A Critical Review of Photo-Based Advanced Oxidation Processes to Pharmaceutical Degradation
Abstract
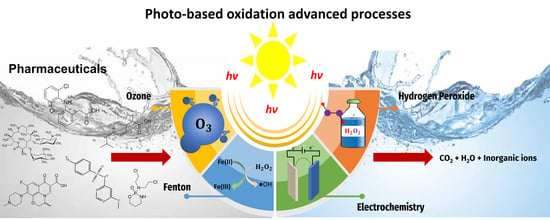
:1. Introduction
2. Status of Pharmaceutical Pollution
3. Photocatalysis as a Tool for the Degradation of Different Pharmaceuticals
3.1. Nitrogenous-Based Compounds
3.2. Chlorinated-Based Compounds
3.3. Fluorinated-Based Compounds
3.4. Sulfurized-Based Compounds
3.5. Phosphorus-Based Compounds

3.6. Oxygenated-Based Compounds
4. Conclusions and Outlook
- More studies need to be carried out using real wastewater samples to evaluate the effectiveness of the combined advanced oxidative processes, since the matrix of real wastewater samples is complex due to the presence of organic and inorganic substances besides the variations of wastewater characteristics.
- Advanced oxidation processes need to be optimized to improve their adaptability and practicability, such as enhancing the efficiency and dosage of the photocatalysts and the utilization efficiency of O3 or H2O2.
- Energy costs must also be reduced. In this context, the search for novel, affordable photocatalysts that can use a broader part of the light spectrum instead of only UV is a priority. Furthermore, the application of renewable energy sources in the treatment plants should also be investigated.
- The generation mechanism of free radicals and the degradation pathways of pollutants are not yet clear. More attention should be given to the study of mechanisms, combining experimental measurements with theoretical calculations.
- The generation of waste (e.g., sludge in the photo-Fenton process and/or exhausted or poisoned catalysts in photocatalyzed AOPs) should be minimized and possible alternatives for the valorization of such wastes should be explored.
- It is recommended that future studies should focus on the evaluation of treated water toxicity, employing ecotoxicity tests to monitor the toxicity of the by-products formed during the degradation.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karimi-Maleh, H.; Karimi, F.; Fu, L.; Sanati, A.; Alizadeh, M.; Karaman, C.; Orooji, Y. Cyanazine herbicide monitoring as a hazardous substance by a DNA nanostructure biosensor. J. Hazard. Mater. 2022, 423, 127058. [Google Scholar] [CrossRef] [PubMed]
- Chandarana, H.; Kumar, P.S.; Seenuvasan, M.; Kumar, M.A. Kinetics, equilibrium and thermodynamic investigations of methylene blue dye removal using Casuarina equisetifolia pines. Chemosphere 2021, 285, 131480. [Google Scholar] [CrossRef] [PubMed]
- Talbot, J.; Nilsson, B. Pharmacovigilance in the pharmaceutical industry. Br. J. Clin. Pharmacol. 1998, 45, 427–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansouri, F.; Chouchene, K.; Roche, N.; Ksibi, M. Removal of pharmaceuticals from water by adsorption and advanced oxidation processes: State of the art and trends. Appl. Sci. 2021, 11, 6659. [Google Scholar] [CrossRef]
- Szabó, R.; Megyeri, C.; Illés, E.; Gajda-Schrantz, K.; Mazellier, P.; Dombi, A. Phototransformation of ibuprofen and ketoprofen in aqueous solutions. Chemosphere 2011, 84, 1658–1663. [Google Scholar] [CrossRef]
- Majumder, A.; Gupta, B.; Gupta, A. Pharmaceutically active compounds in aqueous environment: A status, toxicity and insights of remediation. Environ. Res. 2019, 176, 108542. [Google Scholar] [CrossRef]
- Velempini, T.; Prabakaran, E.; Pillay, K. Recent developments in the use of metal oxides for photocatalytic degradation of pharmaceutical pollutants in water—A review. Mater. Today Chem. 2021, 19, 100380. [Google Scholar] [CrossRef]
- Brillas, E. A review on the degradation of organic pollutants in waters by UV photoelectro-fenton and solar photoelectro-fenton. J. Braz. Chem. Soc. 2014, 25, 393–417. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.; Rodrigo, M.; Sirés, I.; Scialdone, O. Single and Coupled Electrochemical Processes and Reactors for the Abatement of Organic Water Pollutants: A Critical Review. Chem. Rev. 2015, 115, 13362–13407. [Google Scholar] [CrossRef]
- de O.S. Santos, G.; Gonzaga, I.; Dória, A.; Moratalla, A.; da Silva, R.; Eguiluz, K.; Salazar-Banda, G.; Saez, C.; Rodrigo, M. Testing and scaling-up of a novel Ti/Ru0.7Ti0.3O2 mesh anode in a microfluidic flow-through reactor. Chem. Eng. J. 2020, 398, 125568. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Ranjbari, S.; Tanhaei, B.; Ayati, A.; Orooji, Y.; Alizadeh, M.; Karimi, F.; Salmanpour, S.; Rouhi, J.; Sillanpää, M.; et al. Novel 1-butyl-3-methylimidazolium bromide impregnated chitosan hydrogel beads nanostructure as an efficient nanobio-adsorbent for cationic dye removal: Kinetic study. Environ. Res. 2021, 195, 110809. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, A.; Kumar, P.; Jeevanantham, S.; Anubha, M.; Jayashree, S. Degradation of toxic agrochemicals and pharmaceutical pollutants: Effective and alternative approaches toward photocatalysis. Environ. Pollut. 2022, 298, 118844. [Google Scholar] [CrossRef] [PubMed]
- Moratalla, Á.; Cotillas, S.; Lacasa, E.; Fernández-Marchante, C.; Ruiz, S.; Valladolid, A.; Cañizares, P.; Rodrigo, M.; Sáez, C. Occurrence and toxicity impact of pharmaceuticals in hospital effluents: Simulation based on a case of study. Process Saf. Environ. Prot. 2022, 168, 10–21. [Google Scholar] [CrossRef]
- Jelic, A.; Cruz-Morató, C.; Marco-Urrea, E.; Sarrà, M.; Perez, S.; Vicent, T.; Petrović, M.; Barcelo, D. Degradation of carbamazepine by Trametes versicolor in an air pulsed fluidized bed bioreactor and identification of intermediates. Water Res. 2012, 46, 955–964. [Google Scholar] [CrossRef]
- Ahuja, S. Current status of pharmaceutical contamination in water, in: Handb. Water Purity Qual. 2021, 255–270. [Google Scholar] [CrossRef]
- Li, M.; An, Z.; Huo, Y.; Jiang, J.; Zhou, Y.; Cao, H.; Jin, Z.; Xie, J.; Zhan, J.; He, M. Individual and combined degradation of N-heterocyclic compounds under sulfate radical-based advanced oxidation processes. Chem. Eng. J. 2022, 442, 136316. [Google Scholar] [CrossRef]
- Yao, J.; Tang, Y.; Zhang, Y.; Ruan, M.; Wu, W.; Sun, J. New theoretical investigation of mechanism, kinetics, and toxicity in the degradation of dimetridazole and ornidazole by hydroxyl radicals in aqueous phase. J. Hazard. Mater. 2022, 422, 126930. [Google Scholar] [CrossRef]
- Li, B.; Ma, X.; Deng, J.; Li, Q.; Chen, W.; Li, G.; Chen, G.; Wang, J. Comparison of acetaminophen degradation in UV-LED-based advance oxidation processes: Reaction kinetics, radicals contribution, degradation pathways and acute toxicity assessment. Sci. Total Environ. 2020, 723, 137993. [Google Scholar] [CrossRef]
- Cai, H.; Zou, J.; Lin, J.; Li, J.; Huang, Y.; Zhang, S.; Ma, J. Sodium hydroxide-enhanced acetaminophen elimination in heat/peroxymonosulfate system: Production of singlet oxygen and hydroxyl radical. Chem. Eng. J. 2022, 429, 132438. [Google Scholar] [CrossRef]
- Cai, H.; Zou, J.; Lin, J.; Li, Q.; Li, J.; Huang, Y.; Ma, J. Elimination of acetaminophen in sodium carbonate-enhanced thermal/peroxymonosulfate process: Performances, influencing factors and mechanism. Chem. Eng. J. 2022, 449, 137765. [Google Scholar] [CrossRef]
- Li, J.; Zou, J.; Zhang, S.; Cai, H.; Huang, Y.; Lin, J.; Ma, J. Sodium tetraborate simultaneously enhances the degradation of acetaminophen and reduces the formation potential of chlorinated by-products with heat-activated peroxymonosulfate oxidation. Water Res. 2022, 224, 119095. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, M.; Xu, Y.; Quan, X.; Xu, Y.; Liu, W.; Wang, L. Constructing heterojunction interface of Co3O4/TiO2 for efficiently accelerating acetaminophen degradation via photocatalytic activation of sulfite. Chin. Chem. Lett. 2022, 34, 107530. [Google Scholar] [CrossRef]
- Sayadi, M.; Sobhani, S.; Shekari, H. Photocatalytic degradation of azithromycin using GO@Fe3O4/ZnO/SnO2 nanocomposites. J. Clean. Prod. 2019, 232, 127–136. [Google Scholar] [CrossRef]
- Tenzin, T.; Yashas, S.; Anilkumar, K.; Shivaraju, H. UV–LED driven photodegradation of organic dye and antibiotic using strontium titanate nanostructures. J. Mater. Sci. Mater. Electron. 2021, 32, 21093–21105. [Google Scholar] [CrossRef]
- Martins, P.; Salazar, H.; Aoudjit, L.; Gonçalves, R.; Zioui, D.; Fidalgo-Marijuan, A.; Costa, C.; Ferdov, S.; Lanceros-Mendez, S. Crystal morphology control of synthetic giniite for enhanced photo-Fenton activity against the emerging pollutant metronidazole. Chemosphere 2021, 262, 128300. [Google Scholar] [CrossRef] [PubMed]
- Neghi, N.; Krishnan, N.; Kumar, M. Analysis of metronidazole removal and micro-toxicity in photolytic systems: Effects of persulfate dosage, anions and reactor operation-mode. J. Environ. Chem. Eng. 2018, 6, 754–761. [Google Scholar] [CrossRef]
- Leeladevi, K.; Kumar, J.V.; Arunpandian, M.; Thiruppathi, M.; Nagarajan, E. Investigation on photocatalytic degradation of hazardous chloramphenicol drug and amaranth dye by SmVO4 decorated g-C3N4 nanocomposites. Mater. Sci. Semicond. Process. 2021, 123, 105563. [Google Scholar] [CrossRef]
- Hu, X.; Qin, J.; Wang, Y.; Wang, J.; Yang, A.; Tsang, Y.; Liu, B. Synergic degradation Chloramphenicol in photo-electrocatalytic microbial fuel cell over Ni/MXene photocathode. J. Colloid Interface Sci. 2022, 628, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sharma, S.; Kumar, A.; Sharma, G.; AlMasoud, N.; Alomar, T.; Naushad, M.; ALOthman, Z.; Stadler, F. High interfacial charge carrier separation in Fe3O4 modified SrTiO3/Bi4O5I2 robust magnetic nano-heterojunction for rapid photodegradation of diclofenac under simulated solar-light. J. Clean. Prod. 2021, 315, 128137. [Google Scholar] [CrossRef]
- Li, S.; Cui, J.; Wu, X.; Zhang, X.; Hu, Q.; Hou, X. Rapid in situ microwave synthesis of Fe3O4@MIL-100(Fe) for aqueous diclofenac sodium removal through integrated adsorption and photodegradation. J. Hazard. Mater. 2019, 373, 408–416. [Google Scholar] [CrossRef]
- Gonzaga, I.; Dória, A.; Moratalla, A.; Eguiluz, K.; Salazar-Banda, G.; Cañizares, P.; Rodrigo, M.; Saez, C. Electrochemical systems equipped with 2D and 3D microwave-made anodes for the highly efficient degradation of antibiotics in urine. Electrochim. Acta. 2021, 392, 139012. [Google Scholar] [CrossRef]
- Kumari, A.; Kumar, A.; Sharma, G.; Iqbal, J.; Naushad, M.; Stadler, F. Constructing Z-scheme LaTiO2N/g-C3N4@Fe3O4 magnetic nano heterojunctions with promoted charge separation for visible and solar removal of indomethacin. J. Water Process Eng. 2020, 36, 101391. [Google Scholar] [CrossRef]
- Li, R.; Kong, J.; Liu, H.; Chen, P.; Su, Y.; Liu, G.; Lv, W. Removal of indomethacin using UV–vis/peroxydisulfate: Kinetics, toxicity, and transformation pathways. Chem. Eng. J. 2018, 331, 809–817. [Google Scholar] [CrossRef]
- Cao, J.; Li, J.; Chu, W.; Cen, W. Facile synthesis of Mn-doped BiOCl for metronidazole photodegradation: Optimization, degradation pathway, and mechanism. Chem. Eng. J. 2020, 400, 125813. [Google Scholar] [CrossRef]
- Parra-Enciso, C.; Avila, B.; Rubio-Clemente, A.; Peñuela, G. Degradation of diclofenac through ultrasonic-based advanced oxidation processes at low frequency. J. Environ. Chem. Eng. 2022, 10, 108296. [Google Scholar] [CrossRef]
- Naraginti, S.; Yu, Y.; Fang, Z.; Yong, Y. Visible light degradation of macrolide antibiotic azithromycin by novel ZrO2/Ag@TiO2 nanorod composite: Transformation pathways and toxicity evaluation. Process Saf. Environ. Prot. 2019, 125, 39–49. [Google Scholar] [CrossRef]
- Cano, P.; Jaramillo-Baquero, M.; Zúñiga-Benítez, H.; Londoño, Y.; Peñuela, G. Use of simulated sunlight radiation and hydrogen peroxide in azithromycin removal from aqueous solutions: Optimization & mineralization analysis. Emerg. Contam. 2020, 6, 53–61. [Google Scholar] [CrossRef]
- de Almeida, L.; Josué, T.; Fidelis, M.; Abreu, E.; Bechlin, M.; Santos, O.; Lenzi, G. Process Comparison for Caffeine Degradation: Fenton, Photo-Fenton, UV/H2O2 and UV/Fe3+. Water. Air. Soil Pollut. 2021, 232, 147. [Google Scholar] [CrossRef]
- Goulart, L.; Moratalla, A.; Cañizares, P.; Lanza, M.; Sáez, C.; Rodrigo, M. High levofloxacin removal in the treatment of synthetic human urine using Ti/MMO/ZnO photo-electrocatalyst. J. Environ. Chem. Eng. 2022, 10, 107317. [Google Scholar] [CrossRef]
- Geng, C.; Liang, Z.; Cui, F.; Zhao, Z.; Yuan, C.; Du, J.; Wang, C. Energy-saving photo-degradation of three fluoroquinolone antibiotics under VUV/UV irradiation: Kinetics, mechanism, and antibacterial activity reduction. Chem. Eng. J. 2020, 383, 123145. [Google Scholar] [CrossRef]
- Jia, J.; Guan, Y.; Cheng, M.; Chen, H.; He, J.; Wang, S.; Wang, Z. Occurrence and distribution of antibiotics and antibiotic resistance genes in Ba River. China Sci. Total Environ. 2018, 642, 1136–1144. [Google Scholar] [CrossRef]
- Wormser, G.; Tang, Y.-W. Antibiotics in Laboratory Medicine, 5th Edition Edited by Victor Lorain Philadelphia: Lippincott Williams & Wilkins, 2005 832 pp. illustrated. $199.00 (cloth). Clin. Infect. Dis. 2005, 41, 577. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Lu, S.; Guo, W.; Xi, B.; Wang, W. Antibiotics in the aquatic environments: A review of lakes. China Sci. Total Environ. 2018, 627, 1195–1208. [Google Scholar] [CrossRef]
- He, K.; Hain, E.; Timm, A.; Tarnowski, M.; Blaney, L. Occurrence of antibiotics, estrogenic hormones, and UV-filters in water, sediment, and oyster tissue from the Chesapeake Bay. Sci. Total Environ. 2019, 650, 3101–3109. [Google Scholar] [CrossRef]
- Boy-Roura, M.; Mas-Pla, J.; Petrovic, M.; Gros, M.; Soler, D.; Brusi, D.; Menció, A. Towards the understanding of antibiotic occurrence and transport in groundwater: Findings from the Baix Fluvià alluvial aquifer (NE Catalonia, Spain). Sci. Total Environ. 2018, 612, 1387–1406. [Google Scholar] [CrossRef]
- Wu, M.; Que, C.; Xu, G.; Sun, Y.; Ma, J.; Xu, H.; Sun, R.; Tang, L. Occurrence, fate and interrelation of selected antibiotics in sewage treatment plants and their receiving surface water. Ecotoxicol. Environ. Saf. 2016, 132, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Saha, A.; Sinha, A. Removal of ciprofloxacin using modified advanced oxidation processes: Kinetics, pathways and process optimization. J. Clean. Prod. 2018, 171, 1203–1214. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Lu, S.; Wang, Z.; Wang, Y.; Zhang, G.; Guo, X.; Guo, W.; Zhang, T.; Xi, B. Degradation difference of ofloxacin and levofloxacin by UV/H2O2 and UV/PS (persulfate): Efficiency, factors and mechanism. Chem. Eng. J. 2019, 385, 123987. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.; Du, J.; Zhang, P.; Zhao, Z.; Shi, W.; Cui, F. Rapid degradation of norfloxacin by VUV/Fe2+/H2O2 over a wide initial pH: Process parameters, synergistic mechanism, and influencing factors. J. Hazard. Mater. 2021, 416, 125893. [Google Scholar] [CrossRef]
- Yao, W.; Rehman, S.U.; Wang, H.; Yang, H.; Yu, G.; Wang, Y. Pilot-scale evaluation of micropollutant abatements by conventional ozonation, UV/O3, and an electro-peroxone process. Water Res. 2018, 138, 106–117. [Google Scholar] [CrossRef]
- Liu, H.; Gao, Y.; Wang, J.; Ma, D.; Wang, Y.; Gao, B.; Yue, Q.; Xu, X. The application of UV/O3 process on ciprofloxacin wastewater containing high salinity: Performance and its degradation mechanism. Chemosphere 2021, 276, 130220. [Google Scholar] [CrossRef] [PubMed]
- Paucar, N.E.; Kim, I.; Tanaka, H.; Sato, C. Effect of O3 dose on the O3/UV treatment process for the removal of pharmaceuticals and personal care products in secondary effluent. Chem. Eng. 2019, 3, 53. [Google Scholar] [CrossRef] [Green Version]
- Asgari, E.; Sheikhmohammadi, A.; Nourmoradi, H.; Nazari, S.; Aghanaghad, M. Degradation of ciprofloxacin by photocatalytic ozonation process under irradiation with UVA: Comparative study, performance and mechanism. Process Saf. Environ. Prot. 2021, 147, 356–366. [Google Scholar] [CrossRef]
- Ghattavi, S.; Nezamzadeh-Ejhieh, A. A double-Z-scheme ZnO/AgI/WO3 photocatalyst with high visible light activity: Experimental design and mechanism pathway in the degradation of methylene blue. J. Mol. Liq. 2021, 322, 114563. [Google Scholar] [CrossRef]
- Costa, L.; Nobre, F.; Lobo, A.; de Matos, J. Photodegradation of ciprofloxacin using Z-scheme TiO2/SnO2 nanostructures as photocatalyst. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100466. [Google Scholar] [CrossRef]
- Nguyen, L.; Nguyen, H.; Pham, T.; Tran, T.; Chu, H.; Dang, H.; Nguyen, V.; Nguyen, K.; Pham, T.; Van der Bruggen, B. UV–Visible Light Driven Photocatalytic Degradation of Ciprofloxacin by N,S Co-doped TiO2: The Effect of Operational Parameters. Top. Catal. 2020, 63, 985–995. [Google Scholar] [CrossRef]
- Zhang, M.; Lai, C.; Li, B.; Huang, D.; Liu, S.; Qin, L.; Yi, H.; Fu, Y.; Xu, F.; Li, M.; et al. Ultrathin oxygen-vacancy abundant WO3 decorated monolayer Bi2WO6 nanosheet: A 2D/2D heterojunction for the degradation of Ciprofloxacin under visible and NIR light irradiation. J. Colloid Interface Sci. 2019, 556, 557–567. [Google Scholar] [CrossRef]
- Wu, D.; Li, J.; Guan, J.; Liu, C.; Zhao, X.; Zhu, Z.; Ma, C.; Huo, P.; Li, C.; Yan, Y. Improved photoelectric performance via fabricated heterojunction g-C3N4/TiO2/HNTs loaded photocatalysts for photodegradation of ciprofloxacin. J. Ind. Eng. Chem. 2018, 64, 206–218. [Google Scholar] [CrossRef]
- Prabavathi, S.; Saravanakumar, K.; Park, C.; Muthuraj, V. Photocatalytic degradation of levofloxacin by a novel Sm6WO12/g-C3N4 heterojunction: Performance, mechanism and degradation pathways. Sep. Purif. Technol. 2021, 257, 117985. [Google Scholar] [CrossRef]
- Al Balushi, B.; Al Marzouqi, F.; Al Wahaibi, B.; Kuvarega, A.; Al Kindy, S.; Kim, Y.; Selvaraj, R. Hydrothermal synthesis of CdS sub-microspheres for photocatalytic degradation of pharmaceuticals. Appl. Surf. Sci. 2018, 457, 559–565. [Google Scholar] [CrossRef]
- Lu, G.; Lun, Z.; Liang, H.; Wang, H.; Li, Z.; Ma, W. In situ fabrication of BiVO4-CeVO4 heterojunction for excellent visible light photocatalytic degradation of levofloxacin. J. Alloys Compd. 2019, 772, 122–131. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Jia, X.; Zhang, N.; Xia, R.; Zhang, X.; Wang, Z.; Yu, M. In situ fabrication of a novel S-scheme heterojunction photocatalyts Bi2O3/P-C3N4 to enhance levofloxacin removal from water. Sep. Purif. Technol. 2021, 268, 118691. [Google Scholar] [CrossRef]
- Aghaeinejad-Meybodi, A.; Ebadi, A.; Shafiei, S.; Khataee, A.; Kiadehi, A. Degradation of Fluoxetine using catalytic ozonation in aqueous media in the presence of nano-Γ-alumina catalyst: Experimental, modeling and optimization study. Sep. Purif. Technol. 2019, 211, 551–563. [Google Scholar] [CrossRef]
- Pan, C.; Zhu, F.; Wu, M.; Jiang, L.; Zhao, X.; Yang, M. Degradation and toxicity of the antidepressant fluoxetine in an aqueous system by UV irradiation. Chemosphere 2022, 287, 132434. [Google Scholar] [CrossRef] [PubMed]
- Hollman, J.; Dominic, J.; Achari, G. Degradation of pharmaceutical mixtures in aqueous solutions using UV/peracetic acid process: Kinetics, degradation pathways and comparison with UV/H2O2. Chemosphere 2020, 248, 125911. [Google Scholar] [CrossRef]
- Szabó, L.; Mile, V.; Kiss, D.; Kovács, K.; Földes, T.; Németh, T.; Tóth, T.; Homlok, R.; Balogh, G.; Takács, E.; et al. Applicability evaluation of advanced processes for elimination of neurophysiological activity of antidepressant fluoxetine. Chemosphere 2018, 193, 489–497. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, A.; Sharma, G.; Naushad, M.; Vo, D.; Alam, M.; Stadler, F. Fe3O4 mediated Z-scheme BiVO4/Cr2V4O13 strongly coupled nano-heterojunction for rapid degradation of fluoxetine under visible light. Mater. Lett. 2020, 281, 128650. [Google Scholar] [CrossRef]
- Norouzi, R.; Zarei, M.; Khataee, A.; Ebratkhahan, M.; Rostamzadeh, P. Electrochemical removal of fluoxetine via three mixed metal oxide anodes and carbonaceous cathodes from contaminated water. Environ. Res. 2022, 207, 112641. [Google Scholar] [CrossRef]
- Acosta-Rangel, A.; Sánchez-Polo, M.; Polo, A.; Rivera-Utrilla, J.; Berber-Mendoza, M. Sulfonamides degradation assisted by UV, UV/H2O2 and UV/K2S2O8: Efficiency, mechanism and byproducts cytotoxicity. J. Environ. Manag. 2018, 225, 224–231. [Google Scholar] [CrossRef]
- Moradi, M.; Moussavi, G. Investigation of chemical-less UVC/VUV process for advanced oxidation of sulfamethoxazole in aqueous solutions: Evaluation of operational variables and degradation mechanism. Sep. Purif. Technol. 2018, 190, 90–99. [Google Scholar] [CrossRef]
- Wen, D.; Wu, Z.; Tang, Y.; Li, M.; Qiang, Z. Accelerated degradation of sulfamethazine in water by VUV/UV photo-Fenton process: Impact of sulfamethazine concentration on reaction mechanism. J. Hazard. Mater. 2018, 344, 1181–1187. [Google Scholar] [CrossRef]
- Hong, M.; Wang, Y.; Lu, G. UV-Fenton degradation of diclofenac, sulpiride, sulfamethoxazole and sulfisomidine: Degradation mechanisms, transformation products, toxicity evolution and effect of real water matrix. Chemosphere 2020, 258, 127351. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Yang, Z.; Xu, H.; Song, P.; Xiong, W.; Cao, J.; Zhang, Y.; Xiang, Y.; Hu, J.; Zhou, C.; et al. Integrating N and F co-doped TiO2 nanotubes with ZIF-8 as photoelectrode for enhanced photo-electrocatalytic degradation of sulfamethazine. Chem. Eng. J. 2020, 388, 124388. [Google Scholar] [CrossRef]
- Teng, W.; Xu, J.; Cui, Y.; Yu, J. Photoelectrocatalytic degradation of sulfadiazine by Ag3PO4/MoS2/TiO2 nanotube array electrode under visible light irradiation. J. Electroanal. Chem. 2020, 868, 114178. [Google Scholar] [CrossRef]
- She, S.; Wang, Y.; Chen, R.; Yi, F.; Sun, C.; Hu, J.; Li, Z.; Lu, G.; Zhu, M. Ultrathin S-doped graphitic carbon nitride nanosheets for enhanced sulpiride degradation via visible-light-assisted peroxydisulfate activation: Performance and mechanism. Chemosphere 2021, 266, 128929. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, Y.; Zhong, Y.; Lim, T. Comparison of amoxicillin photodegradation in the UV/H2O2 and UV/persulfate systems: Reaction kinetics, degradation pathways, and antibacterial activity. Chem. Eng. J. 2019, 372, 420–428. [Google Scholar] [CrossRef]
- Lu, J.; Ji, Y.; Chovelon, J.; Lu, J. Fluoroquinolone antibiotics sensitized photodegradation of isoproturon. Water Res. 2021, 198, 117136. [Google Scholar] [CrossRef]
- Wan, Z.; Wang, J. Removal of sulfonamide antibiotics from wastewater by gamma irradiation in presence of iron ions. Nucl. Sci. Tech. 2016, 27, 104. [Google Scholar] [CrossRef]
- Lin, C.; Wu, M. Feasibility of using UV/H2O2 process to degrade sulfamethazine in aqueous solutions in a large photoreactor. J. Photochem. Photobiol. A Chem. 2018, 367, 446–451. [Google Scholar] [CrossRef]
- Aryee, A.; Han, R.; Qu, L. Occurrence, detection and removal of amoxicillin in wastewater: A review. J. Clean. Prod. 2022, 368, 133140. [Google Scholar] [CrossRef]
- Yazidi, A.; Atrous, M.; Soetaredjo, F.E.; Sellaoui, L.; Ismadji, S.; Erto, A.; Bonilla-Petriciolet, A.; Dotto, G.L.; Lamine, A.B. Adsorption of amoxicillin and tetracycline on activated carbon prepared from durian shell in single and binary systems: Experimental study and modeling analysis. Chem. Eng. J. 2020, 379, 122320. [Google Scholar] [CrossRef]
- Ighalo, J.; Igwegbe, C.; Aniagor, C.; Oba, S. A review of methods for the removal of penicillins from water. J. Water Process Eng. 2021, 39, 101886. [Google Scholar] [CrossRef]
- Verma, M.; Haritash, A. Degradation of amoxicillin by Fenton and Fenton-integrated hybrid oxidation processes. J. Environ. Chem. Eng. 2019, 7, 102886. [Google Scholar] [CrossRef]
- Le, S.; Zhu, C.; Cao, Y.; Wang, P.; Liu, Q.; Zhou, H.; Chen, C.; Wang, S.; Duan, X. V2O5 nanodot-decorated laminar C3N4 for sustainable photodegradation of amoxicillin under solar light, Appl. Catal. B Environ. 2022, 303, 120903. [Google Scholar] [CrossRef]
- Guerra, M.H.; Alberola, I.O.; Rodriguez, S.M.; López, A.A.; Merino, A.A.; Lopera, A.E.-C.; Alonso, J.Q. Oxidation mechanisms of amoxicillin and paracetamol in the photo-Fenton solar process. Water Res. 2019, 156, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Mmelesi, O.; Patala, R.; Nkambule, T.; Mamba, B.; Kefeni, K.; Kuvarega, A. Effect of Zn doping on physico-chemical properties of cobalt ferrite for the photodegradation of amoxicillin and deactivation of E. coli. Colloids Surf. A Physicochem. Eng. Asp. 2022, 649, 129462. [Google Scholar] [CrossRef]
- Freitas, J.; Quintão, F.; da Silva, J.; de Queiroz, S.; Aquino, S.; de Cássia Franco, R.J. Characterisation of captopril photolysis and photocatalysis by-products in water by direct infusion, electrospray ionisation, high-resolution mass spectrometry and the assessment of their toxicities. Int. J. Environ. Anal. Chem. 2017, 97, 42–55. [Google Scholar] [CrossRef]
- Santos, A.; Cabot, P.; Brillas, E.; Sirés, I. A comprehensive study on the electrochemical advanced oxidation of antihypertensive captopril in different cells and aqueous matrices. Appl. Catal. B Environ. 2020, 277, 119240. [Google Scholar] [CrossRef]
- Osawa, R.A.; Barrocas, B.T.; Monteiro, O.C.; Oliveira, M.; Florêncio, M. Photocatalytic degradation of cyclophosphamide and ifosfamide: Effects of wastewater matrix, transformation products and in silico toxicity prediction. Sci. Total Environ. 2019, 692, 503–510. [Google Scholar] [CrossRef]
- Russo, C.; Lavorgna, M.; Česen, M.; Kosjek, T.; Heath, E.; Isidori, M. Evaluation of acute and chronic ecotoxicity of cyclophosphamide, ifosfamide, their metabolites/transformation products and UV treated samples. Environ. Pollut. 2018, 233, 356–363. [Google Scholar] [CrossRef]
- Janssens, R.; Cristovao, M.; Bronze, M.; Crespo, J.; Pereira, V.; Luis, P. Coupling of nanofiltration and UV, UV/TiO2 and UV/H2O2 processes for the removal of anti-cancer drugs from real secondary wastewater effluent. J. Environ. Chem. Eng. 2019, 7, 103351. [Google Scholar] [CrossRef]
- Graumans, M.; Hoeben, W.; Russel, F.; Scheepers, P. Oxidative degradation of cyclophosphamide using thermal plasma activation and UV/H2O2 treatment in tap water. Environ. Res. 2020, 182, 109046. [Google Scholar] [CrossRef] [PubMed]
- Sabouni, R.; Murtaza, S.; Ghommem, M. Facile Metal Organic Framework Composites as Photocatalysts for Lone/Simultaneous Photodegradation of Naproxen, Ibuprofen and Methyl Orange. SSRN Electron. J. 2022, 27, 102751. [Google Scholar] [CrossRef]
- Wu, T.; Pi, M.; Zhang, D.; Chen, S. Three-dimensional porous structural MoP2 nanoparticles as a novel and superior catalyst for electrochemical hydrogen evolution. J. Power Source 2016, 328, 551–557. [Google Scholar] [CrossRef]
- Mohamed, A.; Salama, A.; Nasser, W.; Uheida, A. Photodegradation of Ibuprofen, Cetirizine, and Naproxen by PAN-MWCNT/TiO2–NH2 nanofiber membrane under UV light irradiation. Environ. Sci. Eur. 2018, 30, 47. [Google Scholar] [CrossRef] [PubMed]
- Zhen, H.; Liu, J.; Zhang, T. The Effect of UV Degradation of Ketoprofen and Its Influencing Factors. J. Phys. Conf. Ser. 2022, 2194, 012049. [Google Scholar] [CrossRef]
- Mofokeng, L.; Hlekelele, L.; Tetana, Z.; Moma, J.; Chauke, V. CuO-doped TiO2 Supported on Graphitic Carbon Nitride for the Photodegradation of Ketoprofen in Drinking and Groundwater: Process Optimization and Energy Consumption evaluation. Chem. Select. 2022, 7, e202101847. [Google Scholar] [CrossRef]
- Chaker, H.; Fourmentin, S.; Chérif-Aouali, L. Efficient Photocatalytic Degradation of Ibuprofen under Visible Light Irradiation Using Silver and Cerium Co-Doped Mesoporous TiO2. Chem. Select. 2020, 5, 11787–11796. [Google Scholar] [CrossRef]
- Wang, P.; Bu, L.; Wu, Y.; Ma, W.; Zhu, S.; Zhou, S. Mechanistic insight into the degradation of ibuprofen in UV/H2O2 process via a combined experimental and DFT study. Chemosphere 2021, 267, 128883. [Google Scholar] [CrossRef]
- Cunha-Filho, F.; Mota-Lima, A.; Ratkievicius, L.; Silva, D.; Silva, D.; Chiavone-Filho, O.; Nascimento, C.O. Rapid mineralization rate of acetylsalicylic acid in a tubular photochemical reactor: The role of the optimized excess of H2O2. J. Water Process Eng. 2019, 31, 100856. [Google Scholar] [CrossRef]
- Zhe, W.; Wenjuan, Z.; Haihan, W.; Zhiwei, W.; Jing, C. Oxidation of acetylsalicylic acid in water by UV/O3 process: Removal, byproduct analysis, and investigation of degradation mechanism and pathway. J. Environ. Chem. Eng. 2021, 9, 106259. [Google Scholar] [CrossRef]
- Karimi, P.; Baneshi, M.; Malakootian, M. Photocatalytic degradation of aspirin from aqueous solutions using the UV/ZnO process: Modelling, analysis and optimization by response surface methodology (RSM), Desalin. Water Treat. 2019, 161, 354–364. [Google Scholar] [CrossRef] [Green Version]
- Mohan, H.; Yoo, S.; Thimmarayan, S.; Oh, H.; Kim, G.; Seralathan, K.; Shin, T. Nickel decorated manganese oxynitride over graphene nanosheets as highly efficient visible light driven photocatalysts for acetylsalicylic acid degradation. Environ. Pollut. 2021, 289, 117864. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Wu, Z.; Tang, C.; Guo, K.; Cao, Y.; Fang, J. Emerging investigators series: Comparative study of naproxen degradation by the UV/chlorine and the UV/H2O2 advanced oxidation processes. Environ. Sci. Water Res. Technol. 2018, 4, 1219–1230. [Google Scholar] [CrossRef]
- Zhou, S.; Li, L.; Wu, Y.; Zhu, S.; Zhu, N.; Bu, L.; Dionysiou, D. UV365 induced elimination of contaminants of emerging concern in the presence of residual nitrite: Roles of reactive nitrogen species. Water Res. 2020, 178, 115829. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.; Sagir, M.; Shahzad, K. Removal of acetylsalicylate and methyl-theobromine from aqueous environment using nano-photocatalyst WO3-TiO2 @g-C3N4 composite. J. Hazard. Mater. 2018, 363, 205–213. [Google Scholar] [CrossRef] [PubMed]








| Class | Pharmaceuticals | Abbreviation | Chemical Formula | Use |
|---|---|---|---|---|
| NITROGENATED | Acetaminophen | ACT | C8H9NO2 | Analgesic and antipyretic for mild pain and fever |
| Tramadol | TRA | C16H25NO2 | Analgesic for rigorous pain | |
| Azithromycin | AZT | C38H72N2O12 | Antibiotic used to treat respiratory, ear, skin or sexually transmitted infections | |
| Doxorubicin | DOX | C27H29NO4 | Antibiotic used for the therapy of lymphoma, leukemia and sarcoma | |
| Metronidazole | MTZ | C6H9N3O3 | Antiparasitics and antiprotozoa | |
| Trimethoprim | TRIM | C14H18N4O3 | Antibiotic used for mild-to-moderate bacterial infections | |
| Caffeine | CAF | C8H10N4O2 | Used for mild pain and is mostly consumed in the form of coffee | |
| Propranolol | PROP | C16H21NO2 | Used for hypertension, cardiac arrhythmias, angina and hyperthyroidism | |
| CHLORINATED | Lamotrigine | LAM | C9H7Cl2N | Used to fight seizures |
| Chloramphenicol | CAP | C11H12Cl2N2O5 | Antibiotics used for severe or life-threatening infections | |
| Diclofenac | DIC | C14H11Cl2NO2 | Used for the therapy of mild-to-moderate acute pain | |
| Indomethacin | IDM | C19H16ClNO | Used for inflammatory arthritis | |
| Chlorhexidine | CLH | C22H30Cl2N10 | Used topical antiseptic and dental practice for the treatment of dental inflammation | |
| Clorazepate | CLZ | C16H11ClN2O3 | Anticonvulsant for therapy in epilepsy and as an anxiolytic | |
| Hydroxyzine | HDZ | C21H27ClN2O2 | Used largely for symptoms of allergy, nausea and anxiety | |
| Indapamide | IDP | C16H16ClNO3 | Antihypertensive and a diuretic | |
| Bezafibrate | BZF | C19H20ClNO4 | Used for hyperlipidemia | |
| FLUORATED | Ciprofloxacin | CIP | C17H18FN3O3 | Used in the therapy of mild-to-moderate urinary or respiratory infections |
| Ofloxacin | OFLO | C18H20FN3O4 | Used for treatment rare instances of hepatocellular injury | |
| Enrofloxacin | ENR | C19H22FN3O3 | Antibiotic used to treat infections of the urinary system | |
| Levofloxacin | LEV | C18H20FN3O4 | Antibiotic used to treat infections, such as pneumonia and sinusitis | |
| Etofinamate | ETO | C18H18F3NO4 | Used in the treatment of muscles and joints | |
| Fluoxetine | FLU | C17H18F3NO | Used to treat major depressive, moderate-to-severe bulimia and obsessive-compulsive disorder | |
| SULFURATED | Eprosartan | EPS | C23H24N2O4S | Used for the treatment of high blood pressure |
| Captopril | CPT | C9H15NO3S | Used in the therapy of hypertension | |
| Sulpiride | SUL | C15H23N3O4S | Used therapy as an antidepressant | |
| Sulfamonomethoxine | SFX | C11H12N4O3S | Long acting antibacterial agent | |
| Amoxicillin | AMX | C16H19N3O5S | Used in the treatment of mild-to-moderate bacterial infections | |
| PHOSPORATED | Ifosfamide | IFD | C7H15Cl2N2O2P | Chemotherapy used to treat certain types of cancer, such as cancer of the ovary and cervix |
| Cyclophosphamide | CFD | C7H15Cl2N2O2P | Cyclophosphamide is used to treat cancer of the ovaries, breast, blood and lymph systems | |
| OXIGENATED | Aspirin | AAS | C9H8O4 | Used as a medicine to treat pain, fever and inflammation |
| Ketoprofen | KET | C16H14O3 | Anti-inflammatory, with analgesic, anti-inflammatory and antirheumatic effects | |
| Naproxen | NAP | C14H14O3 | Analgesic for pain | |
| Ibuprofen | IBU | C13H18O2 | Anti-inflammatory used to treat pain and fever |
| Pharmaceutical | Process | Experimental Conditions | Results | Ref. |
|---|---|---|---|---|
| DC | Photocatalysis | pH = 3; t = 150 min; [Catalyst] = 0.1 g L−1; [DC] = 60 mg L−1 | 99.4% DC and 87.8% TOC removal | [30] |
| CAP | Electrochemical and photo-assisted | pH = 6.8; t = 8h; [CAP] = 50 mg L−1 | 100% CAP removal and complete removal of antibiotic activity | [31] |
| IMT | Photocatalysis | pH = 6.8; t = 45 min; [Catalyst] = 0.03 g mL−1; [IMT] = 20 mg L−1 | 97.3% IMT removal | [32] |
| IMT | UV–vis/peroxydisulfate | pH = 7; t = 45 min; [PDS] = 20 µM [IMT] = 20 µM | ~80% IMT and 30% TOC removal | [33] |
| MET | Photocatalysis | pH = 2; t = 15 min; [Catalyst] = 1.2 g L−1 [MET] = 10 mg L−1 | 94.3% MET removal | [34] |
| DC | US/photo-Fenton | pH = 3; t = 90 min; [Fe2+] = 0.20 mg L−1; [H2O2] = 1 mg L−1; [DC] = 1 mg L−1 | 94.4% DC and 17% TOC removal | [35] |
| AZT | Photocatalysis | t = 8h; [Catalyst] = 2 mg mL−1 [AZT] = 20 mg L−1 | 90% AZT removal and complete removal of toxicity | [36] |
| AZT | UV/H2O2 | pH = 9; t = 120 min; [H2O2] = 482 mg L−1; [DC] = 1 mg L−1 | 53% AZT and 100% TOC removal | [37] |
| CAF | Fenton, photo-Fenton, UV/H2O2 and UV/Fe3+ | pH = 5.8; t = 120 min; [H2O2] = 0.5 mL L−1; [CAF] = 20 mg L−1 | 99% CAF removal | [38] |
| LEV | Photo-electrocatalyst | pH = 5.9; t = 4 h; [LEV] = 20 mg L−1 | 99% LEV and 100% TOC removal | [39] |
| Pharmaceutical Compound | Process | Experimental Conditions | Results | Ref. |
|---|---|---|---|---|
| NOR, CIP and LEV | VUV/UV and UV irradiation | pH = 7 Flow rate: 60 L h−1 [NOR] = [CIP] = [LEV] = 15 µmol L−1 T = 45 °C | The compounds were degraded more efficiently with high temperature and low initial concentration. The degradation pathways of FQs were defluorination, decarboxylation and piperazine ring oxidation, and demethylation was a particular degradation pathway for LEV. | [40] |
| CIP | UV, H2O2, UV/H2O2, modified Fenton and modified photo-Fenton | pH = 7 [CIP] = 10 mg L−1 [H2O2] = 100 mmol L−1 [nZVI] = 5 mmol L−1 | Only 4% of TOC removal for UV radiation TOC removal % increased from 10.47% for only H2O2 to 35.41 % for UV/H2O2. nZVI/H2O2 removed 100% of CIP in 30 min and 60% of the initial TOC. | [47] |
| OFLO and LEV | UV/H2O2 and UV/PS | pH = 3 [LEV] = [OFLO] = 5 mg L−1 t = 30 min [ H2O2] = [PS] = 150 µmol·L−1 | The removal efficiencies of OFLO and LEV were 96.40% and 99.23% with UV/PS. The TOC removal efficiencies of OFLO and LEV were 36.24% and 38.36%, respectively, for the UV/H2O2 system. By contrast, the UV/PS system exhibited mineralization of OFL and LEV, with TOC removal efficiency about 46.43% and 49.74%, respectively. | [48] |
| NOR | UV, UV/H2O2, UV/Fe2+/H2O2, VUV, UV/Fe2+, VUV/H2O2, VUV/Fe2+/H2O2 | pH = 7 [NOR] = 45µmol L−1 [Fe2+] = 90 µmol L−1 [H2O2] = 3 mmol L−1 | VUV/Fe2+/H2O2 process removed 100% of NOR after 4 min, and high mineralization (63.3% at 8 min) and achieved rapid removal of NOR in real waters at neutral pH. | [49] |
| CIP | UV/O3 | pH = 5.6 Gas flow: 0.5 L min−1 [CIP] = 100.0 mg L−1 [O3] = 30.0 ± 2.0 mg L−1 min−1 | Compared with single O3 (37.5%), the DOC reduction was prompted significantly by the introduction of UV irradiation (91.4%) within 40 min. The toxicity of products decreased significantly as compared with parent pollutant. | [51] |
| 38 PPCPs, highlighting CIP and LEV | UV/O3 | Ozone dosage (1−4 and 6 mg L−1) and hydraulic retention time (5 and 10 min) | CIP was degraded below the limit of detection (LOD) at the ozone dose of 1 mg L−1 in 5 min. However, LEV required an O3 dose of 6 mg L−1 to be degraded below LOD. | [52] |
| CIP | Photolysis | pH = 7 catalyst dosage = 50 mg [CIP] = 5 mg L−1 | Photolysis showed a small removal of CIP (4.63%) over 120 min of exposure time under UV-light radiation while the removal of CIP for Na-TiNT, SnO2 and TiO2/SnO2 nanocomposite were 41.55%, 45.83% and 92.8%, respectively. | [55] |
| LEV | Photocatalysis | [LEV] = 10 mg L−1 catalyst dosage = 50 mg | The Sm6WO12/g-C3N4 nanocomposite heterojunction catalyst shows higher photocatalytic efficiency towards LEV degradation (90.8%, after 70 min). | [59] |
| FLU | UV photolysis | pH = 7 T = 20 °C [FLU] = 1 mg L−1 Irradiation source = 300 W | The degradation rates of FLU for five days were approximately 63.6% ± 2.14%, 84.6% ± 0.99%, and 97.5% ± 0.25% after 15, 30 and 60 min of UV irradiation, respectively. | [64] |
| VEN, SFX, FLU and CBZ | UV/PAA and UV/H2O2 | pH = 7 UVC intensity = 3.50 kW m−3 [PAA] = [H2O2] = 50 mg L−1 [compound] = 10 mg L−1 | UV/H2O2 was found to be more efficient than UV/PAA for the degradation of FLU, VEN and CBZ. While UV/PAA was more effective in SFX degradation. | [65] |
| FLU | Photocatalysis | pH = 7 [FLU] = 10 mg L−1 Light source = 280 mW cm−1 catalyst dosage = 0.3 mg mL−1 | Within 60 min of visible exposure, 99.2% of FLU was removed at pH 7. The high total organic carbon removal of 80.3% and 61.4% by FBC under visible and solar light confirmed the mineralization after 180 min of treatment. | [67] |
| FLU | Ti/RuO2, Ti/RuO2-IrO2 and Ti/RuO2-IrO2-SnO2 | pH = 6 i = 500 mA [FLU] = 25 mg L−1 t = 160 min | At optimized conditions, maximum FLU removal efficiency was observed, which was 96.25%. The TOC results showed that about 81.51% of the mineralization of the FLU was achieved after 6 h under optimal experimental conditions. | [68] |
| SMZ, SDZ and SML | UV, UV/H2O2 and UV/K2S2O8 | pH = 7 [SMZ]=[SDZ]=[SML] 15 mg L−1 [H2O2] = [K2S2O8] = 4.4 × 10−4 M | UVC proved inadequate in removing these compounds. A concentration of 4.4 × 10−4 mol L−1 of H2O2 or K2S2O8 increases SMZ degradation up to 100%. UVC/PDS requires less energy than UVC and UVC/H2O2 system. | [69] |
| SMX | UVC/VUV | pH = 7 [SMX] = 100 mg L−1, Fluence = 54.9 mJ cm−2 | Total SMX removal was reached at a fluence of 54.9 mJ cm−2, while the TOC removal of 98.5% was attained at fluence of 109.8 mJ cm−2. | [70] |
| SMT | photo-Fenton | pH = 4 [SMT] = 1.8 × 10−2 mmol L−1 [H2O2] = 0.74 mmol L−1 [Fe3+] = 0.25 mmol L−1 | The mineralization of SMT were significantly enhanced in the VUV/UV photo-Fenton process as compared to the UV and UV photo-Fenton processes after 60 min of treatment, achieving ~60% of TOC removal. | [71] |
| DF, SP, SMX and SIM | photo-Fenton | [H2O2] = 100 mmol L−1 [pharmaceuticals] = 20 mg L−1, Fe2+ dosage = 1/200 mol L−1 of FeSO4 7H2O and H2O2 UV intensity = 75 mW cm−2 | In ultrapure water, all the four pharmaceuticals were degraded by more than 95% within 4 min, the same removal rate expended about 30–60 min. Except for DF, the cytotoxicity increase during the degradation process for SP, SMX and SIM. | [72] |
| SMZ | Photoelectrochemical | [SMZ] = 10 mg L−1 pH = 3.5 Potential = 2 V | The removal efficiency of SMZ by photoelectrochemical process was 81.3%, which was approximately twice the sum of both electrochemical and photochemical processes, and over 40% of TOC was eliminated after 180 min. | [73] |
| SD | Photocatalysis | [SD] = 10 mg L−1 pH = 8 | Nearly 70% of SD was degraded by the Ag3PO4/MoS2/TiO2 NTAs in 240 min, which was higher than that of MoS2/TiO2 (35%) and Ag3PO4/TiO2 NTAs (44%). In addition, the percentage of SD removal over direct photolysis, electrochemical and photochemical was only 16, 20 and 31%, respectively. | [74] |
| SP | US-CN | pH = 7.5 [SP] = 0.03 mmol L−1 catalyst dosage = 32 mg UV intensity = 100 mW cm−2 | The time-dependent removal efficiency of SP over different carbon nitride samples was 90.55%, 50.77% and 26.19% of SP by US-CN, S-CN and CN after 100 min of photocatalytic reaction. The removal rates of SP at 100 min in river water (Shanghai), pharmaceutical water (Guangzhou), domestic wastewater (Guangzhou) and tap water (laboratory) reached up to 84.74, 75.66, 82.06 and 85.26%, respectively. | [75] |
| AMX | UV/H2O2 and UV/persulfate | [AMX] = 20 µmol L−1 [H2O2] = [PS] = 500 µmol L−1 | The direct UV photolysis system alone showed an insignificant AMX degradation. However, the addition of H2O2 or PS increases the degradation efficiency of AMX significantly. Despite the high percentage of AMX removal through UV/AOPs, the mineralization of AMX was insignificant. After 30 min of treatment, only 15.2% and 28.7% of TOC were removed in the UV/H2O2 and UV/persulfate systems, respectively. | [76] |
| AMX | Fenton process, photo-Fenton, solar photo-Fenton, sono-Fenton, and sono-photo-Fenton | pH = 3 [AMX] = 10 mg L−1 [FeSO4] = 3.0 mg L−1 [H2O2] = 375 mg L−1 Ultrasound = 40 kHz, Light source = UV tubes 365 nm | Under the optimized conditions, Fenton’s process was able to remove 100% of AMX within 12 min of reaction time. Coupling the Fenton process with UV-light illumination, solar light illumination and UV light–ultrasound treatment allowed complete antibiotic removal in 3.5, 9 and 6 min, respectively. | [77] |
| AMX | solar photo-Fenton | [Fe3+] = 3 mg L−1 [H2O2] = 2.75 mg L−1 [AMX] = 1 mg L−1 | A total of 94 and 66% of AMX was removed after 60 and 210 min of treatment in simulated and real wastewater, respectively. In addition, the percentage of TOC removal for AMX was 19.5% in simulated wastewater. In the study carried out with real effluent, the removal rate was 6.5%. | [47] |
| AMX | Photolysis | [AMX] = 20 mg L−1 pH = 7 catalyst dosage = 0.5 g L−1 | Photolysis under the simulated sunlight was ineffective in the decomposition of AMX, while pure V2O5 and C3N4 reached 33.2% and 52.7% of AMX removal under 120 min illumination. The V2O5/C3N4 (1 wt% V2O5) nanocomposite increased AMX removal to 91.3%. Moreover, 1-V2O5/C3N4 nanocomposite attained 76.2% of TOC removal under the same conditions. | [48] |
| CP | solar photoelectro-Fenton | [CP] = 0.230 mmol L−1 pH = 3 j = 50 mA cm−2 [Fe2+] = 0.50 mmol L−1 | At the best conditions, the degradation of CP by SPEF reached 100% removal in only 15 min, while using EF, a complete removal was achieved in about 20 min. The AO-H2O2 process was capable of removing only 36% in 30 min of treatment. | [51] |
| Pharmaceutical | Process | Experimental Conditions | Results | Ref. |
|---|---|---|---|---|
| IF and CP | Ru-TNW | [IF] = [CP] = 10 mg L−1 catalyst dosage = 20 mg pH = 7 | The degradation of both compounds presented a higher removal rate independently to the matrix used; DW and WW in secondary treatment. CP and IF degradation experiments followed the pseudo-first-order kinetics and the degradation rate constant for IF was higher than the CP for both matrices. | [89] |
| IF and CP | UV irradiation | λ = 254 nm [IF] = [CP] = 10 mg L−1 pH = 7 | After 48 h of treatment, IF and CP were degraded only by 36.5% and 28.3%, respectively, containing IF and CP at 10 mg L−1 and revealed no significant decrease in DOC after 48 h of treatment, suggesting that UV degradation was not able to mineralize both compounds. | [90] |
| IF and CP | UV irradiation, UV/TiO2 and UV/H2O2 | [IF] = [CP] = 500 µg L−1 [TiO2] = 100 mg L−1 [H2O2] = 40 mg L−1 λ = 254 nm pH = 7 | Direct photolysis was not able to remove IF and CP in secondary effluent after 180 min of treatment. CP and IF did not degrade negligibly either through UV/TiO2 or through UV/H2O2. | [91] |
| CP | UVC/H2O2/PAW | λ = 254 nm [CP] = 4 ng mL−1 [H2O2] = 10 mg L−1 pH = 7 | The oxidative degradation of CP solutions in tap water by PAW resulted in a complete degradation within 80 min at 150 W. CP was also completely degraded within 60 min applying UVC/H2O2 system. | [92] |
| IBP, NPX and MO | MIL-53(Al)@TiO2 and MIL-53(Al)/ZnO | [compound] = 6 mg L−1 pH = 6.8 catalyst dosage = 2 mg | MIL-53(Al)@TiO2 exhibited high photodegradation efficiency (80.3%) for NPX over 4 h and was recyclable for up to three cycles with only a 13.6% decrease in photodegradability. However, for IBP degradation, MIL-53(Al)@ZnO was observed to be more efficient than MIL-53(Al)@TiO2 and it was found that only 1 h of treatment was sufficient to obtain a considerable COD reduction of 58%. | [93] |
| NPX | UV irradiation, UV/chorine and UV/H2O2 | [NPX] = 5 µmol L−1 [chlorine] = [H2O2] = 50 µmol L−1 λ = 254 nm pH = 7 | NPX was degraded by 27.3% at a UV dosage of 922 mJ cm−2. The degradation of NPX by both AOPs followed pseudo-first-order kinetics, and the first-order rate constant was 4.9 times higher in UV/chlorine than that in UV/H2O2. | [94] |
| IBP, NPX and CTZ | PAN-MWCNT/TiO2–NH2 | [compound] = 5 mg L−1 pH = 2 UV intensity = 40 W catalyst dosage = 15 mg | The complete degradation of IBP required 120 min of treatment, while the same degradation rate for NPX was achieved in 40 min. | [95] |
| KET | UV irradiation | UV intensity = 1700 μW cm−2 [KET] = 16 mg L−1 pH = 7 | The maximum removal rate of KET reached 99.59% within 40 min, which agrees with the pseudo-first-order kinetic equations. | [96] |
| KET | CuO/TiO2@GCN | [KET] = 10 mg L−1 pH = 6.2 (GW) and 7.4 (DW) catalyst dosage = 75 mg | At the best conditions, the impregnation of 1% CuO/TiO2 into GCN presented an efficiency of 94.7% in the photodegradation of KET in deionized water under simulated light. A lower removal efficiency (~50%) was achieved in ground (GW) and drinking water (DW). | [97] |
| IBP | Ag-Ce/TiO2 (Co-DPU) and Ag-Ce/TiO2 (C-IMP) | Visible light = 239 W m−2 λ > 400 nmcatalyst dosage = 0.1 g L−1 [IBP] = 10 mg L−1pH = 5.3–5.6 | The obtained TOC conversion followed the decreasing order: Ag-Ce/TiO2 (Co-DPU) > Ag-Ce/TiO2 (C-IMP) >TiO2. After 4 h, up to 98% mineralization of IBP was obtained for Ag-Ce/TiO2 (Co-DPU). | [98] |
| IBP | UV/H2O2 | [IBP] = 10 µmol L−1 [H2O2] = 0.5 mmol L−1 pH = 7–7.5 λ = 254 nm | IBP was degraded by 8 and 3% under UV and H2O2 only, while the combined UV/H2O2 process removed 78% of IBP within 4 min. | [99] |
| ASA | Photo-Fenton | [ASA] = 10 μg L−1 [Fe2+] = 1.5 mmol L−1 [H2O2] = 45 mmol L−1 UV intensity = 40 W pH = 7 | Using optimized conditions, 90% of mineralization was reached in 10 min. | [100] |
| ASA | UV/O3 | [ASA] = 10 mg L−1 [O3] = 2.4 mg L−1 UV intensity = 2600 μW cm−2 pH = 4.3 | The UV/O3 process was able to remove 99% of ASA after 7 min of treatment. Moreover, the combined process exhibited higher mineralization rate, 47% of TOC reduction, compared with the individual ozonation process (25% of TOC) after 30 min. | [101] |
| ASA | UV/ZnO | UV Intensity = 6 W catalyst dosage = 375.16 mg L−1 [IBP] = 33.84 mg L−1 pH = 5.05 | At the optimized conditions, the ASA removal of 83.11% was achieved. The kinetic studies showed that the pseudo-first-order model had the highest correlation with aspirin removal using the UV/ZnO photocatalytic process. | [102] |
| ASA | 1-Ni-MnON/NG | [ASA] = 75 mg L−1 catalyst dose = 10 mg L−1 pH = 3 UV Intensity = 4.2 W | Almost complete degradation was achieved for the nanocomposites of 1-Ni-MnON/NG after 90 min of treatment. | [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzaga, I.M.D.; Almeida, C.V.S.; Mascaro, L.H. A Critical Review of Photo-Based Advanced Oxidation Processes to Pharmaceutical Degradation. Catalysts 2023, 13, 221. https://doi.org/10.3390/catal13020221
Gonzaga IMD, Almeida CVS, Mascaro LH. A Critical Review of Photo-Based Advanced Oxidation Processes to Pharmaceutical Degradation. Catalysts. 2023; 13(2):221. https://doi.org/10.3390/catal13020221
Chicago/Turabian StyleGonzaga, Isabelle M. D., Caio V. S. Almeida, and Lucia H. Mascaro. 2023. "A Critical Review of Photo-Based Advanced Oxidation Processes to Pharmaceutical Degradation" Catalysts 13, no. 2: 221. https://doi.org/10.3390/catal13020221