Technical–Economic Assessment—The Missing Piece for Increasing the Attractiveness of Applied Biocatalysis in Ester Syntheses?
Abstract
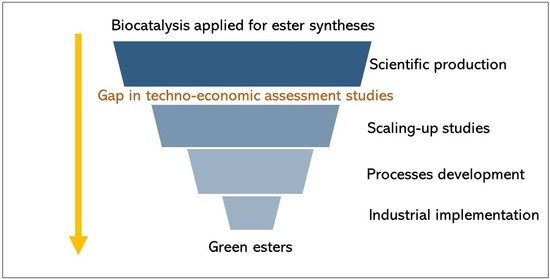
:1. Introduction
2. Discussion
2.1. Bibliometric Survey on the Economic Assessment of Enzymatic Esterification
2.2. The Context of Enzymatic Biodiesel and Enzymatic Specialty Ester Production
2.3. The Relevance of Lipase Immobilization and Its Challenges
2.4. Economic Drawbacks of Biocatalytic Ester Syntheses—Identification and Quantification of Critical Points
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sousa, R.R.; Silva, A.S.; Fernandez-Lafuente, R.; Ferreira-Leitão, V.S. Solvent-Free Esterifications Mediated by Immobilized Lipases: A Review from Thermodynamic and Kinetics Perspective. Catal. Sci. Technol. 2021, 11, 5696–5711. [Google Scholar] [CrossRef]
- de Sousa, R.R.; da Silva, A.S.; Fernandez-Lafuente, R.; Ferreira-Leitão, V.S. Simplified Method to Optimize Enzymatic Esters Syntheses in Solvent-Free Systems: Validation Using Literature and Experimental Data. Catalysts 2021, 11, 1357. [Google Scholar] [CrossRef]
- Ansorge-Schumacher, M.B.; Thum, O. Immobilised Lipases in the Cosmetics Industry. Chem. Soc. Rev. 2013, 42, 6475–6490. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.R.; Rathod, V.K. Enzyme Catalyzed Synthesis of Cosmetic Esters and Its Intensification: A Review. Process Biochem. 2015, 50, 1793–1806. [Google Scholar] [CrossRef]
- Dutra, L.d.S.; Costa Cerqueira Pinto, M.; Cipolatti, E.P.; Aguieiras, E.C.G.; Manoel, E.A.; Greco-Duarte, J.; Guimarães Freire, D.M.; Pinto, J.C. How the Biodiesel from Immobilized Enzymes Production Is Going on: An Advanced Bibliometric Evaluation of Global Research. Renew. Sustain. Energy Rev. 2022, 153, 111765. [Google Scholar] [CrossRef]
- Aguieiras, E.C.G.; de Barros, D.S.N.; Sousa, H.; Fernandez-Lafuente, R.; Freire, D.M.G. Influence of the Raw Material on the Final Properties of Biodiesel Produced Using Lipase from Rhizomucor miehei Grown on Babassu Cake as Biocatalyst of Esterification Reactions. Renew. Energy 2017, 113, 112–118. [Google Scholar] [CrossRef]
- Tufvesson, P.; Lima-Ramos, J.; Nordblad, M.; Woodley, J.M. Guidelines and Cost Analysis for Catalyst Production in Biocatalytic Processes. Org. Process Res. Dev. 2011, 15, 266–274. [Google Scholar] [CrossRef]
- Abdelmoez, W.; Mustafa, A. Oleochemical Industry Future through Biotechnology. J. Oleo Sci. 2014, 63, 545–554. [Google Scholar] [CrossRef] [Green Version]
- Girelli, A.M.; Astolfi, M.L.; Scuto, F.R. Agro-Industrial Wastes as Potential Carriers for Enzyme Immobilization: A Review. Chemosphere 2020, 244, 125368. [Google Scholar] [CrossRef]
- De Sousa, R.R.; Costa, M.; Pinto, C.; Cristina, E.; Aguieiras, G.; Cipolatti, E.P.; Andrade, E.; Ayla, M.; Ana, S.; Pinto, J.C.; et al. Comparative Performance and Reusability Studies of Lipases on Syntheses of Octyl Esters with an Economic Approach. Bioprocess Biosyst. Eng. 2021, 45, 131–145. [Google Scholar] [CrossRef]
- Hills, G. Industrial Use of Lipases to Produce Fatty Acid Esters. Eur. J. Lipid Sci. Technol. 2003, 105, 601–607. [Google Scholar] [CrossRef]
- Thum, O. Enzymatic Production of Care Specialities Based on Fatty Acid Esters. Tens. Surf. Det. 2004, 41, 287–290. [Google Scholar] [CrossRef]
- Thum, O.; Oxenbøll, K.M. Biocatalysis—A Sustainable Method for the Production of Emollient Esters. SÖFW-J. 2008, 134, 44–47. [Google Scholar]
- Delgove, M.A.F.; Laurent, A.B.; Woodley, J.M.; De Wildeman, S.M.A.; Bernaerts, K.V.; van der Meer, Y. A Prospective Life Cycle Assessment (LCA) of Monomer Synthesis: Comparison of Biocatalytic and Oxidative Chemistry. ChemSusChem 2019, 12, 1349–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, M.; Ziemińska-Stolarska, A.; Markowska, D.; Lütz, S.; Rosenthal, K. Comparative Life Cycle Assessment of Chemical and Biocatalytic 2’3’-Cyclic GMP-AMP Synthesis. ChemSusChem 2022. early view. [Google Scholar] [CrossRef] [PubMed]
- Jegannathan, K.R.; Nielsen, P.H. Environmental Assessment of Enzyme Use in Industrial Production-a Literature Review. J. Clean. Prod. 2013, 42, 228–240. [Google Scholar] [CrossRef] [Green Version]
- Mohamad, N.; Huyop, F.; Aboul-Enein, H.Y.; Mahat, N.A.; Waha, R.A. Response Surface Methodological Approach for Optimizing Production of Geranyl Propionate Catalysed by Carbon Nanotubes Nanobioconjugates. Biotechnol. Biotechnol. Equip. 2015, 29, 732–739. [Google Scholar] [CrossRef]
- Gumel, A.M.; Annuar, M.S.M.; Heidelberg, T.; Chisti, Y. Lipase Mediated Synthesis of Sugar Fatty Acid Esters. Process Biochem. 2011, 46, 2079–2090. [Google Scholar] [CrossRef]
- Ferrer, M.; Soliveri, J.; Plou, F.J.; López-Cortés, N.; Reyes-Duarte, D.; Christensen, M.; Copa-Patiño, J.L.; Ballesteros, A. Synthesis of Sugar Esters in Solvent Mixtures by Lipases from Thermomyces lanuginosus and Candida antarctica B, and Their Antimicrobial Properties. Enzyme Microb. Technol. 2005, 36, 391–398. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, T.; Shimizu, M.; Sugiura, M.; Sato, M.; Kohori, J.; Yamada, N.; Nakanishi, K. Optimization of Reaction Conditions for the Production of DAG Using Immobilized 1,3-Regiospecific Lipase Lipozyme RM IM. JAOCS J. Am. Oil Chem. Soc. 2003, 80, 1201–1207. [Google Scholar] [CrossRef]
- Romero, M.D.; Calvo, L.; Alba, C.; Daneshfar, A.; Ghaziaskar, H.S. Enzymatic Synthesis of Isoamyl Acetate with Immobilized Candida antarctica Lipase in N-Hexane. Enzyme Microb. Technol. 2005, 37, 42–48. [Google Scholar] [CrossRef]
- Wu, S.; Snajdrova, R.; Moore, J.C.; Baldenius, K.; Bornscheuer, U.T. Biocatalysis: Enzymatic Synthesis for Industrial Applications. Angew. Chemie Int. Ed. 2021, 60, 88–119. [Google Scholar] [CrossRef]
- Technology Readiness Levels—NASA Earth Science and Technology Office. Available online: https://esto.nasa.gov/trl/ (accessed on 29 November 2022).
- Tinôco, D.; Borschiver, S.; Coutinho, P.L.; Freire, D.M.G. Technological Development of the Bio-Based 2,3-Butanediol Process. Biofuels Bioprod. Biorefining 2021, 15, 357–376. [Google Scholar] [CrossRef]
- Serrano-Arnaldos, M.; Montiel, M.C.; Ortega-Requena, S.; Máximo, F.; Bastida, J. Development and Economic Evaluation of an Eco-Friendly Biocatalytic Synthesis of Emollient Esters. Bioprocess Biosyst. Eng. 2020, 43, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Al-Zuhair, S.; Almenhali, A.; Hamad, I.; Alshehhi, M.A.N.; Mohamed, S. Enzymatic Production of Biodiesel from Used/Waste Vegetable Oils: Design of a Pilot Plant. Renew. Energy 2011, 36, 2605–2614. [Google Scholar] [CrossRef]
- Pasha, M.K.; Dai, L.; Liu, D.; Guo, M.; Du, W. An Overview to Process Design, Simulation and Sustainability Evaluation of Biodiesel Production. Biotechnol. Biofuels 2021, 14, 129. [Google Scholar] [CrossRef]
- Taher, H.; Giwa, A.; Abusabiekeh, H.; Al-Zuhair, S. Biodiesel Production from Nannochloropsis gaditana Using Supercritical CO2 for Lipid Extraction and Immobilized Lipase Transesterification: Economic and Environmental Impact Assessments. Fuel Process. Technol. 2020, 198, 106249. [Google Scholar] [CrossRef]
- Lisboa, P.; Rodrigues, A.R.; Martín, J.L.; Simões, P.; Barreiros, S.; Paiva, A. Economic Analysis of a Plant for Biodiesel Production from Waste Cooking Oil via Enzymatic Transesterification Using Supercritical Carbon Dioxide. J. Supercrit. Fluids 2014, 85, 31–40. [Google Scholar] [CrossRef]
- Zhao, X.; Qi, F.; Yuan, C.; Du, W.; Liu, D. Lipase-Catalyzed Process for Biodiesel Production: Enzyme Immobilization, Process Simulation and Optimization. Renew. Sustain. Energy Rev. 2015, 44, 182–197. [Google Scholar] [CrossRef]
- Andrade, T.A.; Martín, M.; Errico, M.; Christensen, K.V. Biodiesel Production Catalyzed by Liquid and Immobilized Enzymes: Optimization and Economic Analysis. Chem. Eng. Res. Des. 2019, 141, 1–14. [Google Scholar] [CrossRef]
- Anwar, M. Biodiesel Feedstocks Selection Strategies Based on Economic, Technical, and Sustainable Aspects. Fuel 2021, 283, 119204. [Google Scholar] [CrossRef]
- Aemetis Commercializes Advanced Enzymatic Biodiesel Process. Available online: https://www.aemetis.com/aemetis-commercializes-advanced-enzymatic-biodiesel-process/ (accessed on 29 November 2022).
- Blue Sun Launches Commercial-Scale Enzymatic Biodiesel Process. Available online: https://biodieselmagazine.com/articles/9512/blue-sun-launches-commercial-scale-enzymatic-biodiesel-process (accessed on 29 November 2022).
- ALTERNATIVA SOSTENIBLE A LOS COMBUSTIBLES SÓLIDOS. Available online: https://www.oleofat.es/biodiesel/ (accessed on 29 November 2022).
- Enzymocore—Biodiesel. Available online: https://enzymocore.com/ (accessed on 29 November 2022).
- Da Silva, A.S.; De Sá, L.R.V.; Aguieiras, E.C.G.; de Souza, M.F.; Teixeira, R.S.S.; Cammarota, M.C.; Bon, E.P.S.; Freire, D.M.G.; Ferreira-Leitao, V.S. Productive Chain of Biofuels and Industrial Biocatalysis: Two Important Opportunities for Brazilian Sustainable Development. Biotechnol. Microb. Enzym. Prod. Biocatal. Ind. Appl. 2017, 545–581. [Google Scholar] [CrossRef]
- Zahan, K.A.; Kano, M. Biodiesel Production from Palm Oil, Its by-Products, and Mill Effluent: A Review. Energies 2018, 11, 2132. [Google Scholar] [CrossRef] [Green Version]
- Liese, A.; Hilterhaus, L. Evaluation of Immobilized Enzymes for Industrial Applications. Chem. Soc. Rev. 2013, 42, 6236–6249. [Google Scholar] [CrossRef] [PubMed]
- Aguieiras, E.C.G.; Cavalcanti-Oliveira, E.D.; De Castro, A.M.; Langone, M.A.P.; Freire, D.M.G. Biodiesel Production from Acrocomia Aculeata Acid Oil by (Enzyme/Enzyme) Hydroesterification Process: Use of Vegetable Lipase and Fermented Solid as Low-Cost Biocatalysts. Fuel 2014, 135, 315–321. [Google Scholar] [CrossRef]
- Cipolatti, E.P.; Pinto, M.C.C.; Robert, J.d.M.; da Silva, T.P.; Beralto, T.d.C.; Santos, J.G.F.; de Castro, R.d.P.V.; Fernandez-Lafuente, R.; Manoel, E.A.; Pinto, J.C.; et al. Pilot-Scale Development of Core–shell Polymer Supports for the Immobilization of Recombinant Lipase B from Candida antarctica and Their Application in the Production of Ethyl Esters from Residual Fatty Acids. J. Appl. Polym. Sci. 2018, 135, 46727. [Google Scholar] [CrossRef]
- Brito e Cunha, D.A.; Bartkevihi, L.; Robert, J.M.; Cipolatti, E.P.; Ferreira, A.T.S.; Oliveira, D.M.P.; Gomes-Neto, F.; Almeida, R.V.; Fernandez-Lafuente, R.; Freire, D.M.G.; et al. Structural Differences of Commercial and Recombinant Lipase B from Candida antarctica: An Important Implication on Enzymes Thermostability. Int. J. Biol. Macromol. 2019, 140, 761–770. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Woodley, J.M.; Fernandez-Lafuente, R. Is Enzyme Immobilization a Mature Discipline? Some Critical Considerations to Capitalize on the Benefits of Immobilization. Chem. Soc. Rev. 2022, 51, 6251–6290. [Google Scholar] [CrossRef]
- Collaço, A.C.A.; Aguieiras, E.C.G.; Santos, J.G.; de Oliveira, R.A.; de Paula Vieira de Castro, R.; Freire, D.M.G. Experimental Study and Preliminary Economic Evaluation of Enzymatic Biodiesel Production by an Integrated Process Using Co-Products from Palm (Elaeais guineensis jaquim) Industry. Ind. Crops Prod. 2020, 157, 112904. [Google Scholar] [CrossRef]
- Quayson, E.; Amoah, J.; Hama, S.; Kondo, A.; Ogino, C. Immobilized Lipases for Biodiesel Production: Current and Future Greening Opportunities. Renew. Sustain. Energy Rev. 2020, 134, 110355. [Google Scholar] [CrossRef]
- Di Cosimo, R.; Mc Auliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial Use of Immobilized Enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.R.C.; Arana-Peña, S.; da Rocha, T.N.; Miranda, L.P.; Berenguer-Murcia, Á.; Tardioli, P.W.; dos Santos, J.C.S.; Fernandez-Lafuente, R. Liquid Lipase Preparations Designed for Industrial Production of Biodiesel. Is It Really an Optimal Solution? Renew. Energy 2021, 164, 1566–1587. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Strategies for the One-Step Immobilization-Purification of Enzymes as Industrial Biocatalysts. Biotechnol. Adv. 2015, 33, 435–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, R.C.; Berenguer-Murcia, Á.; Carballares, D.; Morellon-Sterling, R.; Fernandez-Lafuente, R. Stabilization of Enzymes via Immobilization: Multipoint Covalent Attachment and Other Stabilization Strategies. Biotechnol. Adv. 2021, 52, 107821. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of Enzyme Activity, Stability and Selectivity via Immobilization Techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying Enzyme Activity and Selectivity by Immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of Different Enzyme Immobilization Strategies to Improve Enzyme Performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Monteiro, R.R.C.; da Silva, S.S.O.; Cavalcante, C.L.; de Luna, F.M.T.; Bolivar, J.M.; Vieira, R.S.; Fernandez-Lafuente, R. Biosynthesis of Alkanes/Alkenes from Fatty Acids or Derivatives (Triacylglycerols or Fatty Aldehydes). Biotechnol. Adv. 2022, 61, 108045. [Google Scholar] [CrossRef]
- Manoel, E.A.; dos Santos, J.C.S.; Freire, D.M.G.; Rueda, N.; Fernandez-Lafuente, R. Immobilization of Lipases on Hydrophobic Supports Involves the Open Form of the Enzyme. Enzyme Microb. Technol. 2015, 71, 53–57. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Virgen-Ortíz, J.J.; dos Santos, J.C.S.; Berenguer-Murcia, Á.; Alcantara, A.R.; Barbosa, O.; Ortiz, C.; Fernandez-Lafuente, R. Immobilization of Lipases on Hydrophobic Supports: Immobilization Mechanism, Advantages, Problems, and Solutions. Biotechnol. Adv. 2019, 37, 746–770. [Google Scholar] [CrossRef] [Green Version]
- Santos, J.C.S.D.; Barbosa, O.; Ortiz, C.; Berenguer-Murcia, A.; Rodrigues, R.C.; Fernandez-Lafuente, R. Importance of the Support Properties for Immobilization or Purification of Enzymes. ChemCatChem 2015, 7, 2413–2432. [Google Scholar] [CrossRef]
- Dossat, V.; Combes, D.; Marty, A. Continuous Enzymatic Transesterification of High Oleic Sunflower Oil in a Packed Bed Reactor: Influence of the Glycerol Production. Enzyme Microb. Technol. 1999, 25, 194–200. [Google Scholar] [CrossRef]
- Séverac, E.; Galy, O.; Turon, F.; Pantel, C.A.; Condoret, J.S.; Monsan, P.; Marty, A. Selection of CalB Immobilization Method to Be Used in Continuous Oil Transesterification: Analysis of the Economical Impact. Enzyme Microb. Technol. 2011, 48, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Marty, A.; Dossat, V.; Condoret, J.S. Continuous Operation of Lipase-Catalyzed Reactions in Nonaqueous Solvents: Influence of the Production of Hydrophilic Compounds. Biotechnol. Bioeng. 1997, 56, 232–237. [Google Scholar] [CrossRef]
- Colombié, S.; Tweddell, R.J.; Condoret, J.S.; Marty, A. Water Activity Control: A Way to Improve the Efficiency of Continuous Lipase Esterification. Biotechnol. Bioeng. 1998, 60, 362–368. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Torrestiana-Sánchez, B.; Dal Magro, L.; Virgen-Ortíz, J.J.; Suárez-Ruíz, F.J.; Rodrigues, R.C.; Fernandez-Lafuente, R. Comparison of Acid, Basic and Enzymatic Catalysis on the Production of Biodiesel after RSM Optimization. Renew. Energy 2019, 135, 1–9. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Virgen-ortíz, J.J.; Jiménez-pérez, M.; Yates, M.; Torrestiana-sanchez, B.; Rosales-quintero, A.; Fernandez-lafuente, R. Evaluation of Different Lipase Biocatalysts in the Production of Biodiesel from Used Cooking Oil: Critical Role of the Immobilization Support. Fuel 2017, 200, 1–10. [Google Scholar] [CrossRef]
- Friedrich, J.L.R.; Peña, F.P.; Garcia-Galan, C.; Fernandez-Lafuente, R.; Ayub, M.A.Z.; Rodrigues, R.C. Effect of Immobilization Protocol on Optimal Conditions of Ethyl Butyrate Synthesis Catalyzed by Lipase B from Candida antarctica. J. Chem. Technol. Biotechnol. 2012, 88, 1089–1095. [Google Scholar] [CrossRef]
- Graebin, N.G.; Martins, A.B.; Lorenzoni, A.S.G.; Garcia-Galan, C.; Fernandez-Lafuente, R.; Ayub, M.A.Z.; Rodrigues, R.C. Immobilization of Lipase B from Candida antarctica on Porous Styrene-Divinylbenzene Beads Improves Butyl Acetate Synthesis. Biotechnol. Prog. 2012, 28, 406–412. [Google Scholar] [CrossRef]
- Eby, J.M.; Peretti, S.W. Performance in Synthetic Applications of a Yeast Surface Display-Based Biocatalyst. RSC Adv. 2015, 5, 30425–30432. [Google Scholar] [CrossRef]
- Lopresto, C.G.; De Paola, M.G.; Albo, L.; Policicchio, M.F.; Chakraborty, S.; Calabro, V. Comparative Analysis of Immobilized Biocatalyst: Study of Process Variables in Trans-Esterification Reaction. 3 Biotech 2019, 9, 443. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ma, X.; Zhang, Z.; Chen, B.; Li, S.; Wang, G. Lipase Immobilized by Modification-Coupled and Adsorption-Cross-Linking Methods: A Comparative Study. Biotechnol. Adv. 2010, 28, 644–650. [Google Scholar] [CrossRef]
- Bähner, F.D.; Prado-Rubio, O.A.; Huusom, J.K. Challenges in Optimization and Control of Biobased Process Systems: An Industrial-Academic Perspective. Ind. Eng. Chem. Res. 2021, 60, 14985–15003. [Google Scholar] [CrossRef]
- Sotoft, L.F.; Rong, B.G.; Christensen, K.V.; Norddahl, B. Process Simulation and Economical Evaluation of Enzymatic Biodiesel Production Plant. Bioresour. Technol. 2010, 101, 5266–5274. [Google Scholar] [CrossRef] [PubMed]
- Brigagão, G.V.; de Queiroz Fernandes Araújo, O.; de Medeiros, J.L.; Mikulcic, H.; Duic, N. A Techno-Economic Analysis of Thermochemical Pathways for Corncob-to-Energy: Fast Pyrolysis to Bio-Oil, Gasification to Methanol and Combustion to Electricity. Fuel Process. Technol. 2019, 193, 102–113. [Google Scholar] [CrossRef]
- Karimi Alavijeh, M.; Meyer, A.S.; Gras, S.L.; Kentish, S.E. Simulation and Economic Assessment of Large-Scale Enzymatic N-Acetyllactosamine Manufacture. Biochem. Eng. J. 2020, 154, 107459. [Google Scholar] [CrossRef]
- Lima-Ramos, J.; Tufvesson, P.; Woodley, J.M. Application of Environmental and Economic Metrics to Guide the Development of Biocatalytic Processes. Green Process. Synth. 2014, 3, 195–213. [Google Scholar] [CrossRef] [Green Version]
- Gunukula, S.; Runge, T.; Anex, R. Assessment of Biocatalytic Production Parameters to Determine Economic and Environmental Viability. ACS Sustain. Chem. Eng. 2017, 5, 8119–8126. [Google Scholar] [CrossRef]
- Keng, P.S.; Basri, M.; Ariff, A.B.; Abdul Rahman, M.B.; Abdul Rahman, R.N.Z.; Salleh, A.B. Scale-up Synthesis of Lipase-Catalyzed Palm Esters in Stirred-Tank Reactor. Bioresour. Technol. 2008, 99, 6097–6104. [Google Scholar] [CrossRef]
- Price, J.; Hofmann, B.; Silva, V.T.L.; Nordblad, M.; Woodley, J.M.; Huusom, J.K. Mechanistic Modeling of Biodiesel Production Using a Liquid Lipase Formulation. Biotechnol. Prog. 2014, 30, 1277–1290. [Google Scholar] [CrossRef]
- Wancura, J.H.C.; Fantinel, A.L.; Ugalde, G.A.; Donato, F.F.; Vladimir de Oliveira, J.; Tres, M.V.; Jahn, S.L. Semi-Continuous Production of Biodiesel on Pilot Scale via Enzymatic Hydroesterification of Waste Material: Process and Economics Considerations. J. Clean. Prod. 2021, 285, 124838. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Sousa, R.R.; de Castro, R.d.P.V.; Assis, N.M.; da Silva, A.S.; Freire, D.M.G.; Fernandez-Lafuente, R.; Ferreira-Leitão, V.S. Technical–Economic Assessment—The Missing Piece for Increasing the Attractiveness of Applied Biocatalysis in Ester Syntheses? Catalysts 2023, 13, 223. https://doi.org/10.3390/catal13020223
de Sousa RR, de Castro RdPV, Assis NM, da Silva AS, Freire DMG, Fernandez-Lafuente R, Ferreira-Leitão VS. Technical–Economic Assessment—The Missing Piece for Increasing the Attractiveness of Applied Biocatalysis in Ester Syntheses? Catalysts. 2023; 13(2):223. https://doi.org/10.3390/catal13020223
Chicago/Turabian Stylede Sousa, Ronaldo Rodrigues, Rui de Paula Vieira de Castro, Nadinne Medeiros Assis, Ayla Sant’Ana da Silva, Denise Maria Guimarães Freire, Roberto Fernandez-Lafuente, and Viridiana Santana Ferreira-Leitão. 2023. "Technical–Economic Assessment—The Missing Piece for Increasing the Attractiveness of Applied Biocatalysis in Ester Syntheses?" Catalysts 13, no. 2: 223. https://doi.org/10.3390/catal13020223