; Nie, K.; Wang, X.; Zhao, F.; Li, Y.; et al. Supported Cobalt Polyphthalocyanine for High-Performance Electrocatalytic CO2 Reduction. Chem 2017, 3, 652–664. [Google Scholar] [CrossRef]
- Ren, D.; Deng, Y.; Handoko, A.D.; Chen, C.S.; Malkhandi, S.; Yeo, B.S. Selective Electrochemical Reduction of Carbon Dioxide to Ethylene and Ethanol on Copper(I) Oxide Catalysts. ACS Catal. 2015, 5, 2814–2821. [Google Scholar] [CrossRef]
- Reller, C.; Krause, R.; Volkova, E.; Schmid, B.; Neubauer, S.; Rucki, A.; Schuster, M.; Schmid, G. Selective Electroreduction of CO2 toward Ethylene on Nano Dendritic Copper Catalysts at High Current Density. Adv. Energy Mater. 2017, 7, 1602114. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, W.; Fu, J.; Zhang, H.; Wu, G.; Sun, S. Controlled Assembly of Cu Nanoparticles on Pyridinic-N Rich Graphene for Electrochemical Reduction of CO2 to Ethylene. Nano Energy 2016, 24, 1–9. [Google Scholar] [CrossRef]
- Clark, E.L.; Hahn, C.; Jaramillo, T.F.; Bell, A.T. Electrochemical CO2 Reduction over Compressively Strained CuAg Surface Alloys with Enhanced Multi-Carbon Oxygenate Selectivity. J. Am. Chem. Soc. 2017, 139, 15848–15857. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.; Quan, X.; Yu, H. Efficient Electrochemical Reduction of Carbon Dioxide to Acetate on Nitrogen-Doped Nanodiamond. J. Am. Chem. Soc. 2015, 137, 11631–11636. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Dares, C.J.; Marquard, S.L.; Sheridan, M.V.; Meyer, T.J. CO2 Reduction to Acetate in Mixtures of Ultrasmall (Cu)n,(Ag)m Bimetallic Nanoparticles. Proc. Natl. Acad. Sci. USA 2018, 115, 278–283. [Google Scholar] [CrossRef]
- Ren, D.; Wong, N.T.; Handoko, A.D.; Huang, Y.; Yeo, B.S. Mechanistic Insights into the Enhanced Activity and Stability of Agglomerated Cu Nanocrystals for the Electrochemical Reduction of Carbon Dioxide to N-Propanol. J. Phys. Chem. Lett. 2016, 7, 20–24. [Google Scholar] [CrossRef]
- Wang, R.; Kapteijn, F.; Gascon, J. Engineering Metal–Organic Frameworks for the Electrochemical Reduction of CO2: A Minireview. Chem. Asian J. 2019, 14, 3452–3461. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Kas, R.; Smith, W.A.; Burdyny, T. Role of the Carbon-Based Gas Diffusion Layer on Flooding in a Gas Diffusion Electrode Cell for Electrochemical CO2 Reduction. ACS Energy Lett. 2021, 6, 33–40. [Google Scholar] [CrossRef]
- Rabiee, H.; Ge, L.; Zhang, X.; Hu, S.; Li, M.; Yuan, Z. Gas Diffusion Electrodes (GDEs) for Electrochemical Reduction of Carbon Dioxide, Carbon Monoxide, and Dinitrogen to Value-Added Products: A Review. Energy Environ. Sci. 2021, 14, 1959–2008. [Google Scholar] [CrossRef]
- Lu, Q.; Jiao, F. Electrochemical CO2 Reduction: Electrocatalyst, Reaction Mechanism, and Process Engineering. Nano Energy 2016, 29, 439–456. [Google Scholar] [CrossRef]
- Kim, C.; Dionigi, F.; Beermann, V.; Wang, X.; Möller, T.; Strasser, P. Alloy Nanocatalysts for the Electrochemical Oxygen Reduction (ORR) and the Direct Electrochemical Carbon Dioxide Reduction Reaction (CO2RR). Adv. Mater. 2019, 31, 1805617. [Google Scholar] [CrossRef]
- He, J.; Huang, A.; Johnson, N.J.J.; Dettelbach, K.E.; Weekes, D.M.; Cao, Y.; Berlinguette, C.P. Stabilizing Copper for CO2 Reduction in Low-Grade Electrolyte. Inorg. Chem. 2018, 57, 14624–14631. [Google Scholar] [CrossRef] [PubMed]
- Mistry, H.; Varela, A.S.; Kühl, S.; Strasser, P.; Cuenya, B.R. Nanostructured Electrocatalysts with Tunable Activity and Selectivity. Nat. Rev. Mater. 2016, 1, 16009. [Google Scholar] [CrossRef]
- Prabhu, P.; Lee, J.-M. Metallenes as Functional Materials in Electrocatalysis. Chem. Soc. Rev. 2021, 50, 6700–6719. [Google Scholar] [CrossRef]
- Chen, H.; Liang, X.; Liu, Y.; Ai, X.; Asefa, T.; Zou, X. Active Site Engineering in Porous Electrocatalysts. Adv. Mater. 2020, 32, 2002435. [Google Scholar] [CrossRef]
- Rasul, S.; Pugnant, A.; Xiang, H.; Fontmorin, J.-M.; Yu, E.H. Low Cost and Efficient Alloy Electrocatalysts for CO2 Reduction to Formate. J. CO2 Util. 2019, 32, 1–10. [Google Scholar] [CrossRef]
- Huang, S.; Meng, Y.; Cao, Y.; He, S.; Li, X.; Tong, S.; Wu, M. N-, O- and P-Doped Hollow Carbons: Metal-Free Bifunctional Electrocatalysts for Hydrogen Evolution and Oxygen Reduction Reactions. Appl. Catal. B Environ. 2019, 248, 239–248. [Google Scholar] [CrossRef]
- Jia, Y.; Jiang, K.; Wang, H.; Yao, X. The Role of Defect Sites in Nanomaterials for Electrocatalytic Energy Conversion. Chem 2019, 5, 1371–1397. [Google Scholar] [CrossRef]
- Cabrera, C.R.; Abruña, H.D. Electrocatalysis of CO2 Reduction at Surface Modified Metallic and Semiconducting Electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1986, 209, 101–107. [Google Scholar] [CrossRef]
- Sun, S.; Li, H.; Xu, Z.J. Impact of Surface Area in Evaluation of Catalyst Activity. Joule 2018, 2, 1024–1027. [Google Scholar] [CrossRef]
- Guo, S.-X.; Liu, Y.; Lee, C.-Y.; Bond, A.M.; Zhang, J.; Geletii, Y.V.; Hill, C.L. Graphene-Supported [{Ru4O4(OH)2(H2O)4}(γ-SiW10O36)2]10− for Highly Efficient Electrocatalytic Water Oxidation. Energy Environ. Sci. 2013, 6, 2654–2663. [Google Scholar] [CrossRef]
- Dunwell, M.; Yan, Y.; Xu, B. Understanding the Influence of the Electrochemical Double-Layer on Heterogeneous Electrochemical Reactions. Curr. Opin. Chem. Eng. 2018, 20, 151–158. [Google Scholar] [CrossRef]
- Wang, Y.; Han, P.; Lv, X.; Zhang, L.; Zheng, G. Defect and Interface Engineering for Aqueous Electrocatalytic CO2 Reduction. Joule 2018, 2, 2551–2582. [Google Scholar] [CrossRef]
- Lum, Y.; Cheng, T.; Goddard, W.A.I.I.I.; Ager, J.W. Electrochemical CO Reduction Builds Solvent Water into Oxygenate Products. J. Am. Chem. Soc. 2018, 140, 9337–9340. [Google Scholar] [CrossRef]
- Zhu, D.D.; Liu, J.L.; Qiao, S.Z. Recent Advances in Inorganic Heterogeneous Electrocatalysts for Reduction of Carbon Dioxide. Adv. Mater. 2016, 28, 3423–3452. [Google Scholar] [CrossRef] [PubMed]
- Benson, E.E.; Kubiak, C.P.; Sathrum, A.J.; Smieja, J.M. Electrocatalytic and Homogeneous Approaches to Conversion of CO2 to Liquid Fuels. Chem. Soc. Rev. 2009, 38, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Jung, H.; Kim, N.-K.; Oh, H.-S.; Min, B.K.; Hwang, Y.J. Mixed Copper States in Anodized Cu Electrocatalyst for Stable and Selective Ethylene Production from CO2 Reduction. J. Am. Chem. Soc. 2018, 140, 8681–8689. [Google Scholar] [CrossRef]
- Yan, D.; Li, Y.; Huo, J.; Chen, R.; Dai, L.; Wang, S. Defect Chemistry of Nonprecious-Metal Electrocatalysts for Oxygen Reactions. Adv. Mater. 2017, 29, 1606459. [Google Scholar] [CrossRef]
- Jia, J.; Qian, C.; Dong, Y.; Li, Y.F.; Wang, H.; Ghoussoub, M.; Butler, K.T.; Walsh, A.; Ozin, G.A. Heterogeneous Catalytic Hydrogenation of CO2 by Metal Oxides: Defect Engineering—Perfecting Imperfection. Chem. Soc. Rev. 2017, 46, 4631–4644. [Google Scholar] [CrossRef]
- Eckmann, A.; Felten, A.; Mishchenko, A.; Britnell, L.; Krupke, R.; Novoselov, K.S.; Casiraghi, C. Probing the Nature of Defects in Graphene by Raman Spectroscopy. Nano Lett. 2012, 12, 3925–3930. [Google Scholar] [CrossRef]
- Estrade-Szwarckopf, H. XPS Photoemission in Carbonaceous Materials: A “Defect” Peak beside the Graphitic Asymmetric Peak. Carbon 2004, 42, 1713–1721. [Google Scholar] [CrossRef]
- Wang, M.; Árnadóttir, L.; Xu, Z.J.; Feng, Z. In Situ X-Ray Absorption Spectroscopy Studies of Nanoscale Electrocatalysts. Nano-Micro Lett. 2019, 11, 47. [Google Scholar] [CrossRef]
- Stevens, K.T.; Halliburton, L.E.; Setzler, S.D.; Schunemann, P.G.; Pollak, T.M. Electron Paramagnetic Resonance and Electron-Nuclear Double Resonance Study of the Neutral Copper Acceptor in ZnGeP2 Crystals. J. Phys. Condens. Matter 2003, 15, 1625. [Google Scholar] [CrossRef]
- Hemraj-Benny, T.; Banerjee, S.; Sambasivan, S.; Balasubramanian, M.; Fischer, D.A.; Eres, G.; Puretzky, A.A.; Geohegan, D.B.; Lowndes, D.H.; Han, W.; et al. Near-Edge X-Ray Absorption Fine Structure Spectroscopy as a Tool for Investigating Nanomaterials. Small 2006, 2, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Liu, T.; Li, H.; Wei, P.; Song, Y.; Cheng, C.; Gao, D.; Song, Y.; Wang, G.; Bao, X. In Situ Reconstruction of Defect-Rich SnO2 through an Analogous Disproportionation Process for CO2 Electroreduction. Chem. Eng. J. 2022, 446, 137444. [Google Scholar] [CrossRef]
- Domínguez-Gutiérrez, F.J.; Byggmästar, J.; Nordlund, K.; Djurabekova, F.; von Toussaint, U. Computational Study of Crystal Defect Formation in Mo by a Machine Learning Molecular Dynamics Potential. Model. Simul. Mater. Sci. Eng. 2021, 29, 55001. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, Q. Nanocarbon for Oxygen Reduction Electrocatalysis: Dopants, Edges, and Defects. Adv. Mater. 2017, 29, 1604103. [Google Scholar] [CrossRef] [PubMed]
- Moshe, M.; Levin, I.; Aharoni, H.; Kupferman, R.; Sharon, E. Geometry and Mechanics of Two-Dimensional Defects in Amorphous Materials. Proc. Natl. Acad. Sci. USA 2015, 112, 10873–10878. [Google Scholar] [CrossRef]
- Sun, Z.; Ma, T.; Tao, H.; Fan, Q.; Han, B. Fundamentals and Challenges of Electrochemical CO2 Reduction Using Two-Dimensional Materials. Chem 2017, 3, 560–587. [Google Scholar] [CrossRef]
- Sharma, P.P.; Wu, J.; Yadav, R.M.; Liu, M.; Wright, C.J.; Tiwary, C.S.; Yakobson, B.I.; Lou, J.; Ajayan, P.M.; Zhou, X.-D. Nitrogen-Doped Carbon Nanotube Arrays for High-Efficiency Electrochemical Reduction of CO2: On the Understanding of Defects, Defect Density, and Selectivity. Angew. Chem. Int. Ed. 2015, 54, 13701–13705. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Resasco, J.; Yu, Y.; Asiri, A.M.; Yang, P. Synergistic Geometric and Electronic Effects for Electrochemical Reduction of Carbon Dioxide Using Gold–Copper Bimetallic Nanoparticles. Nat. Commun. 2014, 5, 4948. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Pan, Z.; Zhang, L.; Peng, H.; Zheng, G. Selective Etching of Nitrogen-Doped Carbon by Steam for Enhanced Electrochemical CO2 Reduction. Adv. Energy Mater. 2017, 7, 1701456. [Google Scholar] [CrossRef]
- Mistry, H.; Choi, Y.; Bagger, A.; Scholten, F.; Bonifacio, C.S.; Sinev, I.; Divins, N.J.; Zegkinoglou, I.; Jeon, H.S.; Kisslinger, K.; et al. Enhanced Carbon Dioxide Electroreduction to Carbon Monoxide over Defect-Rich Plasma-Activated Silver Catalysts. Angew. Chem. 2017, 129, 11552–11556. [Google Scholar] [CrossRef]
- Yu, X.; Han, P.; Wei, Z.; Huang, L.; Gu, Z.; Peng, S.; Ma, J.; Zheng, G. Boron-Doped Graphene for Electrocatalytic N2 Reduction. Joule 2018, 2, 1610–1622. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, Y.; Zhou, Z.; Cai, F.; Zhao, X.; Huang, W.; Li, Y.; Zhu, J.; Liu, P.; Yang, F.; et al. Enhancing CO2 Electroreduction with the Metal–Oxide Interface. J. Am. Chem. Soc. 2017, 139, 5652–5655. [Google Scholar] [CrossRef]
- Zhang, W.; Jia, B.; Liu, X.; Ma, T. Surface and Interface Chemistry in Metal-Free Electrocatalysts for Electrochemical CO2 Reduction. SmartMat 2022, 3, 5–34. [Google Scholar] [CrossRef]
- Yang, K.D.; Ko, W.R.; Lee, J.H.; Kim, S.J.; Lee, H.; Lee, M.H.; Nam, K.T. Morphology-Directed Selective Production of Ethylene or Ethane from CO2 on a Cu Mesopore Electrode. Angew. Chem. 2017, 129, 814–818. [Google Scholar] [CrossRef]
- Zoubir, O.; Atourki, L.; Ait Ahsaine, H.; BaQais, A. Current State of Copper-Based Bimetallic Materials for Electrochemical CO2 Reduction: A Review. RSC Adv. 2022, 12, 30056–30075. [Google Scholar] [CrossRef]
- Hansen, H.A.; Shi, C.; Lausche, A.C.; Peterson, A.A.; Nørskov, J.K. Bifunctional Alloys for the Electroreduction of CO2 and CO. Phys. Chem. Chem. Phys. 2016, 18, 9194–9201. [Google Scholar] [CrossRef] [PubMed]
- Hirunsit, P.; Soodsawang, W.; Limtrakul, J. CO2 Electrochemical Reduction to Methane and Methanol on Copper-Based Alloys: Theoretical Insight. J. Phys. Chem. C 2015, 119, 8238–8249. [Google Scholar] [CrossRef]
- Kuhl, K.P.; Cave, E.R.; Abram, D.N.; Jaramillo, T.F. New Insights into the Electrochemical Reduction of Carbon Dioxide on Metallic Copper Surfaces. Energy Environ. Sci. 2012, 5, 7050–7059. [Google Scholar] [CrossRef]
- Hori, Y.; Kikuchi, K.; Murata, A.; Suzuki, S. Production of Methane and Ethylene in Electrochemical Reduction of Carbon Dioxide at Copper Electrode in Aqueous Hydrogencarbonate Solution. Chem. Lett. 1986, 15, 897–898. [Google Scholar] [CrossRef]
- Kuhl, K.P.; Hatsukade, T.; Cave, E.R.; Abram, D.N.; Kibsgaard, J.; Jaramillo, T.F. Electrocatalytic Conversion of Carbon Dioxide to Methane and Methanol on Transition Metal Surfaces. J. Am. Chem. Soc. 2014, 136, 14107–14113. [Google Scholar] [CrossRef]
- Ma, M.; Djanashvili, K.; Smith, W.A. Controllable Hydrocarbon Formation from the Electrochemical Reduction of CO2 over Cu Nanowire Arrays. Angew. Chem. Int. Ed. 2016, 55, 6680–6684. [Google Scholar] [CrossRef]
- Sen, S.; Liu, D.; Palmore, G.T.R. Electrochemical Reduction of CO2 at Copper Nanofoams. ACS Catal. 2014, 4, 3091–3095. [Google Scholar] [CrossRef]
- Wang, A.; Li, J.; Zhang, T. Heterogeneous Single-Atom Catalysis. Nat. Rev. Chem. 2018, 2, 65–81. [Google Scholar] [CrossRef]
- Li, M.; Wang, H.; Luo, W.; Sherrell, P.C.; Chen, J.; Yang, J. Heterogeneous Single-Atom Catalysts for Electrochemical CO2 Reduction Reaction. Adv. Mater. 2020, 32, 2001848. [Google Scholar] [CrossRef] [PubMed]
- Pander, J.E.I.I.I.; Baruch, M.F.; Bocarsly, A.B. Probing the Mechanism of Aqueous CO2 Reduction on Post-Transition-Metal Electrodes Using ATR-IR Spectroelectrochemistry. ACS Catal. 2016, 6, 7824–7833. [Google Scholar] [CrossRef]
- Bi, W.; Wu, C.; Xie, Y. Atomically Thin Two-Dimensional Solids: An Emerging Platform for CO2 Electroreduction. ACS Energy Lett. 2018, 3, 624–633. [Google Scholar] [CrossRef]
- Li, F.; MacFarlane, D.R.; Zhang, J. Recent Advances in the Nanoengineering of Electrocatalysts for CO2 Reduction. Nanoscale 2018, 10, 6235–6260. [Google Scholar] [CrossRef]
- Gao, D.; Cai, F.; Wang, G.; Bao, X. Nanostructured Heterogeneous Catalysts for Electrochemical Reduction of CO2. Curr. Opin. Green Sustain. Chem. 2017, 3, 39–44. [Google Scholar] [CrossRef]
- Peterson, A.A.; Abild-Pedersen, F.; Studt, F.; Rossmeisl, J.; Nørskov, J.K. How Copper Catalyzes the Electroreduction of Carbon Dioxide into Hydrocarbon Fuels. Energy Environ. Sci. 2010, 3, 1311–1315. [Google Scholar] [CrossRef]
- Xiang, K.; Zhu, F.; Liu, Y.; Pan, Y.; Wang, X.; Yan, X.; Liu, H. A Strategy to Eliminate Carbon Deposition on a Copper Electrode in Order to Enhance Its Stability in CO2RR Catalysis by Introducing Crystal Defects. Electrochem. Commun. 2019, 102, 72–77. [Google Scholar] [CrossRef]
- Mi, Y.; Shen, S.; Peng, X.; Bao, H.; Liu, X.; Luo, J. Selective Electroreduction of CO2 to C2 Products over Cu3N-Derived Cu Nanowires. ChemElectroChem 2019, 6, 2393–2397. [Google Scholar] [CrossRef]
- Hoang, T.T.H.; Verma, S.; Ma, S.; Fister, T.T.; Timoshenko, J.; Frenkel, A.I.; Kenis, P.J.A.; Gewirth, A.A. Nanoporous Copper–Silver Alloys by Additive-Controlled Electrodeposition for the Selective Electroreduction of CO2 to Ethylene and Ethanol. J. Am. Chem. Soc. 2018, 140, 5791–5797. [Google Scholar] [CrossRef]
- Fu, X.; Zhang, P.; Sun, T.; Xu, L.; Gong, L.; Chen, B.; Xu, Q.; Zheng, T.; Yu, Z.; Chen, X.; et al. Atomically Dispersed Ni–N3 Sites on Highly Defective Micro-Mesoporous Carbon for Superior CO2 Electroreduction. Small 2022, 18, 2107997. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, W.; Ding, L.; Wu, Z.; Gao, F. Au Nanocrystals@Defective Amorphous MnO2 Nanosheets Core/Shell Nanostructure with Effective CO2 Adsorption and Activation toward CO2 Electroreduction to CO. ACS Sustain. Chem. Eng. 2021, 9, 5230–5239. [Google Scholar] [CrossRef]
- Wu, S.; Yi, F.; Ping, D.; Huang, S.; Zhang, Y.; Han, L.; Wang, S.; Wang, H.; Yang, X.; Guo, D.; et al. Constructing Single-Atomic Nickel Sites in Carbon Nanotubes for Efficient CO2 Electroreduction. Carbon 2022, 196, 1–9. [Google Scholar] [CrossRef]
- Ali, S.; Yasin, G.; Iqbal, R.; Huang, X.; Su, J.; Ibraheem, S.; Zhang, Z.; Wu, X.; Wahid, F.; Ismail, P.M.; et al. Porous Aza-Doped Graphene-Analogous 2D Material a Unique Catalyst for CO2 Conversion to Formic-Acid by Hydrogenation and Electroreduction Approaches. Mol. Catal. 2022, 524, 112285. [Google Scholar] [CrossRef]
- Ni, W.; Liu, Z.; Zhang, Y.; Ma, C.; Deng, H.; Zhang, S.; Wang, S. Electroreduction of Carbon Dioxide Driven by the Intrinsic Defects in the Carbon Plane of a Single Fe–N4 Site. Adv. Mater. 2021, 33, 2003238. [Google Scholar] [CrossRef]
- Zhao, X.-H.; Chen, Q.-S.; Zhuo, D.-H.; Lu, J.; Xu, Z.-N.; Wang, C.-M.; Tang, J.-X.; Sun, S.-G.; Guo, G.-C. Oxygen Vacancies Enriched Bi Based Catalysts for Enhancing Electrocatalytic CO2 Reduction to Formate. Electrochim. Acta 2021, 367, 137478. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Ling, Y.; Li, F.; Bond, A.M.; Zhang, J. Controllable Synthesis of Few-Layer Bismuth Subcarbonate by Electrochemical Exfoliation for Enhanced CO2 Reduction Performance. Angew. Chem. 2018, 130, 13467–13471. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, Y.; Ma, L.; Zhu, G.; Zhao, P.; Xue, X.; Chen, R.; Yang, S.; Ma, J.; Liu, J.; et al. Liquid-Phase Exfoliated Ultrathin Bi Nanosheets: Uncovering the Origins of Enhanced Electrocatalytic CO2 Reduction on Two-Dimensional Metal Nanostructure. Nano Energy 2018, 53, 808–816. [Google Scholar] [CrossRef]
- Won, D.H.; Choi, C.H.; Chung, J.; Chung, M.W.; Kim, E.-H.; Woo, S.I. Rational Design of a Hierarchical Tin Dendrite Electrode for Efficient Electrochemical Reduction of CO2. ChemSusChem 2015, 8, 3092–3098. [Google Scholar] [CrossRef]
- Li, F.; Xue, M.; Li, J.; Ma, X.; Chen, L.; Zhang, X.; MacFarlane, D.R.; Zhang, J. Unlocking the Electrocatalytic Activity of Antimony for CO2 Reduction by Two-Dimensional Engineering of the Bulk Material. Angew. Chem. 2017, 129, 14910–14914. [Google Scholar] [CrossRef]
- Dutta, A.; Rahaman, M.; Luedi, N.C.; Mohos, M.; Broekmann, P. Morphology Matters: Tuning the Product Distribution of CO2 Electroreduction on Oxide-Derived Cu Foam Catalysts. ACS Catal. 2016, 6, 3804–3814. [Google Scholar] [CrossRef]
- Hori, Y.; Takahashi, I.; Koga, O.; Hoshi, N. Electrochemical Reduction of Carbon Dioxide at Various Series of Copper Single Crystal Electrodes. J. Mol. Catal. A Chem. 2003, 199, 39–47. [Google Scholar] [CrossRef]
- Chen, C.S.; Handoko, A.D.; Wan, J.H.; Ma, L.; Ren, D.; Yeo, B.S. Stable and Selective Electrochemical Reduction of Carbon Dioxide to Ethylene on Copper Mesocrystals. Catal. Sci. Technol. 2015, 5, 161–168. [Google Scholar] [CrossRef]
- Lv, W.; Zhou, J.; Bei, J.; Zhang, R.; Wang, L.; Xu, Q.; Wang, W. Electrodeposition of Nano-Sized Bismuth on Copper Foil as Electrocatalyst for Reduction of CO2 to Formate. Appl. Surf. Sci. 2017, 393, 191–196. [Google Scholar] [CrossRef]
- Bai, X.; Chen, W.; Zhao, C.; Li, S.; Song, Y.; Ge, R.; Wei, W.; Sun, Y. Exclusive Formation of Formic Acid from CO2 Electroreduction by a Tunable Pd-Sn Alloy. Angew. Chem. Int. Ed. 2017, 56, 12219–12223. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Jeong, S.K.; Kim, H.J.; Baek, I.-H.; Park, K.T. Electrochemical Reduction of Carbon Dioxide to Formate on Tin–Lead Alloys. ACS Sustain. Chem. Eng. 2016, 4, 1311–1318. [Google Scholar] [CrossRef]
- Luc, W.; Collins, C.; Wang, S.; Xin, H.; He, K.; Kang, Y.; Jiao, F. Ag–Sn Bimetallic Catalyst with a Core–Shell Structure for CO2 Reduction. J. Am. Chem. Soc. 2017, 139, 1885–1893. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, X.; Williams, T.; Bourgeois, L.; MacFarlane, D.R. Electrochemical Reduction of CO2 on Core-Shell Cu/Au Nanostructure Arrays for Syngas Production. Electrochim. Acta 2017, 239, 84–89. [Google Scholar] [CrossRef]
- Yin, Z.; Gao, D.; Yao, S.; Zhao, B.; Cai, F.; Lin, L.; Tang, P.; Zhai, P.; Wang, G.; Ma, D.; et al. Highly Selective Palladium-Copper Bimetallic Electrocatalysts for the Electrochemical Reduction of CO2 to CO. Nano Energy 2016, 27, 35–43. [Google Scholar] [CrossRef]
- Watanabe, M.; Shibata, M.; Kato, A.; Azuma, M.; Sakata, T. Design of Alloy Electrocatalysts for CO2 Reduction: III. The Selective and Reversible Reduction of on Cu Alloy Electrodes. J. Electrochem. Soc. 1991, 138, 3382. [Google Scholar] [CrossRef]
- Rasul, S.; Anjum, D.H.; Jedidi, A.; Minenkov, Y.; Cavallo, L.; Takanabe, K. A Highly Selective Copper–Indium Bimetallic Electrocatalyst for the Electrochemical Reduction of Aqueous CO2 to CO. Angew. Chem. Int. Ed. 2015, 54, 2146–2150. [Google Scholar] [CrossRef]
- Ma, S.; Sadakiyo, M.; Heima, M.; Luo, R.; Haasch, R.T.; Gold, J.I.; Yamauchi, M.; Kenis, P.J.A. Electroreduction of Carbon Dioxide to Hydrocarbons Using Bimetallic Cu–Pd Catalysts with Different Mixing Patterns. J. Am. Chem. Soc. 2017, 139, 47–50. [Google Scholar] [CrossRef]
- Xu, Z.; Lai, E.; Shao-Horn, Y.; Hamad-Schifferli, K. Compositional Dependence of the Stability of AuCu Alloy Nanoparticles. Chem. Commun. 2012, 48, 5626–5628. [Google Scholar] [CrossRef]
- Tang, H.; Liu, Y.; Zhou, Y.; Qian, Y.; Lin, B.-L. Boosting the Electroreduction of CO2 to Ethanol via the Synergistic Effect of Cu–Ag Bimetallic Catalysts. ACS Appl. Energy Mater. 2022, 5, 14045–14052. [Google Scholar] [CrossRef]
- Ma, X.; Shen, Y.; Yao, S.; An, C.; Zhang, W.; Zhu, J.; Si, R.; Guo, C.; An, C. Core–Shell Nanoporous AuCu3@Au Monolithic Electrode for Efficient Electrochemical CO2 Reduction. J. Mater. Chem. A 2020, 8, 3344–3350. [Google Scholar] [CrossRef]
- Zhu, S.; Qin, X.; Wang, Q.; Li, T.; Tao, R.; Gu, M.; Shao, M. Composition-Dependent CO2 Electrochemical Reduction Activity and Selectivity on Au–Pd Core–Shell Nanoparticles. J. Mater. Chem. A 2019, 7, 16954–16961. [Google Scholar] [CrossRef]
- Liu, F.; Wu, C.; Yang, S. Strain and Ligand Effects on CO2 Reduction Reactions over Cu–Metal Heterostructure Catalysts. J. Phys. Chem. C 2017, 121, 22139–22146. [Google Scholar] [CrossRef]
- Shan, J.; Sun, K.; Li, H.; Xu, P.; Sun, J.; Wang, Z. Composition Regulation and Defects Introduction via Amorphous CuEu Alloy Shell for Efficient CO2 Electroreduction toward Methane. J. CO2 Util. 2020, 41, 101285. [Google Scholar] [CrossRef]
- Xing, Y.; Kong, X.; Guo, X.; Liu, Y.; Li, Q.; Zhang, Y.; Sheng, Y.; Yang, X.; Geng, Z.; Zeng, J. Bi@Sn Core–Shell Structure with Compressive Strain Boosts the Electroreduction of CO2 into Formic Acid. Adv. Sci. 2020, 7, 1902989. [Google Scholar] [CrossRef]
- Hou, X.; Cai, Y.; Zhang, D.; Li, L.; Zhang, X.; Zhu, Z.; Peng, L.; Liu, Y.; Qiao, J. 3D Core–Shell Porous-Structured Cu@Sn Hybrid Electrodes with Unprecedented Selective CO2-into-Formate Electroreduction Achieving 100%. J. Mater. Chem. A 2019, 7, 3197–3205. [Google Scholar] [CrossRef]
- Feng, X.; Jiang, K.; Fan, S.; Kanan, M.W. Grain-Boundary-Dependent CO2 Electroreduction Activity. J. Am. Chem. Soc. 2015, 137, 4606–4609. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, T.; Liu, B.; Cheng, D.; Hu, C.; Zhang, G.; Zhu, W.; Wang, H.; Zhao, Z.-J.; Gong, J. Grain-Boundary-Rich Copper for Efficient Solar-Driven Electrochemical CO2 Reduction to Ethylene and Ethanol. J. Am. Chem. Soc. 2020, 142, 6878–6883. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, S.; Li, Y.; Liao, X.; Zhao, T.; Cheng, F.; Wang, H. Galvanic-Cell Deposition Enables the Exposure of Bismuth Grain Boundary for Efficient Electroreduction of Carbon Dioxide. Small 2022, 18, 2201633. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, C.W.; Kanan, M.W. Aqueous CO2 Reduction at Very Low Overpotential on Oxide-Derived Au Nanoparticles. J. Am. Chem. Soc. 2012, 134, 19969–19972. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.; Mukhopadhyay, S.; Hod, I. Metal–Organic Framework Derived Nanomaterials for Electrocatalysis: Recent Developments for CO2 and N2 Reduction. Nano Converg. 2021, 8, 1. [Google Scholar] [CrossRef]
- Keerthiga, G.; Chetty, R. Electrochemical Reduction of Carbon Dioxide on Zinc-Modified Copper Electrodes. J. Electrochem. Soc. 2017, 164, H164. [Google Scholar] [CrossRef]
- Gattrell, M.; Gupta, N.; Co, A. A Review of the Aqueous Electrochemical Reduction of CO2 to Hydrocarbons at Copper. J. Electroanal. Chem. 2006, 594, 1–19. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S.; Winè, G.; Gangeri, M. Electrocatalytic Conversion of CO2 to Long Carbon-Chain Hydrocarbons. Green Chem. 2007, 9, 671–678. [Google Scholar] [CrossRef]
- Li, H.; Oloman, C. Development of a Continuous Reactor for the Electro-Reduction of Carbon Dioxide to Formate—Part 2: Scale-Up. J. Appl. Electrochem. 2007, 37, 1107–1117. [Google Scholar] [CrossRef]
- Kwon, Y.; Lee, J. Formic Acid from Carbon Dioxide on Nanolayered Electrocatalyst. Electrocatalysis 2010, 1, 108–115. [Google Scholar] [CrossRef]
- Ye, K.; Zhou, Z.; Shao, J.; Lin, L.; Gao, D.; Ta, N.; Si, R.; Wang, G.; Bao, X. In Situ Reconstruction of a Hierarchical Sn-Cu/SnOx Core/Shell Catalyst for High-Performance CO2 Electroreduction. Angew. Chem. Int. Ed. 2020, 59, 4814–4821. [Google Scholar] [CrossRef]
- Birdja, Y.Y.; Vos, R.E.; Wezendonk, T.A.; Jiang, L.; Kapteijn, F.; Koper, M.T.M. Effects of Substrate and Polymer Encapsulation on CO2 Electroreduction by Immobilized Indium(III) Protoporphyrin. ACS Catal. 2018, 8, 4420–4428. [Google Scholar] [CrossRef]
- Lee, C.H.; Kanan, M.W. Controlling H+ vs. CO2 Reduction Selectivity on Pb Electrodes. ACS Catal. 2015, 5, 465–469. [Google Scholar] [CrossRef]
- Gao, S.; Jiao, X.; Sun, Z.; Zhang, W.; Sun, Y.; Wang, C.; Hu, Q.; Zu, X.; Yang, F.; Yang, S.; et al. Ultrathin Co3O4 Layers Realizing Optimized CO2 Electroreduction to Formate. Angew. Chem. Int. Ed. 2016, 55, 698–702. [Google Scholar] [CrossRef]
- Noda, H.; Ikeda, S.; Oda, Y.; Imai, K.; Maeda, M.; Ito, K. Electrochemical Reduction of Carbon Dioxide at Various Metal Electrodes in Aqueous Potassium Hydrogen Carbonate Solution. Bull. Chem. Soc. Jpn. 1990, 63, 2459–2462. [Google Scholar] [CrossRef]
- Christophe, J.; Doneux, T.; Buess-Herman, C. Electroreduction of Carbon Dioxide on Copper-Based Electrodes: Activity of Copper Single Crystals and Copper–Gold Alloys. Electrocatalysis 2012, 3, 139–146. [Google Scholar] [CrossRef]
- Imani, R.; Qiu, Z.; Younesi, R.; Pazoki, M.; Fernandes, D.L.A.; Mitev, P.D.; Edvinsson, T.; Tian, H. Unravelling In-Situ Formation of Highly Active Mixed Metal Oxide CuInO2 Nanoparticles during CO2 Electroreduction. Nano Energy 2018, 49, 40–50. [Google Scholar] [CrossRef]
- Zhang, S.; Kang, P.; Meyer, T.J. Nanostructured Tin Catalysts for Selective Electrochemical Reduction of Carbon Dioxide to Formate. J. Am. Chem. Soc. 2014, 136, 1734–1737. [Google Scholar] [CrossRef]
- Kumar, B.; Atla, V.; Brian, J.P.; Kumari, S.; Nguyen, T.Q.; Sunkara, M.; Spurgeon, J.M. Reduced SnO2 Porous Nanowires with a High Density of Grain Boundaries as Catalysts for Efficient Electrochemical CO2-into-HCOOH Conversion. Angew. Chem. Int. Ed. 2017, 56, 3645–3649. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chen, L.; Knowles, G.P.; MacFarlane, D.R.; Zhang, J. Hierarchical Mesoporous SnO(2) Nanosheets on Carbon Cloth: A Robust and Flexible Electrocatalyst for CO(2) Reduction with High Efficiency and Selectivity. Angew. Chem. Int. Ed. Engl. 2017, 56, 505–509. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, R.; Wang, H.; Zhang, K.H.L.; Song, Y.; Wu, F.; Fang, F.; Sun, D. Embedding ZnSe Nanodots in Nitrogen-Doped Hollow Carbon Architectures for Superior Lithium Storage. Nano Res. 2018, 11, 966–978. [Google Scholar] [CrossRef]
- Cao, Z.; Yin, Y.; Fu, P.; Li, D.; Zhou, Y.; Deng, Y.; Peng, Y.; Wang, W.; Zhou, W.; Tang, D. TiO2 Nanosheet Arrays with Layered SnS2 and CoOx Nanoparticles for Efficient Photoelectrochemical Water Splitting. Nanoscale Res. Lett. 2019, 14, 342. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, K.; Takahashi, H.; Watters, E.P.J.; Taguchi, M. In-Situ Analysis of CO2 Electroreduction on Pt and Pt Oxide Cathodes. Electrochemistry 2020, 88, 210–217. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Zhang, J.; Wang, Y.; Wu, H.; Zheng, X.; Ding, J.; Han, X.; Deng, Y.; Hu, W. Inversely Tuning the CO2 Electroreduction and Hydrogen Evolution Activity on Metal Oxide via Heteroatom Doping. Angew. Chem. Int. Ed. 2021, 60, 7602–7606. [Google Scholar] [CrossRef]
- Zhong, X.; Liang, S.; Yang, T.; Zeng, G.; Zhong, Z.; Deng, H.; Zhang, L.; Sun, X. Sn Dopants with Synergistic Oxygen Vacancies Boost CO2 Electroreduction on CuO Nanosheets to CO at Low Overpotential. ACS Nano 2022, 16, 19210–19219. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, R.; Shao, Q.; Zhu, T.; Huang, X. Oxygen Vacancies in Amorphous InOx Nanoribbons Enhance CO2 Adsorption and Activation for CO2 Electroreduction. Angew. Chem. Int. Ed. 2019, 58, 5609–5613. [Google Scholar] [CrossRef]
- Sun, S.; Cheng, H.; Li, X.; Wu, X.; Zhen, D.; Wang, Y.; Jin, R.; He, G. Improving CO2 Electroreduction Activity by Creating an Oxygen Vacancy-Rich Surface with One-Dimensional In–SnO2 Hollow Nanofiber Architecture. Ind. Eng. Chem. Res. 2021, 60, 1164–1174. [Google Scholar] [CrossRef]
- Wang, K.; Liu, D.; Liu, L.; Liu, J.; Hu, X.; Li, P.; Li, M.; Vasenko, A.S.; Xiao, C.; Ding, S. Tuning the Local Electronic Structure of Oxygen Vacancies over Copper-Doped Zinc Oxide for Efficient CO2 Electroreduction. EScience 2022, 2, 518–528. [Google Scholar] [CrossRef]
- Geng, Z.; Kong, X.; Chen, W.; Su, H.; Liu, Y.; Cai, F.; Wang, G.; Zeng, J. Oxygen Vacancies in ZnO Nanosheets Enhance CO2 Electrochemical Reduction to CO. Angew. Chem. 2018, 130, 6162–6167. [Google Scholar] [CrossRef]
- Zhang, B.; Chang, Y.; Wu, Y.; Fan, Z.; Zhai, P.; Wang, C.; Gao, J.; Sun, L.; Hou, J. Regulating *OCHO Intermediate as Rate-Determining Step of Defective Oxynitride Nanosheets Enabling Robust CO2 Electroreduction. Adv. Energy Mater. 2022, 12, 2200321. [Google Scholar] [CrossRef]
- Du, X.; Qin, Y.; Gao, B.; Wang, K.; Li, D.; Li, Y.; Ding, S.; Song, Z.; Su, Y.; Xiao, C. Plasma-Assisted and Oxygen Vacancy-Engineered In2O3 Films for Enhanced Electrochemical Reduction of CO2. Appl. Surf. Sci. 2021, 563, 150405. [Google Scholar] [CrossRef]
- Zong, X.; Jin, Y.; Li, Y.; Zhang, X.; Zhang, S.; Xie, H.; Zhang, J.; Xiong, Y. Morphology-Controllable ZnO Catalysts Enriched with Oxygen-Vacancies for Boosting CO2 Electroreduction to CO. J. CO2 Util. 2022, 61, 102051. [Google Scholar] [CrossRef]
- Ren, X.; Gao, Y.; Zheng, L.; Wang, Z.; Wang, P.; Zheng, Z.; Liu, Y.; Cheng, H.; Dai, Y.; Huang, B. Oxygen Vacancy Enhancing CO2 Electrochemical Reduction to CO on Ce-Doped ZnO Catalysts. Surf. Interfaces 2021, 23, 100923. [Google Scholar] [CrossRef]
- Shao, Q.; Wang, P.; Huang, X. Opportunities and Challenges of Interface Engineering in Bimetallic Nanostructure for Enhanced Electrocatalysis. Adv. Funct. Mater. 2019, 29, 1806419. [Google Scholar] [CrossRef]
- Qin, B.; Li, Y.; Wang, H.; Yang, G.; Cao, Y.; Yu, H.; Zhang, Q.; Liang, H.; Peng, F. Efficient Electrochemical Reduction of CO2 into CO Promoted by Sulfur Vacancies. Nano Energy 2019, 60, 43–51. [Google Scholar] [CrossRef]
- Zhang, A.; He, R.; Li, H.; Chen, Y.; Kong, T.; Li, K.; Ju, H.; Zhu, J.; Zhu, W.; Zeng, J. Nickel Doping in Atomically Thin Tin Disulfide Nanosheets Enables Highly Efficient CO2 Reduction. Angew. Chem. Int. Ed. 2018, 57, 10954–10958. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Y.; Zeng, J.; Zhao, X.; Chen, C.; Wu, Q.; Chen, L.; Chen, Z.-Y.; Lei, Y. Bimetallic Chalcogenides for Electrocatalytic CO2 Reduction. Rare Met. 2021, 40, 3442–3453. [Google Scholar] [CrossRef]
- Xing, Z.; Rongjian, S.; Feng, Z.; Yuan, R.; Ruixia, L.; Zhenhai, W.; Ruihu, W. Metal–Organic Framework-Derived CuS Nanocages for Selective CO2 Electroreduction to Formate. CCS Chem. 2021, 3, 199–207. [Google Scholar] [CrossRef]
- Lv, K.; Suo, W.; Shao, M.; Zhu, Y.; Wang, X.; Feng, J.; Fang, M.; Zhu, Y. Nitrogen Doped MoS2 and Nitrogen Doped Carbon Dots Composite Catalyst for Electroreduction CO2 to CO with High Faradaic Efficiency. Nano Energy 2019, 63, 103834. [Google Scholar] [CrossRef]
- He, C.; Chen, S.; Long, R.; Song, L.; Xiong, Y. Design of CuInS2 Hollow Nanostructures toward CO2 Electroreduction. Sci. China Chem. 2020, 63, 1721–1726. [Google Scholar] [CrossRef]
- Zhou, J.-H.; Yuan, K.; Zhou, L.; Guo, Y.; Luo, M.-Y.; Guo, X.-Y.; Meng, Q.-Y.; Zhang, Y.-W. Boosting Electrochemical Reduction of CO2 at a Low Overpotential by Amorphous Ag-Bi-S-O Decorated Bi0 Nanocrystals. Angew. Chem. Int. Ed. 2019, 58, 14197–14201. [Google Scholar] [CrossRef]
- Zhang, A.; Liang, Y.; Li, H.; Zhao, X.; Chen, Y.; Zhang, B.; Zhu, W.; Zeng, J. Harmonizing the Electronic Structures of the Adsorbate and Catalysts for Efficient CO(2) Reduction. Nano Lett. 2019, 19, 6547–6553. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, Y.; Shao, J.; Ye, K.; Wang, Q.; Wang, G. Boosting CO2 Electroreduction via Construction of a Stable ZnS/ZnO Interface. ACS Appl. Mater. Interfaces 2022, 14, 20368–20374. [Google Scholar] [CrossRef]
- Gao, F.-Y.; Hu, S.-J.; Zhang, X.-L.; Zheng, Y.-R.; Wang, H.-J.; Niu, Z.-Z.; Yang, P.-P.; Bao, R.-C.; Ma, T.; Dang, Z.; et al. High-Curvature Transition-Metal Chalcogenide Nanostructures with a Pronounced Proximity Effect Enable Fast and Selective CO2 Electroreduction. Angew. Chem. Int. Ed. 2020, 59, 8706–8712. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, J.; Goodenough, J.B. Comparison of Electrocatalytic Reduction of CO2 to HCOOH with Different Tin Oxides on Carbon Nanotubes. Electrochem. Commun. 2016, 65, 9–13. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, B.; Gao, Y.; Luo, Y.; Zhao, J.; Zhang, Z.; Zhao, C. Oxygen Vacancy and Facet Engineering of Cuprous Oxide by Doping Transition Metal Oxides for Boosting Alcohols Selectivity in Electrochemical CO2 Reduction. J. Power Sources 2023, 556, 232468. [Google Scholar] [CrossRef]
- Li, Q.; Fu, J.; Zhu, W.; Chen, Z.; Shen, B.; Wu, L.; Xi, Z.; Wang, T.; Lu, G.; Zhu, J.; et al. Tuning Sn-Catalysis for Electrochemical Reduction of CO2 to CO via the Core/Shell Cu/SnO2 Structure. J. Am. Chem. Soc. 2017, 139, 4290–4293. [Google Scholar] [CrossRef]
- Hussain, N.; Abdelkareem, M.A.; Alawadhi, H.; Begum, S.; Elsaid, K.; Olabi, A.G. Novel Ternary CuO–ZnO–MoS2 Composite Material for Electrochemical CO2 Reduction to Alcohols. J. Power Sources 2022, 549, 232128. [Google Scholar] [CrossRef]
- Wu, J.; Risalvato, F.G.; Ma, S.; Zhou, X.-D. Electrochemical Reduction of Carbon Dioxide III. The Role of Oxide Layer Thickness on the Performance of Sn Electrode in a Full Electrochemical Cell. J. Mater. Chem. A 2014, 2, 1647–1651. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, L.; Li, L.; Deng, W.; Hu, C.; Zhao, Z.-J.; Gong, J. Abundant Ce3+ Ions in Au-CeOx Nanosheets to Enhance CO2 Electroreduction Performance. Small 2019, 15, 1900289. [Google Scholar] [CrossRef]
- Mi, Y.; Qiu, Y.; Liu, Y.; Peng, X.; Hu, M.; Zhao, S.; Cao, H.; Zhuo, L.; Li, H.; Ren, J.; et al. Cobalt−Iron Oxide Nanosheets for High-Efficiency Solar-Driven CO2−H2O Coupling Electrocatalytic Reactions. Adv. Funct. Mater. 2020, 30, 2003438. [Google Scholar] [CrossRef]
- Wen, G.; Ren, B.; Park, M.G.; Yang, J.; Dou, H.; Zhang, Z.; Deng, Y.-P.; Bai, Z.; Yang, L.; Gostick, J.; et al. Ternary Sn-Ti-O Electrocatalyst Boosts the Stability and Energy Efficiency of CO2 Reduction. Angew. Chem. Int. Ed. 2020, 59, 12860–12867. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-W.; Scholten, F.; Sinev, I.; Roldan Cuenya, B. Enhanced Stability and CO/Formate Selectivity of Plasma-Treated SnOx/AgOx Catalysts during CO2 Electroreduction. J. Am. Chem. Soc. 2019, 141, 5261–5266. [Google Scholar] [CrossRef]
- Xie, H.; Chen, S.; Ma, F.; Liang, J.; Miao, Z.; Wang, T.; Wang, H.-L.; Huang, Y.; Li, Q. Boosting Tunable Syngas Formation via Electrochemical CO2 Reduction on Cu/In2O3 Core/Shell Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 36996–37004. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Gao, J.; Pan, L.; Wang, Z.; Luo, J.; Zakeeruddin, S.M.; Hagfeldt, A.; Grätzel, M. Atomic Layer Deposition of ZnO on CuO Enables Selective and Efficient Electroreduction of Carbon Dioxide to Liquid Fuels. Angew. Chem. Int. Ed. 2019, 58, 15036–15040. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, H.; Guo, X.; Luo, J.; Zakeeruddin, S.M.; Ren, D.; Grätzel, M. Selective C–C Coupling in Carbon Dioxide Electroreduction via Efficient Spillover of Intermediates As Supported by Operando Raman Spectroscopy. J. Am. Chem. Soc. 2019, 141, 18704–18714. [Google Scholar] [CrossRef]
- Zhu, S.; Ren, X.; Li, X.; Niu, X.; Wang, M.; Xu, S.; Wang, Z.; Han, Y.; Wang, Q. Core-Shell ZnO@Cu2O as Catalyst to Enhance the Electrochemical Reduction of Carbon Dioxide to C2 Products. Catalysts 2021, 11, 535. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Han, P.; Du, Y.; Gu, Z.; Xu, X.; Zheng, G. Single-Atomic Cu with Multiple Oxygen Vacancies on Ceria for Electrocatalytic CO2 Reduction to CH4. ACS Catal. 2018, 8, 7113–7119. [Google Scholar] [CrossRef]
- He, R.; Yuan, X.; Shao, P.; Duan, T.; Zhu, W. Hybridization of Defective Tin Disulfide Nanosheets and Silver Nanowires Enables Efficient Electrochemical Reduction of CO2 into Formate and Syngas. Small 2019, 15, 1904882. [Google Scholar] [CrossRef] [PubMed]
- Ning, H.; Fei, X.; Tan, Z.; Wang, W.; Yang, Z.; Wu, M. In Situ-Fabricated In2S3-Reduced Graphene Oxide Nanosheet Composites for Enhanced CO2 Electroreduction to Formate. ACS Appl. Nano Mater. 2022, 5, 2335–2342. [Google Scholar] [CrossRef]
- Liu, F.; Ren, X.; Zhao, J.; Wu, H.; Wang, J.; Han, X.; Deng, Y.; Hu, W. Inhibiting Sulfur Dissolution and Enhancing Activity of SnS for CO2 Electroreduction via Electronic State Modulation. ACS Catal. 2022, 12, 13533–13541. [Google Scholar] [CrossRef]
- Zeng, L.; Shi, J.; Luo, J.; Chen, H. Silver Sulfide Anchored on Reduced Graphene Oxide as a High -Performance Catalyst for CO2 Electroreduction. J. Power Sources 2018, 398, 83–90. [Google Scholar] [CrossRef]
- Li, S.; Duan, H.; Yu, J.; Qiu, C.; Yu, R.; Chen, Y.; Fang, Y.; Cai, X.; Yang, S. Cu Vacancy Induced Product Switching from Formate to CO for CO2 Reduction on Copper Sulfide. ACS Catal. 2022, 12, 9074–9082. [Google Scholar] [CrossRef]
- Cheng, J.; Yang, X.; Xuan, X.; Zhou, J. Efficient Hybrid Solar-to-Alcohol System via Synergistic Catalysis between Well-Defined Cu–N4 Sites and Its Sulfide (CuS). Chem. Eng. J. 2020, 392, 123799. [Google Scholar] [CrossRef]
- Xu, J.; Li, X.; Liu, W.; Sun, Y.; Ju, Z.; Yao, T.; Wang, C.; Ju, H.; Zhu, J.; Wei, S.; et al. Carbon Dioxide Electroreduction into Syngas Boosted by a Partially Delocalized Charge in Molybdenum Sulfide Selenide Alloy Monolayers. Angew. Chem. Int. Ed. 2017, 56, 9121–9125. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chen, L.; Xue, M.; Williams, T.; Zhang, Y.; MacFarlane, D.R.; Zhang, J. Towards a Better Sn: Efficient Electrocatalytic Reduction of CO2 to Formate by Sn/SnS2 Derived from SnS2 Nanosheets. Nano Energy 2017, 31, 270–277. [Google Scholar] [CrossRef]
- Xue, D.; Xia, H.; Yan, W.; Zhang, J.; Mu, S. Defect Engineering on Carbon-Based Catalysts for Electrocatalytic CO2 Reduction. Nano-Micro Lett. 2020, 13, 5. [Google Scholar] [CrossRef]
- Titirici, M.-M.; White, R.J.; Brun, N.; Budarin, V.L.; Su, D.S.; del Monte, F.; Clark, J.H.; MacLachlan, M.J. Sustainable Carbon Materials. Chem. Soc. Rev. 2015, 44, 250–290. [Google Scholar] [CrossRef]
- Jia, Y.; Chen, J.; Yao, X. Defect Electrocatalytic Mechanism: Concept{,} Topological Structure and Perspective. Mater. Chem. Front. 2018, 2, 1250–1268. [Google Scholar] [CrossRef]
- Amiinu, I.S.; Liu, X.; Pu, Z.; Li, W.; Li, Q.; Zhang, J.; Tang, H.; Zhang, H.; Mu, S. From 3D ZIF Nanocrystals to Co–Nx/C Nanorod Array Electrocatalysts for ORR, OER, and Zn–Air Batteries. Adv. Funct. Mater. 2018, 28, 1704638. [Google Scholar] [CrossRef]
- Xie, C.; Yan, D.; Chen, W.; Zou, Y.; Chen, R.; Zang, S.; Wang, Y.; Yao, X.; Wang, S. Insight into the Design of Defect Electrocatalysts: From Electronic Structure to Adsorption Energy. Mater. Today 2019, 31, 47–68. [Google Scholar] [CrossRef]
- Liu, S.; Yang, H.; Su, X.; Ding, J.; Mao, Q.; Huang, Y.; Zhang, T.; Liu, B. Rational Design of Carbon-Based Metal-Free Catalysts for Electrochemical Carbon Dioxide Reduction: A Review. J. Energy Chem. 2019, 36, 95–105. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, C.; Jin, Z.; Wang, D.-W.; Yan, X.; Chen, Z.; Zhu, G.; Yao, X. Carbon for the Oxygen Reduction Reaction: A Defect Mechanism. J. Mater. Chem. A 2015, 3, 11736–11739. [Google Scholar] [CrossRef]
- Todorova, T.K.; Schreiber, M.W.; Fontecave, M. Mechanistic Understanding of CO2 Reduction Reaction (CO2RR) Toward Multicarbon Products by Heterogeneous Copper-Based Catalysts. ACS Catal. 2020, 10, 1754–1768. [Google Scholar] [CrossRef]
- Yang, H.; Lin, Q.; Zhang, C.; Yu, X.; Cheng, Z.; Li, G.; Hu, Q.; Ren, X.; Zhang, Q.; Liu, J.; et al. Carbon Dioxide Electroreduction on Single-Atom Nickel Decorated Carbon Membranes with Industry Compatible Current Densities. Nat. Commun. 2020, 11, 593. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, J.; Shi, J.; Tan, D.; Liu, L.; Zhang, F.; Lu, C.; Su, Z.; Tan, X.; Cheng, X.; et al. Manganese Acting as a High-Performance Heterogeneous Electrocatalyst in Carbon Dioxide Reduction. Nat. Commun. 2019, 10, 2980. [Google Scholar] [CrossRef]
- Wang, W.; Shang, L.; Chang, G.; Yan, C.; Shi, R.; Zhao, Y.; Waterhouse, G.I.N.; Yang, D.; Zhang, T. Intrinsic Carbon-Defect-Driven Electrocatalytic Reduction of Carbon Dioxide. Adv. Mater. 2019, 31, 1808276. [Google Scholar] [CrossRef]
- Liu, S.; Yang, H.; Huang, X.; Liu, L.; Cai, W.; Gao, J.; Li, X.; Zhang, T.; Huang, Y.; Liu, B. Identifying Active Sites of Nitrogen-Doped Carbon Materials for the CO2 Reduction Reaction. Adv. Funct. Mater. 2018, 28, 1800499. [Google Scholar] [CrossRef]
- Ju, W.; Bagger, A.; Hao, G.-P.; Varela, A.S.; Sinev, I.; Bon, V.; Roldan Cuenya, B.; Kaskel, S.; Rossmeisl, J.; Strasser, P. Understanding Activity and Selectivity of Metal-Nitrogen-Doped Carbon Catalysts for Electrochemical Reduction of CO2. Nat. Commun. 2017, 8, 944. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-Doped Carbon Nanotube Arrays with High Electrocatalytic Activity for Oxygen Reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhang, S.; Du, X.; Hong, S.; Zhao, S.; Chen, Y.; Chen, X.; Song, H. Boosting the Electrical Double-Layer Capacitance of Graphene by Self-Doped Defects through Ball-Milling. Adv. Funct. Mater. 2019, 29, 1901127. [Google Scholar] [CrossRef]
- Xue, L.; Li, Y.; Liu, X.; Liu, Q.; Shang, J.; Duan, H.; Dai, L.; Shui, J. Zigzag Carbon as Efficient and Stable Oxygen Reduction Electrocatalyst for Proton Exchange Membrane Fuel Cells. Nat. Commun. 2018, 9, 3819. [Google Scholar] [CrossRef]
- Dou, S.; Tao, L.; Wang, R.; El Hankari, S.; Chen, R.; Wang, S. Plasma-Assisted Synthesis and Surface Modification of Electrode Materials for Renewable Energy. Adv. Mater. 2018, 30, 1705850. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Wang, Q.; Dou, S.; Ma, Z.; Huo, J.; Wang, S.; Dai, L. Edge-Rich and Dopant-Free Graphene as a Highly Efficient Metal-Free Electrocatalyst for the Oxygen Reduction Reaction. Chem. Commun. 2016, 52, 2764–2767. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lei, Y.; Wang, D.; Li, Y. Defect Engineering in Earth-Abundant Electrocatalysts for CO2 and N2 Reduction. Energy Environ. Sci. 2019, 12, 1730–1750. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, Y.; Mei, W.; Zhao, C.; Zhang, C.; Zhang, J.; Amiinu, I.S.; Mu, S. Effects of Intrinsic Pentagon Defects on Electrochemical Reactivity of Carbon Nanomaterials. Angew. Chem. Int. Ed. 2019, 58, 3859–3864. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Long, P.; Feng, Y.; Li, Y. Two-Dimensional Fluorinated Graphene: Synthesis, Structures, Properties and Applications. Adv. Sci. 2016, 3, 1500413. [Google Scholar] [CrossRef]
- Varela, A.S.; Ranjbar Sahraie, N.; Steinberg, J.; Ju, W.; Oh, H.-S.; Strasser, P. Metal-Doped Nitrogenated Carbon as an Efficient Catalyst for Direct CO2 Electroreduction to CO and Hydrocarbons. Angew. Chem. Int. Ed. 2015, 54, 10758–10762. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Mu, S. Defect Engineering in Carbon-Based Electrocatalysts: Insight into Intrinsic Carbon Defects. Adv. Funct. Mater. 2020, 30, 2001097. [Google Scholar] [CrossRef]
- Cheng, W.; Yuan, P.; Lv, Z.; Guo, Y.; Qiao, Y.; Xue, X.; Liu, X.; Bai, W.; Wang, K.; Xu, Q.; et al. Boosting Defective Carbon by Anchoring Well-Defined Atomically Dispersed Metal-N4 Sites for ORR, OER, and Zn-Air Batteries. Appl. Catal. B Environ. 2020, 260, 118198. [Google Scholar] [CrossRef]
- Liu, T.; Ali, S.; Lian, Z.; Si, C.; Su, D.S.; Li, B. Phosphorus-Doped Onion-like Carbon for CO2 Electrochemical Reduction: The Decisive Role of the Bonding Configuration of Phosphorus. J. Mater. Chem. A 2018, 6, 19998–20004. [Google Scholar] [CrossRef]
- Nakata, K.; Ozaki, T.; Terashima, C.; Fujishima, A.; Einaga, Y. High-Yield Electrochemical Production of Formaldehyde from CO2 and Seawater. Angew. Chem. Int. Ed. 2014, 53, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, M.; Sharma, P.P.; Yadav, R.M.; Ma, L.; Yang, Y.; Zou, X.; Zhou, X.-D.; Vajtai, R.; Yakobson, B.I.; et al. Incorporation of Nitrogen Defects for Efficient Reduction of CO2 via Two-Electron Pathway on Three-Dimensional Graphene Foam. Nano Lett. 2016, 16, 466–470. [Google Scholar] [CrossRef]
- Xie, J.; Zhao, X.; Wu, M.; Li, Q.; Wang, Y.; Yao, J. Metal-Free Fluorine-Doped Carbon Electrocatalyst for CO2 Reduction Outcompeting Hydrogen Evolution. Angew. Chem. Int. Ed. 2018, 57, 9640–9644. [Google Scholar] [CrossRef] [PubMed]
- Qin, R.; Liu, P.; Fu, G.; Zheng, N. Strategies for Stabilizing Atomically Dispersed Metal Catalysts. Small Methods 2018, 2, 1700286. [Google Scholar] [CrossRef]
- Huang, F.; Deng, Y.; Chen, Y.; Cai, X.; Peng, M.; Jia, Z.; Xie, J.; Xiao, D.; Wen, X.; Wang, N.; et al. Anchoring Cu1 Species over Nanodiamond-Graphene for Semi-Hydrogenation of Acetylene. Nat. Commun. 2019, 10, 4431. [Google Scholar] [CrossRef]
- Yoo, M.; Yu, Y.-S.; Ha, H.; Lee, S.; Choi, J.-S.; Oh, S.; Kang, E.; Choi, H.; An, H.; Lee, K.-S.; et al. A Tailored Oxide Interface Creates Dense Pt Single-Atom Catalysts with High Catalytic Activity. Energy Environ. Sci. 2020, 13, 1231–1239. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, S.; Zhao, S.; Chen, W.; Dong, J.; Cheong, W.-C.; Shen, R.; Wen, X.; Zheng, L.; Rykov, A.I.; et al. Enhanced Oxygen Reduction with Single-Atomic-Site Iron Catalysts for a Zinc-Air Battery and Hydrogen-Air Fuel Cell. Nat. Commun. 2018, 9, 5422. [Google Scholar] [CrossRef]
- Zhao, C.; Dai, X.; Yao, T.; Chen, W.; Wang, X.; Wang, J.; Yang, J.; Wei, S.; Wu, Y.; Li, Y. Ionic Exchange of Metal–Organic Frameworks to Access Single Nickel Sites for Efficient Electroreduction of CO2. J. Am. Chem. Soc. 2017, 139, 8078–8081. [Google Scholar] [CrossRef]
- Wei, S.; Wang, Y.; Chen, W.; Li, Z.; Cheong, W.-C.; Zhang, Q.; Gong, Y.; Gu, L.; Chen, C.; Wang, D.; et al. Atomically Dispersed Fe Atoms Anchored on COF-Derived N-Doped Carbon Nanospheres as Efficient Multi-Functional Catalysts. Chem. Sci. 2020, 11, 786–790. [Google Scholar] [CrossRef]
- Zhang, S.; Kang, P.; Ubnoske, S.; Brennaman, M.K.; Song, N.; House, R.L.; Glass, J.T.; Meyer, T.J. Polyethylenimine-Enhanced Electrocatalytic Reduction of CO2 to Formate at Nitrogen-Doped Carbon Nanomaterials. J. Am. Chem. Soc. 2014, 136, 7845–7848. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Hou, X.; Ma, C.; Tan, T. Nitrogen-Doped Graphenes as Efficient Electrocatalysts for the Selective Reduction of Carbon Dioxide to Formate in Aqueous Solution. Green Chem. 2016, 18, 3250–3256. [Google Scholar] [CrossRef]
- Chai, G.-L.; Guo, Z.-X. Highly Effective Sites and Selectivity of Nitrogen-Doped Graphene/CNT Catalysts for CO2 Electrochemical Reduction. Chem. Sci. 2016, 7, 1268–1275. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wu, Y.; Lin, Q.; Fan, L.; Chai, X.; Zhang, Q.; Liu, J.; He, C.; Lin, Z. Composition Tailoring via N and S Co-Doping and Structure Tuning by Constructing Hierarchical Pores: Metal-Free Catalysts for High-Performance Electrochemical Reduction of CO2. Angew. Chem. Int. Ed. 2018, 57, 15476–15480. [Google Scholar] [CrossRef]
- Wu, J.; Sharifi, T.; Gao, Y.; Zhang, T.; Ajayan, P.M. Emerging Carbon-Based Heterogeneous Catalysts for Electrochemical Reduction of Carbon Dioxide into Value-Added Chemicals. Adv. Mater. 2019, 31, 1804257. [Google Scholar] [CrossRef]
- Sreekanth, N.; Nazrulla, M.A.; Vineesh, T.V.; Sailaja, K.; Phani, K.L. Metal-Free Boron-Doped Graphene for Selective Electroreduction of Carbon Dioxide to Formic Acid/Formate. Chem. Commun. 2015, 51, 16061–16064. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, T.; Ye, Y.-Q.; Wu, C.-Y.; Xiao, K.; Liu, Z.-Q. Heterostructures Composed of N-Doped Carbon Nanotubes Encapsulating Cobalt and β-Mo2C Nanoparticles as Bifunctional Electrodes for Water Splitting. Angew. Chem. Int. Ed. 2019, 58, 4923–4928. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jin, H.; Meng, T.; Liao, K.; Meng, W.; Yang, J.; He, D.; Xiong, Y.; Mu, S. Fe, Cu-Coordinated ZIF-Derived Carbon Framework for Efficient Oxygen Reduction Reaction and Zinc–Air Batteries. Adv. Funct. Mater. 2018, 28, 1802596. [Google Scholar] [CrossRef]
- Pan, Y.; Lin, R.; Chen, Y.; Liu, S.; Zhu, W.; Cao, X.; Chen, W.; Wu, K.; Cheong, W.-C.; Wang, Y.; et al. Design of Single-Atom Co–N5 Catalytic Site: A Robust Electrocatalyst for CO2 Reduction with Nearly 100% CO Selectivity and Remarkable Stability. J. Am. Chem. Soc. 2018, 140, 4218–4221. [Google Scholar] [CrossRef]
- Yan, C.; Li, H.; Ye, Y.; Wu, H.; Cai, F.; Si, R.; Xiao, J.; Miao, S.; Xie, S.; Yang, F.; et al. Coordinatively Unsaturated Nickel–Nitrogen Sites towards Selective and High-Rate CO2 Electroreduction. Energy Environ. Sci. 2018, 11, 1204–1210. [Google Scholar] [CrossRef]
- Bi, W.; Li, X.; You, R.; Chen, M.; Yuan, R.; Huang, W.; Wu, X.; Chu, W.; Wu, C.; Xie, Y. Surface Immobilization of Transition Metal Ions on Nitrogen-Doped Graphene Realizing High-Efficient and Selective CO2 Reduction. Adv. Mater. 2018, 30, 1706617. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, S.; Li, H.; He, S.; Veder, J.-P.; Johannessen, B.; Xiao, J.; Lu, S.; Pan, J.; Chisholm, M.F.; et al. Unsaturated Edge-Anchored Ni Single Atoms on Porous Microwave Exfoliated Graphene Oxide for Electrochemical CO2. Appl. Catal. B Environ. 2019, 243, 294–303. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Z.; Zhao, X.; Yao, T.; Chen, W.; You, R.; Zhao, C.; Wu, G.; Wang, J.; Huang, W.; et al. Regulation of Coordination Number over Single Co Sites: Triggering the Efficient Electroreduction of CO2. Angew. Chem. 2018, 130, 1962–1966. [Google Scholar] [CrossRef]
- Lu, C.; Yang, J.; Wei, S.; Bi, S.; Xia, Y.; Chen, M.; Hou, Y.; Qiu, M.; Yuan, C.; Su, Y.; et al. Atomic Ni Anchored Covalent Triazine Framework as High Efficient Electrocatalyst for Carbon Dioxide Conversion. Adv. Funct. Mater. 2019, 29, 1806884. [Google Scholar] [CrossRef]
- He, Q.; Lee, J.H.; Liu, D.; Liu, Y.; Lin, Z.; Xie, Z.; Hwang, S.; Kattel, S.; Song, L.; Chen, J.G. Accelerating CO2 Electroreduction to CO Over Pd Single-Atom Catalyst. Adv. Funct. Mater. 2020, 30, 2000407. [Google Scholar] [CrossRef]
- Rogers, C.; Perkins, W.S.; Veber, G.; Williams, T.E.; Cloke, R.R.; Fischer, F.R. Synergistic Enhancement of Electrocatalytic CO2 Reduction with Gold Nanoparticles Embedded in Functional Graphene Nanoribbon Composite Electrodes. J. Am. Chem. Soc. 2017, 139, 4052–4061. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Cheng, K.; Quan, X.; Fan, X.; Su, Y.; Chen, S.; Zhao, H.; Zhang, Y.; Yu, H.; et al. Selective Electrochemical Reduction of Carbon Dioxide to Ethanol on a Boron- and Nitrogen-Co-Doped Nanodiamond. Angew. Chem. Int. Ed. 2017, 56, 15607–15611. [Google Scholar] [CrossRef]
- Ni, W.; Xue, Y.; Zang, X.; Li, C.; Wang, H.; Yang, Z.; Yan, Y.-M. Fluorine Doped Cagelike Carbon Electrocatalyst: An Insight into the Structure-Enhanced CO Selectivity for CO2 Reduction at High Overpotential. ACS Nano 2020, 14, 2014–2023. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, Q.; Tian, Z.; Li, B.; Yan, W.; Wang, S.; Jiang, K.; Su, J.; Oloman, C.W.; Gyenge, E.L.; et al. Ammonia Thermal Treatment toward Topological Defects in Porous Carbon for Enhanced Carbon Dioxide Electroreduction. Adv. Mater. 2020, 32, 2001300. [Google Scholar] [CrossRef]
- Xue, X.; Yang, H.; Yang, T.; Yuan, P.; Li, Q.; Mu, S.; Zheng, X.; Chi, L.; Zhu, J.; Li, Y.; et al. N{,}P-Coordinated Fullerene-like Carbon Nanostructures with Dual Active Centers toward Highly-Efficient Multi-Functional Electrocatalysis for CO2RR{,} ORR and Zn-Air Battery. J. Mater. Chem. A 2019, 7, 15271–15277. [Google Scholar] [CrossRef]
- Wu, Q.; Gao, J.; Feng, J.; Liu, Q.; Zhou, Y.; Zhang, S.; Nie, M.; Liu, Y.; Zhao, J.; Liu, F.; et al. A CO2 Adsorption Dominated Carbon Defect-Based Electrocatalyst for Efficient Carbon Dioxide Reduction. J. Mater. Chem. A 2020, 8, 1205–1211. [Google Scholar] [CrossRef]
- Wu, J.; Yadav, R.M.; Liu, M.; Sharma, P.P.; Tiwary, C.S.; Ma, L.; Zou, X.; Zhou, X.-D.; Yakobson, B.I.; Lou, J.; et al. Achieving Highly Efficient, Selective, and Stable CO2 Reduction on Nitrogen-Doped Carbon Nanotubes. ACS Nano 2015, 9, 5364–5371. [Google Scholar] [CrossRef]
- Ye, L.; Ying, Y.; Sun, D.; Zhang, Z.; Fei, L.; Wen, Z.; Qiao, J.; Huang, H. Highly Efficient Porous Carbon Electrocatalyst with Controllable N-Species Content for Selective CO2 Reduction. Angew. Chem. Int. Ed. 2020, 59, 3244–3251. [Google Scholar] [CrossRef]
- Chen, C.; Sun, X.; Yan, X.; Wu, Y.; Liu, H.; Zhu, Q.; Bediako, B.B.A.; Han, B. Boosting CO2 Electroreduction on N,P-Co-Doped Carbon Aerogels. Angew. Chem. Int. Ed. 2020, 59, 11123–11129. [Google Scholar] [CrossRef]
- Wu, J.; Ma, S.; Sun, J.; Gold, J.I.; Tiwary, C.; Kim, B.; Zhu, L.; Chopra, N.; Odeh, I.N.; Vajtai, R.; et al. A Metal-Free Electrocatalyst for Carbon Dioxide Reduction to Multi-Carbon Hydrocarbons and Oxygenates. Nat. Commun. 2016, 7, 13869. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, L.; Liu, S.; Li, A.; Yuan, X.; Hu, C.; Zhang, G.; Deng, W.; Zang, K.; Luo, J.; et al. Enhanced CO2 Electroreduction on Neighboring Zn/Co Monomers by Electronic Effect. Angew. Chem. Int. Ed. 2020, 59, 12664–12668. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Li, B.; Sarnello, E.; Fei, Y.; Gang, Y.; Xiang, X.; Du, Z.; Zhang, P.; Wang, G.; Nguyen, H.T.; et al. Atomically Dispersed Iron–Nitrogen Sites on Hierarchically Mesoporous Carbon Nanotube and Graphene Nanoribbon Networks for CO2 Reduction. ACS Nano 2020, 14, 5506–5516. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Tan, X.; Yang, W.; Jia, C.; Xu, S.; Wang, K.; Smith, S.C.; Zhao, C. Isolated Diatomic Ni-Fe Metal–Nitrogen Sites for Synergistic Electroreduction of CO2. Angew. Chem. Int. Ed. 2019, 58, 6972–6976. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Jiao, L.; Qian, Y.; Pan, C.; Zheng, L.; Cai, X.; Liu, B.; Yu, S.; Jiang, H. Regulating the Coordination Environment of MOF-Templated Single-Atom Nickel Electrocatalysts for Boosting CO2 Reduction. Angew. Chem. 2020, 132, 2727–2731. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, D.; Biswas, A.N.; Xie, Z.; Hwang, S.; Lee, J.H.; Meng, H.; Chen, J.G. Transition Metal Nitrides as Promising Catalyst Supports for Tuning CO/H2 Syngas Production from Electrochemical CO2 Reduction. Angew. Chem. Int. Ed. 2020, 59, 11345–11348. [Google Scholar] [CrossRef]
- Tang, S.; Zhou, X.; Zhang, S.; Li, X.; Yang, T.; Hu, W.; Jiang, J.; Luo, Y. Metal-Free Boron Nitride Nanoribbon Catalysts for Electrochemical CO2 Reduction: Combining High Activity and Selectivity. ACS Appl. Mater. Interfaces 2019, 11, 906–915. [Google Scholar] [CrossRef]
- Yin, Z.; Yu, C.; Zhao, Z.; Guo, X.; Shen, M.; Li, N.; Muzzio, M.; Li, J.; Liu, H.; Lin, H.; et al. Cu3N Nanocubes for Selective Electrochemical Reduction of CO2 to Ethylene. Nano Lett. 2019, 19, 8658–8663. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.-Q.; Zhuang, T.-T.; Seifitokaldani, A.; Li, J.; Huang, C.-W.; Tan, C.-S.; Li, Y.; De Luna, P.; Dinh, C.T.; Hu, Y.; et al. Copper-on-Nitride Enhances the Stable Electrosynthesis of Multi-Carbon Products from CO2. Nat. Commun. 2018, 9, 3828. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xi, S.; Sun, L.; Dou, S.; Huang, Z.; Su, T.; Wang, X. Isolated FeN4 Sites for Efficient Electrocatalytic CO2 Reduction. Adv. Sci. 2020, 7, 2001545. [Google Scholar] [CrossRef] [PubMed]










| Single Metals and Alloys for CO2-RR | |||||
|---|---|---|---|---|---|
| Catalyst | Type of Defect | Electrolyte/E vs. RHE | Product | FE (%) | Ref. |
| AuNPs/CNT | Metal doping | 0.25 M Na2CO3/−0.5 V | CO | 94 | [142] |
| Cu NPs (solar-driven) | Grain boundary | 1 M KOH/−1.0 V to −1.3 V | C2 | 73.1 | [143] |
| Bi nanodendrites | Low-angle grain boundary | 0.5 M KHCO3/−0.76 V | HCOO− | 92 | [144] |
| CuAg wire | Alloying/intrinsic defects (point, line, and plane) | 1 M KOH/−0.7 V | C2H4 | 60 | [110] |
| C2H5OH | 25 | ||||
| Au-Pd Core–shell | Core–shell structures | 0.5 M KHCO3/−0.6 V | CO | 96.7 | [137] |
| AuCu3@Au | Oxidative etching/alloying | 0.5 M KHCO3/−0.6 V | CO | 97.27 | [136] |
| Cu@CuEu NPs | Point (Eu) defect | 0.5 M KHCO3/−1.2 V | CH4 | 74.7 | [139] |
| Bi@Sn NPs | Compressive strain effect | 0.5 M KHCO3/−1.1 V | HCOOH | 91 | [140] |
| Cu@Sn | Metal doping | 0.5 M KHCO3/−0.93 V | HCOO− | 100 | [141] |
| Metal-Oxide-Based Electrocatalysts | |||||
|---|---|---|---|---|---|
| Catalysts | Type of Defect | Electrolyte/E vs. RHE | Products | * FE% | Ref. |
| SnOx | Thermal treatment/ annealing in O2 atm | 0.1 M KHCO3/−0.76 V | COOH | 64 | [174] |
| Cu2O/ZnO | Oxygen vacancies | 0.5 M KHCO3/−0.3 V | Alcohols | 64.27 | [175] |
| 7.0 nm SnOx layer of Sn nanoparticles | Core–shell structures/ alloying | 0.5 M KHCO3/−0.7 V | CO | 35 | [176] |
| CuO/ZnO/MoS2 | Grain boundaries | 0.5 M KHCO3/−0.6 V | CH3OH | 24.6 | [177] |
| 3.5 nm SnOx layer of Sn nanoparticles | Layer thickness | 0.1 M KHCO3/−1.2 V | COOH | 64 | [178] |
| Sn-Cu/SnOx | Core–shell structures | 1 M KOH/−0.7 V | COOH | 98 | [152] |
| Au-CeOx nanosheet | Metal doping | 0.1 M KHCO3/−0.5 V | CO | 90.1 | [179] |
| Co2FeO4 nanosheets | Metal (Co) incorporation | 0.1M KHCO3/−1.0 V | CO | 92 | [180] |
| Sn-Ti–O | O2 defect sites | 0.5M KHCO3/−0.54 V | CO | 94.5 | [181] |
| SnOx/AgOx | Plasma oxidation | 0.1M KHCO3/−0.80 V | C1 | 91 | [182] |
| Cu/In2O3 | Core–shell structures | 0.5M KHCO3/−0.40 V to −0.90 V | Syngas | 90 | [183] |
| ZnO shell/CuO core | Core–shell structures | 1M KOH/−0.68 V | CH3CH=OH | 49 | [184] |
| Cu2O-Ag | Grain boundaries | 0.2M KHCO3/−0.60 V −1.2 V | C2H4 | 52 | [185] |
| In-doped Cu@Cu2O | Metal doping/core–shell structures | 0.1M KHCO3/−0.45 V to −0.84 V | CO | 2.2 | [186] |
| Cu-CeO2–4% | O2 vacancy | 0.1M KHCO3/−1.8 V | CH4 | 58 | [187] |
| Ag-Bi-S–O-decorated BiO | Bimetal (Ag, Bi) doping/decoration | 0.5M KHCO3/−0.45 V | HCOOH | 94.3 | [188] |
| Metal-Sulfide-Based Electrocatalysts | |||||
|---|---|---|---|---|---|
| Catalysts | Type of Defect | Electrolyte/ E vs. RHE | Products | * FE% | Ref. |
| Ag-SnS2 | Chemical-induced defective sites | 0.5M KHCO3/−1.0 V | HCOOH | 65.5 | [199] |
| 5%Ni-SnS2 | Metal (Ni) doping | KHCO3/−0.9 V | CO | 93 | [191] |
| Mn-In2S3 | Metal (Mn) doping | 0.1M KHCO3/−0.9 V | HCOOH | 86 | [196] |
| Nitrogen-doped MoS2 | The heteroatom (N) doping | EMIM-BF4/−0.9 V | CO | 90.2 | [194] |
| ZnS/ZnO | Oxide–sulfide interface | 1M KOH/−0.73 V | CO | 91.9 | [197] |
| In2S3-rGO | Layer thickness | 0.1M KHCO3/−1.2 V | - | 91% | [200] |
| SnS | The heteroatom (In) doping | 1M KOH/−0.60 V | HCOOH | 96.6 | [201] |
| Ag2S/N, S-rGO | Heteroatom (N, S) co-doping | 0.1M KHCO3/−0.76 V | CO | 87.4 | [202] |
| Lattice defect/metal (Cu) vacancy | 0.5M KHCO3/−0.84 V | COOH | - | [203] | |
| CuS/CuPor | Surface structural defects | 0.5M NaHCO3/−2.0 V | C2H5OH | 74.4 | [204] |
| MoSeS | Alloying (TMD alloy) | −/−1.15 V | CO | 45 | [205] |
| SnS2/rGO | O2 defects | 0.5M NaHCO3/−0.75 V | CO | 84.5 | [206] |
| Carbon-Based Nanomaterials for CO2-RR | |||||
|---|---|---|---|---|---|
| Catalyst | Type of Defect | Electrolyte/E vs. RHE | Product | * FE (%) | Ref. |
| GNR AuNPs | Metal doping | 0.5M KHCO3/−0.2 V | CO | >90 | [255] |
| BND | B, N co-doping | 0.5M KHCO3/−1.0 V | C2H5OH | 93.2 | [256] |
| B-graphene | B doping | 0.1M KHCO3/−1.1 V | HCOOH | - | [245] |
| N-graphene | N doping | 0.5M KHCO3/- | HCOO− | - | [242] |
| F-CPC | F doping | 0.5M KHCO3/−1.0 V | CO | 88.3 | [257] |
| DPC-NH3-950 | Thermal N removal, topological effects | 0.1M KHCO3/−0.6 V | CO | 95.2 | [258] |
| N-GRW | N doping | 0.5M KHCO3/−0.49 V | CO | 87.6 | [239] |
| NCNTs-ACN-850 | N doping | 0.1M KHCO3/−1.05 V | CO | 80 | [85] |
| P-OLC | P doping | 0.5M KHCO3/−0.9 V | CO | 81 | [228] |
| FC | F doping | 0.1M KHCO3/−1.22 V | CO | 93.1 | [259] |
| Ni-SAs/N-C | Metal (Ni) doping | 0.5M KHCO3/−1.0 V | CO | 71.9 | [236] |
| N, P-FC | N, P co-doping | 0.5M KHCO3/−0.8 V | CO | 83.3 | [260] |
| D-NC-1100 | Heteroatom doping, porous carbon | 0.1M KHCO3/−0.6 V | CO | 94.5 | [238] |
| DHPC | Thermal treatment | 0.5M KHCO3/−0.5 V | CO | 99.5 | [261] |
| NG-800 | N doping | 0.1M KHCO3/−0.58 V | CO | 85 | [230] |
| NCNTs | N doping | 0.1M KHCO3/−0.78 V | CO | 80 | [262] |
| NPC-1000 | N doping | 0.5M KHCO3/−0.55 V | CO | 98.4 | [263] |
| NDD | N doping | 0.5M NaHCO3/−1.0 V | Acetate | 91.8 | [49] |
| NPCA | N, P co-doping | 0.5M KHCO3/−2.49 V | CO | 99.1 | [264] |
| NGQDs | N doping | 1M KOH/−0.75 V | (Total) | 90 | [265] |
| −0.75 V | C2H4 | 31 | |||
| −0.86 V | CH4 | 15 | |||
| −0.74 V | C2H5OH | 11.8 | |||
| Co-N5/HNPCSs | N doping | 0.2M NaHCO3/−0.79 V | CO | 99.4 | [248] |
| Ni-N-C | Metal (Ni, Fe) and N co-doping | 0.1M KHCO3/−0.78 V | CO | 85 | [240] |
| Fe-N-C | −0.55V | 65 | |||
| Co-N2 | Metal (Co) and N co-doping | 0.5M KHCO3/−0.63 V | CO | 94 | [252] |
| Ni-N-MPGO | Metal (Ni) doping | 0.5M KHCO3/−0.70 V | CO | 92.1 | [251] |
| Zn/Co-N-C | N doping | 0.5M KHCO3/−0.50 V | CO | 93.2 | [266] |
| Fe-N/CNT@GNR | Fe, N co-doping | 0.5M KHCO3/−0.76 V | CO | 96 | [267] |
| C-Zn1Ni4ZIF-8 | Ni, N co-doping | 0.5M KHCO3/−1.03 V | CO | 98 | [249] |
| Ni/Fe-NC | Ni, Fe, and N doping | 0.5M KHCO3/−0.70 V | CO | 98 | [268] |
| NiSA-N2-C | Metal (Ni) doping | 0.5M KHCO3/−0.80 V | CO | 98 | [269] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balu, S.; Hanan, A.; Venkatesvaran, H.; Chen, S.-W.; Yang, T.C.-K.; Khalid, M. Recent Progress in Surface-Defect Engineering Strategies for Electrocatalysts toward Electrochemical CO2 Reduction: A Review. Catalysts 2023, 13, 393. https://doi.org/10.3390/catal13020393
Balu S, Hanan A, Venkatesvaran H, Chen S-W, Yang TC-K, Khalid M. Recent Progress in Surface-Defect Engineering Strategies for Electrocatalysts toward Electrochemical CO2 Reduction: A Review. Catalysts. 2023; 13(2):393. https://doi.org/10.3390/catal13020393
Chicago/Turabian StyleBalu, Sridharan, Abdul Hanan, Harikrishnan Venkatesvaran, Shih-Wen Chen, Thomas C.-K. Yang, and Mohammad Khalid. 2023. "Recent Progress in Surface-Defect Engineering Strategies for Electrocatalysts toward Electrochemical CO2 Reduction: A Review" Catalysts 13, no. 2: 393. https://doi.org/10.3390/catal13020393