Electrocatalytic Treatment of Pharmaceutical Wastewater by Transition Metals Encapsulated by B, N-Doped CNTs
Abstract
:1. Introduction
2. Results
2.1. The Morphology of M@BN-C
2.2. The Catalytic Performance of M@BN-C
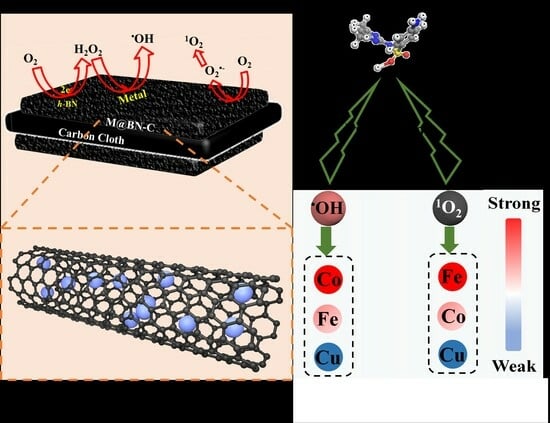
2.3. Catalytic Mechanism of M@BN-C Cathode
2.4. The Effect of pH on Catalytic Performance by M@BN-C
2.5. Cycling Performance of the M@BN-C Cathode
2.6. Application Prospect of the M@BN-C Cathode
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Synthesis of Bifunctional Cathodes
3.3. Experimental Procedure
3.4. Electrochemical Analysis
3.5. Characterization and Analytical Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Phoon, B.L.; Ong, C.C.; Saheed, M.S.M.; Show, P.-L.; Chang, J.-S.; Ling, T.C.; Lam, S.S.; Juan, J.C. Conventional and emerging technologies for removal of antibiotics from wastewater. J. Hazard. Mater. 2020, 400, 122961. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jiao, Y.; Xu, X.; Pan, Y.; Su, C.; Duan, X.; Sun, H.; Liu, S.; Wang, S.; Shao, Z. Superstructures with atomic-level arranged perovskite and oxide layers for advanced oxidation with an enhanced non-free radical pathway. ACS Sustain. Chem. Eng. 2022, 10, 1899–1909. [Google Scholar] [CrossRef]
- Xu, X.; Zhong, Y.; Shao, Z. Double perovskites in catalysis, electrocatalysis, and photo(electro)catalysis. Trends Chem. 2019, 1, 410–424. [Google Scholar] [CrossRef]
- Da Silva, S.W.; Welter, J.B.; Albornoz, L.L.; Heberle, A.N.A.; Ferreira, J.Z.; Bernardes, A.M. Advanced electrochemical oxidation processes in the treatment of pharmaceutical containing water and wastewater: A Review. Curr. Pollut. Rep. 2021, 7, 146–159. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Gandhimathi, R. Trends in electro-Fenton process for water and wastewater treatment: An overview. Desalination 2012, 299, 1–15. [Google Scholar] [CrossRef]
- Barhoumi, N.; Oturan, N.; Ammar, S.; Gadri, A.; Oturan, M.A.; Brillas, E. Enhanced degradation of the antibiotic tetracycline by heterogeneous electro-Fenton with pyrite catalysis. Environ. Chem. Lett. 2017, 15, 689–693. [Google Scholar] [CrossRef]
- Pei, D.-N.; Liu, C.; Zhang, A.-Y.; Pan, X.-Q.; Yu, H.-Q. In situ organic Fenton-like catalysis triggered by anodic polymeric intermediates for electrochemical water purification. Proc. Natl. Acad. Sci. USA 2020, 117, 30966–30972. [Google Scholar] [CrossRef]
- Du, X.; Wang, S.; Ye, F.; Qingrui, Z. Derivatives of metal-organic frameworks for heterogeneous Fenton-like processes: From preparation to performance and mechanisms in wastewater purification—A mini review. Environ. Res. 2022, 206, 112414. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Huong Le, T.X.; Bechelany, M.; Esposito, G.; van Hullebusch, E.D.; Oturan, M.A.; Cretin, M. A hierarchical CoFe-layered double hydroxide modified carbon-felt cathode for heterogeneous electro-Fenton process. J. Mater. Chem. A 2017, 5, 3655–3666. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, G.; Chai, S.; Zhao, H.; Wang, Y. Three-dimensional homogeneous ferrite-carbon aerogel: One pot fabrication and enhanced electro-Fenton reactivity. ACS Appl. Mater. Interfaces 2013, 5, 842–852. [Google Scholar] [CrossRef]
- Chen, W.; Yang, X.; Huang, J.; Zhu, Y.; Zhou, Y.; Yao, Y.; Li, C. Iron oxide containing graphene/carbon nanotube based carbon aerogel as an efficient E-Fenton cathode for the degradation of methyl blue. Electrochim. Acta 2016, 200, 75–83. [Google Scholar] [CrossRef]
- Ri, K.C.; Pak, S.S.; Sun, D.; Zhong, Q.; Yang, S.; Sin, S.I.; Wu, L.; Sun, Y.; Cao, H.; Han, C.; et al. Boron-doped rGO electrocatalyst for high effective generation of hydrogen peroxide: Mechanism and effect of oxygen-enriched air. App. Catal. B: Environ. 2024, 343, 123471. [Google Scholar] [CrossRef]
- Divyapriya, G.; Srinivasan, R.; Nambi, I.M.; Senthilnathan, J. Highly active and stable ferrocene functionalized graphene encapsulated carbon felt array—A novel rotating disc electrode for electro-Fenton oxidation of pharmaceutical compounds. Electrochim. Acta 2018, 283, 858–870. [Google Scholar] [CrossRef]
- Su, P.; Zhou, M.; Lu, X.; Yang, W.; Ren, G.; Cai, J. Electrochemical catalytic mechanism of N-doped graphene for enhanced H2O2 yield and in-situ degradation of organic pollutant. Appl. Catal. B Environ. 2019, 245, 583–595. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, Y.; Chen, Z.; Duan, Y.; Lai, Y.; Fang, Q.; Wang, F.; Li, S. Performance of boron-doped graphene aerogel modified gas diffusion electrode for in-situ metal-free electrochemical advanced oxidation of Bisphenol A. Appl. Catal. B Environ. 2019, 255, 117784. [Google Scholar] [CrossRef]
- Su, P.; Zhou, M.; Ren, G.; Lu, X.; Du, X.; Song, G. A carbon nanotube-confined iron modified cathode with prominent stability and activity for heterogeneous electro-Fenton reactions. J. Mater. Chem. A 2019, 7, 24408–24419. [Google Scholar] [CrossRef]
- Zhao, H.; Qian, L.; Chen, Y.; Wang, Q.; Zhao, G. Selective catalytic two-electron O2 reduction for onsite efficient oxidation reaction in heterogeneous electro-Fenton process. Chem. Eng. J. 2018, 332, 486–498. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Q.; Chen, Y.; Tian, Q.; Zhao, G. Efficient removal of dimethyl phthalate with activated iron-doped carbon aerogel through an integrated adsorption and electro-Fenton oxidation process. Carbon 2017, 124, 111–122. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, Z.; Fan, J.; Majima, T.; Zhao, H.; Zhao, G. Selective electrocatalytic reduction of oxygen to hydroxyl radicals via 3-electron pathway with FeCo alloy encapsulated carbon aerogel for fast and complete removing pollutants. Angew. Chem. Int. Edit. 2021, 60, 10375–10383. [Google Scholar] [CrossRef]
- Su, P.; Fu, W.; Hu, Z.; Jing, J.; Zhou, M. Insights into transition metal encapsulated N-doped CNTs cathode for self-sufficient electrocatalytic degradation. Appl. Catal. B Environ. 2022, 313, 121457. [Google Scholar] [CrossRef]
- Yang, Z.; Qian, J.; Yu, A.; Pan, B. Singlet oxygen mediated iron-based Fenton-like catalysis under nanoconfinement. Proc. Natl. Acad. Sci. USA 2019, 116, 6659–6664. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Qian, L.; Guan, X.; Wu, D.; Zhao, G. Continuous bulk FeCuC aerogel with ultradispersed metal nanoparticles: An efficient 3D heterogeneous electro-Fenton cathode over a wide range of pH 3−9. Environ. Sci. Technol. 2016, 50, 5225–5233. [Google Scholar] [CrossRef] [PubMed]
- Koblenz, T.S.; Wassenaar, J.; Reek, J.N.H. Reactivity within a confined self-assembled nanospace. Chem. Soc. Rev. 2008, 37, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Fu, W.; Du, X.; Guo, J.; Zhou, M. Cost-effective degradation of pollutants by in-situ electrocatalytic process on Fe@BN-C bifunctional cathode: Formation of 1O2 with high selectivity under nanoconfinement. Chem. Eng. J. 2023, 452, 139693. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, T.; Yang, C.; Ma, C.; Zhao, Z.; Wu, Z.; Cao, S.; Geng, W.; Wang, Y.; Yao, Y.; et al. Activity trends and mechanisms in peroxymonosulfate-assisted catalytic production of singlet oxygen over atomic metal-N-C catalysts. Angew. Chem. Int. Ed. 2021, 60, 22513–22521. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, X.; Han, Y.; Yue, D.; Cao, X.; Zhao, Y.; Qian, X. Highly efficient utilization of nano-Fe(0) embedded in mesoporous carbon for activation of peroxydisulfate. Environ. Sci. Technol. 2019, 53, 9081–9090. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ren, N.; Xi, J.; Liu, Y.; Kong, T.; Chen, C.; Xie, Y.; Duan, X.; Wang, S. Mechanistic investigations of the pyridinic N−Co structures in Co embedded N-doped carbon nanotubes for catalytic ozonation. ACS EST Eng. 2021, 1, 32–45. [Google Scholar] [CrossRef]
- Fu, W.; Hu, Z.; Zheng, Y.; Su, P.; Zhang, Q.; Jiao, Y.; Zhou, M. Tuning mobility of intermediate and electron transfer to enhance electrochemical reduction of nitrate to ammonia on Cu2O/Cu interface. Chem. Eng. J. 2021, 433, 133680. [Google Scholar] [CrossRef]
- Ding, D.; Yang, S.; Qian, X.; Chen, L.; Cai, T. Nitrogen-doping positively whilst sulfur-doping negatively affect the catalytic activity of biochar for the degradation of organic contaminant. Appl. Catal. B Environ. 2020, 263, 118348. [Google Scholar] [CrossRef]
- Liu, C.; Li, H.; Liu, F.; Chen, J.; Yu, Z.; Yuan, Z.; Wang, C.; Zheng, H.; Henkelman, G.; Wei, L.; et al. Intrinsic activity of metal centers in metal–nitrogen–carbon single-atom catalysts for hydrogen peroxide synthesis. J. Am. Chem. Soc. 2020, 142, 21861–21871. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, M.; Ren, G.; Li, Y.; Li, Y.; Du, X. Highly efficient electrosynthesis of hydrogen peroxide on a superhydrophobic three-phase interface by natural air diffusion. Nat. Commun. 2020, 11, 1731. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Quan, X.; Zhao, K.; Chen, S.; Yu, H.; Su, Y. High-eefficiency electrocatalysis of molecular oxygen toward hydroxyl radicals enabled by an atomically dispersed iron catalyst. Environ. Sci. Technol. 2020, 54, 12662–12672. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Quan, X.; Zhao, K.; Chen, S.; Yu, H.; Niu, J. Selective electrochemical H2O2 generation and activation on a bifunctional catalyst for heterogeneous electro-Fenton catalysis. J. Hazard. Mater. 2020, 382, 121102. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Fu, W.; Wang, Q.; Tang, Y.; Zhou, M. High electron transfer rate and efficiency on Fe0 modified by sulfidation and pre-magnetization for carbamazepine degradation by heterogeneous electro-Fenton in wide pH ranges. Chem. Eng. J. 2022, 427, 131694. [Google Scholar] [CrossRef]
- Feng, Z.; Tian, Q.; Yang, Q.; Zhou, Y.; Zhao, H.; Zhao, G. Selectively photoelectrocatalytic reduction of oxygen to hydroxyl radical and singlet oxygen: Mechanism and validation in coal wastewater. Appl. Catal. B Environ. 2021, 286, 119908. [Google Scholar] [CrossRef]
- Zhang, L.-S.; Jiang, X.-H.; Zhong, Z.-A.; Tian, L.; Sun, Q.; Cui, Y.-T.; Lu, X.; Zou, J.-P.; Luo, S.-L. Carbon nitride supported high-loading Fe single-atom catalyst for activation of peroxymonosulfate to generate 1O2 with 100 % selectivity. Angew. Chem. Int. Edit. 2021, 60, 21751–21755. [Google Scholar] [CrossRef]
- Zuo, S.; Jin, X.; Wang, X.; Lu, Y.; Zhu, Q.; Wang, J.; Liu, W.; Du, Y.; Wang, J. Sandwich structure stabilized atomic Fe catalyst for highly efficient Fenton-like reaction at all pH values. Appl. Catal. B Environ. 2021, 282, 119551. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, M.; Wang, X.; Wang, C.; Lu, D.; Ma, W.; Kube, S.A.; Ma, J.; Elimelech, M. Janus electrocatalytic flow-through membrane enables highly selective singlet oxygen production. Nat. Commun. 2020, 11, 6228. [Google Scholar] [CrossRef]
- Ramel, F.; Birtic, S.; Ginies, C.; Soubigou-Taconnat, L.; Triantaphylidès, C.; Havaux, M. Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc. Natl. Acad. Sci. USA 2012, 109, 5535–5540. [Google Scholar] [CrossRef]
- Fan, Y.; Ji, Y.; Kong, D.; Lu, J.; Zhou, Q. Kinetic and mechanistic investigations of the degradation of sulfamethazine in heat-activated persulfate oxidation process. J. Hazard. Mater. 2015, 300, 39–47. [Google Scholar] [CrossRef]
- Chen, C.; Liu, L.; Li, Y.; Li, W.; Zhou, L.; Lan, Y.; Li, Y. Insight into heterogeneous catalytic degradation of sulfamethazine by peroxymonosulfate activated with CuCo2O4 derived from bimetallic oxalate. Chem. Eng. J. 2020, 384, 123257. [Google Scholar] [CrossRef]
- Ge, L.; Zhang, P.; Halsall, C.; Li, Y.; Chen, C.-E.; Li, J.; Sun, H.; Yao, Z. The importance of reactive oxygen species on the aqueous phototransformation of sulfonamide antibiotics: Kinetics, pathways, and comparisons with direct photolysis. Water Res. 2019, 149, 243–250. [Google Scholar] [CrossRef] [PubMed]
- El Jery, A.; Aldrdery, M.; Shirode, U.R.; Gavilán, J.C.; Elkhaleefa, A.; Sillanpää, M.; Sammen, S.S.; Tizkam, H.H. An efficient investigation and machine learning-based prediction of decolorization of wastewater by using zeolite catalyst in electro-Fenton reaction. Catalysts 2023, 13, 1085. [Google Scholar] [CrossRef]
- Adachi, A.; Ouadrhiri, F.E.; Kara, M.; El Manssouri, I.; Assouguem, A.; Almutairi, M.H.; Bayram, R.; Mohamed, H.R.H.; Peluso, I.; Eloutassi, N.; et al. Decolorization and degradation of methyl orange azo dye in aqueous solution by the electro-Fenton process: Application of optimization. Catalysts 2022, 12, 665. [Google Scholar] [CrossRef]
- Li, T.; Ge, L.; Peng, X.; Wang, W.; Zhang, W. Enhanced degradation of sulfamethoxazole by a novel Fenton-like system with significantly reduced consumption of H2O2 activated by g-C3N4/MgO composite. Water Res. 2021, 190, 116777. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Pervez, M.N.; Sun, P.; Cao, C.; Li, B.; Naddeo, V.; Jin, W.; Zhao, Y. Highly efficient removal of bisphenol A by a novel Co-doped LaFeO3 perovskite/PMS system in salinity water. Sci. Total Environ. 2021, 801, 149490. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wu, Y.; Gao, Y.; Li, H.; Chen, Z. Effect of humic acid, oxalate and phosphate on Fenton-like oxidation of microcystin-LR by nanoscale zero-valent iron. Sep. Purif. Technol. 2016, 170, 337–343. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, D.; Chen, Y.; Hu, Y. Enhanced degradation of triclosan in heterogeneous E-Fenton process with MOF-derived hierarchical Mn/Fe@PC modified cathode. Chem. Eng. J. 2020, 384, 123324. [Google Scholar] [CrossRef]
- Yu, F.; Wang, Y.; Ma, H.; Zhou, M. Hydrothermal synthesis of FeS2 as a highly efficient heterogeneous electro-Fenton catalyst to degrade diclofenac via molecular oxygen effects for Fe(II)/Fe(III) cycle. Sep. Purif. Technol. 2020, 248, 117022. [Google Scholar] [CrossRef]









| Mati Lake | Reverse Osmosis Concentrate | Pharmaceutical Wastewater | |
|---|---|---|---|
| pH | 8.0 | 8.3 | 7.8 |
| Conductivity (μS cm−1) | 1198 | 4879 | 184.3 |
| COD (mg L−1) | 104.4 | 127.4 | 148.3 |
| TOC (mg L−1) | 32.0 | 509.1 | 40.3 |
| Cl− (mg L−1) | 115.2 | 3384.4 | 40.9 |
| HCO3− (mg L−1) | 44.9 | 492.4 | - |
| NO3− (mg L−1) | 54.8 | - | 5.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sha, O.; Lu, X.; Su, P. Electrocatalytic Treatment of Pharmaceutical Wastewater by Transition Metals Encapsulated by B, N-Doped CNTs. Catalysts 2023, 13, 1459. https://doi.org/10.3390/catal13121459
Sha O, Lu X, Su P. Electrocatalytic Treatment of Pharmaceutical Wastewater by Transition Metals Encapsulated by B, N-Doped CNTs. Catalysts. 2023; 13(12):1459. https://doi.org/10.3390/catal13121459
Chicago/Turabian StyleSha, Ou, Xifeng Lu, and Pei Su. 2023. "Electrocatalytic Treatment of Pharmaceutical Wastewater by Transition Metals Encapsulated by B, N-Doped CNTs" Catalysts 13, no. 12: 1459. https://doi.org/10.3390/catal13121459