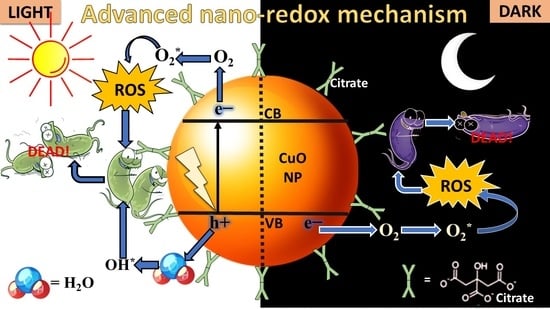
Development of Nanomedicine from Copper Mine Tailing Waste: A Pavement towards Circular Economy with Advanced Redox Nanotechnology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Functionalized CuO Nanoparticles
2.3. Characterization Tools and Techniques
2.4. Antioxidant Activity
2.5. Quantification and Characterization of ROS
2.6. Bacterial Strain and Culture Conditions
2.7. Statistical Analysis
2.8. Method of Computational Biology
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borkow, G.; Gabbay, J. Copper, an ancient remedy returning to fight microbial, fungal and viral infections. Curr. Chem. Biol. 2009, 3, 272–278. [Google Scholar]
- Dollwet, H. Historic uses of copper compounds in medicine. Trace Elem. Med. 1985, 2, 80–87. [Google Scholar]
- Council, N.R. Copper in Drinking Water; National Academies Press: Washington, DC, USA, 2000. [Google Scholar]
- Giannousi, K.; Sarafidis, G.; Mourdikoudis, S.; Pantazaki, A.; Dendrinou-Samara, C. Selective synthesis of Cu2O and Cu/Cu2O NPs: Antifungal activity to yeast Saccharomyces cerevisiae and DNA interaction. Inorg. Chem. 2014, 53, 9657–9666. [Google Scholar] [CrossRef] [PubMed]
- Gandhare, N.V.; Chaudhary, R.G.; Meshram, V.P.; Tanna, J.A.; Lade, S.; Gharpure, M.P.; Juneja, H.D. An efficient and one-pot synthesis of 2,4,5-trisubstituted imidazole compounds catalyzed by copper nanoparticles. J. Chin. Adv. Mater. Soc. 2015, 3, 270–279. [Google Scholar] [CrossRef]
- Tanna, J.A.; Chaudhary, R.G.; Sonkusare, V.N.; Juneja, H.D. CuO nanoparticles: Synthesis, characterization and reusable catalyst for polyhydroquinoline derivatives under ultrasonication. J. Chin. Adv. Mater. Soc. 2016, 4, 110–122. [Google Scholar] [CrossRef]
- Sun, H.; Kim, H.; Song, S.; Jung, W. Copper foam-derived electrodes as efficient electrocatalysts for conventional and hybrid water electrolysis. Mater. Rep. Energy 2022, 2, 100092. [Google Scholar] [CrossRef]
- Han, X.; Liu, P.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z. Non-metal fluorine doping in Ruddlesden–Popper perovskite oxide enables high-efficiency photocatalytic water splitting for hydrogen production. Mater. Today Energy 2022, 23, 100896. [Google Scholar] [CrossRef]
- Kuang, P.; Ni, Z.; Yu, J.; Low, J. New progress on MXenes-based nanocomposite photocatalysts. Mater. Rep. Energy 2022, 2, 100081. [Google Scholar] [CrossRef]
- He, J.; Liu, P.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z. Single-atom catalysts for high-efficiency photocatalytic and photoelectrochemical water splitting: Distinctive roles, unique fabrication methods and specific design strategies. J. Mater. Chem. A 2022, 10, 6835–6871. [Google Scholar] [CrossRef]
- Ssekatawa, K.; Byarugaba, D.K.; Angwe, M.K.; Wampande, E.M.; Ejobi, F.; Nxumalo, E.; Kirabira, J.B. Phyto-Mediated Copper Oxide Nanoparticles for Antibacterial, Antioxidant and Photocatalytic Performances. Front. Bioeng. Biotechnol. 2022, 10, 820218. [Google Scholar] [CrossRef]
- Palmer, A.; Kishony, R. Understanding, predicting and manipulating the genotypic evolution of antibiotic resistance. Nat. Rev. Genet. 2013, 14, 243–248. [Google Scholar] [CrossRef]
- Chaudhary, R.G.; Sonkusare, V.N.; Bhusari, G.S.; Mondal, A.; Shaik, D.P.; Juneja, H.D. Microwave-mediated synthesis of spinel CuAl2O4 nanocomposites for enhanced electrochemical and catalytic performance. Res. Chem. Intermed. 2018, 44, 2039–2060. [Google Scholar] [CrossRef]
- Kaweeteerawat, C.; Chang, C.H.; Roy, K.R.; Liu, R.; Li, R.; Toso, D.; Fischer, H.; Ivask, A.; Ji, Z.; Zink, J.I.; et al. Cu Nanoparticles Have Different Impacts in Escherichia coli and Lactobacillus brevis than Their Microsized and Ionic Analogues. ACS Nano 2015, 9, 7215–7225. [Google Scholar] [CrossRef]
- Bogdanović, U.; Vodnik, V.; Mitrić, M.; Dimitrijević, S.; Škapin, S.D.; Žunič, V.; Budimir, M.; Stoiljković, M. Nanomaterial with High Antimicrobial Efficacy—Copper/Polyaniline Nanocomposite. ACS Appl. Mater. Interfaces 2015, 7, 1955–1966. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, Y.-H.; Tsai, P.-H.; Lin, K.-S. pH-dependent antimicrobial properties of copper oxide nanoparticles in Staphylococcus aureus. Int. J. Mol. Sci. 2017, 18, 793. [Google Scholar] [CrossRef] [PubMed]
- Nishino, F.; Jeem, M.; Zhang, L.; Okamoto, K.; Okabe, S.; Watanabe, S. Formation of CuO nano-flowered surfaces via submerged photo-synthesis of crystallites and their antimicrobial activity. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Weaver, L.; Noyce, J.; Michels, H.; Keevil, C. Potential action of copper surfaces on meticillin-resistant Staphylococcus aureus. J. Appl. Microbiol. 2010, 109, 2200–2205. [Google Scholar] [CrossRef]
- Jadhav, S.; Gaikwad, S.; Nimse, M.; Rajbhoj, A. Copper oxide nanoparticles: Synthesis, characterization and their antibacterial activity. J. Clust. Sci. 2011, 22, 121–129. [Google Scholar] [CrossRef]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef]
- Zhu, Z.; Wan, S.; Zhao, Y.; Gu, Y.; Wang, Y.; Qin, Y.; Bu, Y. Recent advances in bismuth-based multimetal oxide photocatalysts for hydrogen production from water splitting: Competitiveness, challenges, and future perspectives. Mater. Rep. Energy 2021, 1, 100019. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, P.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. Enhancing the photocatalytic activity of Ruddlesden-Popper Sr2TiO4 for hydrogen evolution through synergistic silver doping and moderate reducing pretreatment. Mater. Today Energy 2022, 23, 100899. [Google Scholar] [CrossRef]
- McDonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef]
- Pang, H.; Gao, F.; Lu, Q. Morphology effect on antibacterial activity of cuprous oxide. Chem. Commun. 2009, 9, 1076–1078. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Hu, D.; Cheng, E.W.; Vargas-Reus, M.A.; Reip, P.; Allaker, R.P. Characterisation of copper oxide nanoparticles for antimicrobial applications. Int. J. Antimicrob. Agents 2009, 33, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Woźniak-Budych, M.J.; Przysiecka, Ł.; Maciejewska, B.M.; Wieczorek, D.; Staszak, K.; Jarek, M.; Jesionowski, T.; Jurga, S. Facile Synthesis of Sulfobetaine-Stabilized Cu2O Nanoparticles and Their Biomedical Potential. ACS Biomater. Sci. Eng. 2017, 3, 3183–3194. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xiang, H.; Zabihi, F.; Yu, S.; Sun, B.; Zhu, M. Intriguing anti-superbug Cu2O@ ZrP hybrid nanosheet with enhanced antibacterial performance and weak cytotoxicity. Nano Res. 2019, 12, 1453–1460. [Google Scholar] [CrossRef]
- Hotze, E.M.; Phenrat, T.; Lowry, G.V. Nanoparticle Aggregation: Challenges to Understanding Transport and Reactivity in the Environment. J. Environ. Qual. 2010, 39, 1909–1924. [Google Scholar] [CrossRef]
- Mondal, S.; Ghosh, R.; Adhikari, A.; Pal, U.; Mukherjee, D.; Biswas, P.; Darbar, S.; Singh, S.; Bose, S.; Saha-Dasgupta, T.; et al. In vitro and Microbiological Assay of Functionalized Hybrid Nanomaterials To Validate Their Efficacy in Nanotheranostics: A Combined Spectroscopic and Computational Study. Chemmedchem 2021, 16, 3739–3749. [Google Scholar] [CrossRef]
- Zhang, L.; Thomas, J.C.; Miragaia, M.; Bouchami, O.; Chaves, F.; d’Azevedo, P.A.; Robinson, D.A. Multilocus sequence typing and further genetic characterization of the enigmatic pathogen, Staphylococcus hominis. PLoS ONE 2013, 8, e66496. [Google Scholar] [CrossRef]
- Kim, S.-D.; McDonald, L.C.; Jarvis, W.R.; McAllister, S.K.; Jerris, R.; Carson, L.A.; Miller, J.M. Determining the Significance of Coagulase-Negative Staphylococci Isolated From Blood Cultures at a Community Hospital A Role for Species and Strain Identification. Infect. Control. Hosp. Epidemiology 2000, 21, 213–217. [Google Scholar] [CrossRef]
- Iyer, M.N.; Wirostko, W.J.; Kim, S.H.; Simons, K.B. Staphylococcus hominis Endophthalmitis Associated With a Capsular Hypopyon. Am. J. Ophthalmol. 2005, 139, 930–932. [Google Scholar] [CrossRef]
- Lam, T.H.; Verzotto, D.; Brahma, P.; Ng, A.H.Q.; Hu, P.; Schnell, D.; Tiesman, J.; Kong, R.; Ton, T.M.U.; Li, J.; et al. Understanding the microbial basis of body odor in pre-pubescent children and teenagers. Microbiome 2018, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, D.; Chen, H.; Yang, X.; Lu, L.; Wang, X. Highly dispersed CuO nanoparticles prepared by a novel quick-precipitation method. Mater. Lett. 2004, 58, 3324–3327. [Google Scholar] [CrossRef]
- Mallakpour, S.; Dinari, M.; Azadi, E. Grafting of Citric Acid as a Green Coupling Agent on the Surface of CuO Nanoparticle and its Application for Synthesis and Characterization of Novel Nanocomposites Based on Poly(amide-imide) ContainingN-trimellitylimido-L-valine Linkage. Polym. Technol. Eng. 2015, 54, 594–602. [Google Scholar] [CrossRef]
- Nawaz, A.; Goudarzi, S.; Asghari, M.A.; Pichiah, S.; Selopal, G.S.; Rosei, F.; Wang, Z.M.; Zarrin, H. Review of Hybrid 1D/2D Photocatalysts for Light-Harvesting Applications. ACS Appl. Nano Mater. 2021, 4, 11323–11352. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Hasan, N.; Bagchi, D.; Altass, H.M.; Morad, M.; Althagafi, I.I.; Hameed, A.M.; Sayqal, A.; Khder, A.E.R.S.; Asghar, B.H.; et al. Nano-MOFs as targeted drug delivery agents to combat antibiotic-resistant bacterial infections. R. Soc. Open Sci. 2020, 7, 200959. [Google Scholar] [CrossRef] [PubMed]
- Tinajero-Díaz, E.; Salado-Leza, D.; Gonzalez, C.; Martínez Velázquez, M.; López, Z.; Bravo-Madrigal, J.; Hernández-Gutiérrez, R. Green metallic nanoparticles for cancer therapy: Evaluation models and cancer applications. Pharmaceutics 2021, 13, 1719. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Adhikari, A.; Ghosh, R.; Singh, M.; Das, M.; Darbar, S.; Bhattacharya, S.S.; Pal, D.; Pal, S.K. Synthesis and spectroscopic characterization of a target-specific nanohybrid for redox buffering in cellular milieu. MRS Adv. 2021, 6, 427–433. [Google Scholar] [CrossRef]
- Polley, N.; Saha, S.; Adhikari, A.; Banerjee, S.; Darbar, S.; Das, S.; Pal, S.K. Safe and symptomatic medicinal use of surface-functionalized Mn3O4 nanoparticles for hyperbilirubinemia treatment in mice. Nanomedicine 2015, 10, 2349–2363. [Google Scholar] [CrossRef]
- Bera, A.; Hasan, N.; Pal, U.; Bagchi, D.; Maji, T.K.; Saha-Dasgupta, T.; Das, R.; Pal, S.K. Fabrication of nanohybrids toward improving therapeutic potential of a NIR photo-sensitizer: An optical spectroscopic and computational study. J. Photochem. Photobiol. A Chem. 2022, 424, 113610. [Google Scholar] [CrossRef]
- Hasan, N.; Bera, A.; Maji, T.K.; Mukherjee, D.; Pan, N.; Karmakar, D.; Pal, S.K. Functionalized nano-MOF for NIR induced bacterial remediation: A combined spectroscopic and computational study. Inorganica Chim. Acta 2022, 532, 120733. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Santos, A.; Von Mering, C.; Jensen, L.J.; Bork, P.; Kuhn, M. STITCH 5: Augmenting protein–chemical interaction networks with tissue and affinity data. Nucleic Acids Res. 2016, 44, D380–D384. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, M.; Szklarczyk, D.; Pletscher-Frankild, S.; Blicher, T.H.; von Mering, C.; Jensen, L.J.; Bork, P. STITCH 4: Integration of protein–chemical interactions with user data. Nucleic Acids Res. 2013, 42, D401–D407. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, X.-L.; Shu, C.-Y.; Guo, Y.-G.; Wang, C.-R. Synthesis of CuO/graphene nanocomposite as a high-performance anode material for lithium-ion batteries. J. Mater. Chem. 2010, 20, 10661–10664. [Google Scholar] [CrossRef]
- Asbrink, S.; Waskowska, A. CuO: X-ray single-crystal structure determination at 196 K and room temperature. J. Physics Condens. Matter 1991, 3, 8173–8180. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Ahamed, M.; Alhadlaq, H.A.; Khan, M.A.M.; Karuppiah, P.; Al-Dhabi, N.A. Synthesis, Characterization, and Antimicrobial Activity of Copper Oxide Nanoparticles. J. Nanomater. 2014, 2014, 1–4. [Google Scholar] [CrossRef]
- Moniri, S.; Ghoranneviss, M.; Hantehzadeh, M.R.; Asadabad, M.A. Synthesis and optical characterization of copper nanoparticles prepared by laser ablation. Bull. Mater. Sci. 2017, 40, 37–43. [Google Scholar] [CrossRef]
- Sen, S.; Sarkar, K. Effective Biocidal and Wound Healing Cogency of Biocompatible Glutathione: Citrate-Capped Copper Oxide Nanoparticles Against Multidrug-Resistant Pathogenic Enterobacteria. Microb. Drug Resist. 2021, 27, 616–627. [Google Scholar] [CrossRef]
- Cantu, J.M.; Ye, Y.; Valdes, C.; Cota-Ruiz, K.; Hernandez-Viezcas, J.A.; Gardea-Torresdey, J.L. Citric Acid-Functionalized CuO Nanoparticles Alter Biochemical Responses in Candyland Red Tomato (Solanum lycopersicum). ACS Agric. Sci. Technol. 2022, 2, 359–370. [Google Scholar] [CrossRef]
- Pabisch, S.; Feichtenschlager, B.; Kickelbick, G.; Peterlik, H. Effect of interparticle interactions on size determination of zirconia and silica based systems–A comparison of SAXS, DLS, BET, XRD and TEM. Chem. Phys. Lett. 2012, 521, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Abboud, Y.; Saffaj, T.; Chagraoui, A.; El Bouari, A.; Brouzi, K.; Tanane, O.; Ihssane, B. Biosynthesis, characterization and antimicrobial activity of copper oxide nanoparticles (CONPs) produced using brown alga extract (Bifurcaria bifurcata). Appl. Nanosci. 2014, 4, 571–576. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, N.; Xiang, Y.; Wang, D.; Zhang, P.; Wang, Y.; Lu, S.; Xu, R.; Zhao, J. A flexible non-enzymatic glucose sensor based on copper nanoparticles anchored on laser-induced graphene. Carbon 2020, 156, 506–513. [Google Scholar] [CrossRef]
- Adhikari, A.; Bhutani, V.K.; Mondal, S.; Das, M.; Darbar, S.; Ghosh, R.; Polley, N.; Das, A.K.; Bhattacharya, S.S.; Pal, D.; et al. Chemoprevention of bilirubin encephalopathy with a nanoceutical agent. Pediatr. Res. 2022, 1–11. [Google Scholar] [CrossRef]
- Pirilä, M.; Saouabe, M.; Ojala, S.; Rathnayake, B.; Drault, F.; Valtanen, A.; Huuhtanen, M.; Brahmi, R.; Keiski, R.L. Photocatalytic Degradation of Organic Pollutants in Wastewater. Top. Catal. 2015, 58, 1085–1099. [Google Scholar] [CrossRef]
- Ajmal, A.; Majeed, I.; Malik, R.N.; Idriss, H.; Nadeem, M.A. Principles and mechanisms of photocatalytic dye degradation on TiO2based photocatalysts: A comparative overview. RSC Adv. 2014, 4, 37003–37026. [Google Scholar] [CrossRef]
- Sibhatu, A.K.; Weldegebrieal, G.K.; Sagadevan, S.; Tran, N.N.; Hessel, V. Photocatalytic activity of CuO nanoparticles for organic and inorganic pollutants removal in wastewater remediation. Chemosphere 2022, 300, 134623. [Google Scholar] [CrossRef]
- Sharma, I.; Ahmad, P. Catalase: A versatile antioxidant in plants. In Oxidative Damage to Plants; Elsevier: Amsterdam, The Netherlands, 2014; pp. 131–148. [Google Scholar]
- Wang, T.; Xie, X.; Liu, H.; Chen, F.; Du, J.; Wang, X.; Jiang, X.; Yu, F.; Fan, H. Pyridine nucleotide-disulphide oxidoreductase domain 2 (PYROXD2): Role in mitochondrial function. Mitochondrion 2019, 47, 114–124. [Google Scholar] [CrossRef]
- Moran, J.F.; Sun, Z.; Sarath, G.; Arredondo-Peter, R.; James, E.K.; Becana, M.; Klucas, R.V. Molecular cloning, functional characterization, and subcellular localization of soybean nodule dihydrolipoamide reductase. Plant Physiol. 2002, 128, 300–313. [Google Scholar] [CrossRef]
- Marzi, S.; Knight, W.; Brandi, L.; Caserta, E.; Soboleva, N.; Hill, W.E.; Gualerzi, C.O.; Lodmell, J.S. Ribosomal localization of translation initiation factor IF2. Rna 2003, 9, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Tarry, M.; Arends, S.R.; Roversi, P.; Piette, E.; Sargent, F.; Berks, B.C.; Weiss, D.S.; Lea, S.M. The Escherichia coli Cell Division Protein and Model Tat Substrate SufI (FtsP) Localizes to the Septal Ring and Has a Multicopper Oxidase-Like Structure. J. Mol. Biol. 2009, 386, 504–519. [Google Scholar] [CrossRef] [PubMed]
- Freedman, Z.; Zhu, C.; Barkay, T. Mercury Resistance and Mercuric Reductase Activities and Expression among Chemotrophic Thermophilic Aquificae. Appl. Environ. Microbiol. 2012, 78, 6568–6575. [Google Scholar] [CrossRef] [PubMed]





| Identifier | Corresponding Protein Name | Activity |
|---|---|---|
| HMPREF0798_01720 | Catalase | Catalase, an antioxidant enzyme found in all aerobic organisms, facilitates the transformation of H2O2 into water and oxygen under environmental stress. [59] |
| HMPREF0798_01742 | Pyridine nucleotide-disulfide oxidoreductase family protein | Controls mitochondrial function. [60] |
| HMPREF0798_00999 | Pyridine nucleotide-disulfide oxidoreductase | |
| HMPREF0798_01779 | Dihydrolipoyl dehydrogenase | This oxidoreductase is a key factor in bacterial pathogenesis and is responsible for energy metabolism [61] |
| HMPREF0798_01752 | Dihydrolipoyl dehydrogenase | |
| HMPREF0798_00272 | Dihydrolipoyl dehydrogenase | |
| HMPREF0798_00629 | Dihydrolipoyl dehydrogenase | |
| HMPREF0798_00484, infB | Translation initiation factor, IF-2 | IF2 is a crucial protein that binds GTP and increases the rate of translation. [62] |
| HMPREF0798_01681 | Cell division protein SufI | SufI is a protein responsible for cell division and is also considered a bacterial twin-arginine translocation protein [63] |
| HMPREF0798_01771 | Mercury(II) reductase | This protein helps the organism to withstand the toxic Hg concentrations [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banerjee, A.; Ghosh, R.; Adhikari, T.; Mukhopadhyay, S.; Chattopadhyay, A.; Pal, S.K. Development of Nanomedicine from Copper Mine Tailing Waste: A Pavement towards Circular Economy with Advanced Redox Nanotechnology. Catalysts 2023, 13, 369. https://doi.org/10.3390/catal13020369
Banerjee A, Ghosh R, Adhikari T, Mukhopadhyay S, Chattopadhyay A, Pal SK. Development of Nanomedicine from Copper Mine Tailing Waste: A Pavement towards Circular Economy with Advanced Redox Nanotechnology. Catalysts. 2023; 13(2):369. https://doi.org/10.3390/catal13020369
Chicago/Turabian StyleBanerjee, Amrita, Ria Ghosh, Tapan Adhikari, Subhadipta Mukhopadhyay, Arpita Chattopadhyay, and Samir Kumar Pal. 2023. "Development of Nanomedicine from Copper Mine Tailing Waste: A Pavement towards Circular Economy with Advanced Redox Nanotechnology" Catalysts 13, no. 2: 369. https://doi.org/10.3390/catal13020369