Green Synthesis of Biocatalysts Based on Nanocarriers Promises an Effective Role in Pharmaceutical and Biomedical Fields
Abstract
:1. Introduction
2. Enzyme Immobilization
2.1. Advantages of Enzyme Immobilization
2.2. Enzyme Immobilization Techniques
2.2.1. Physical Adsorption
2.2.2. Covalent Bonding
2.2.3. Entrapment
3. Characteristics of Nanomaterials Used for NBCs
3.1. Nanomaterial-Mediated Charge Transport
3.2. Using Nanomaterials to Deliver Membrane-Impermeable Enzymes
3.3. Nanomaterial-Mediated Remote Control of Enzyme Activity
3.4. Creation of Catalytic Systems with Synergistic Functions
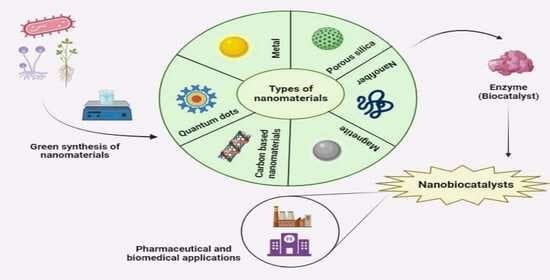
4. Types of Nanomaterials Used for NBCs
4.1. Metal Nanomaterials
4.2. Carbon-Based Nanomaterials
4.3. Porous Silica
4.4. Magnetite Nanoparticles
4.5. Quantum Dots
4.6. Nanofiber
5. Synthesis of Nanomaterials
5.1. Green Synthesis of Nanomaterials
5.1.1. Bacterial Synthesis
5.1.2. Fungal Synthesis
5.1.3. Algal Synthesis
6. Modification of Nanomaterials for NBCs
6.1. Silica Nanoparticle Modification
6.2. Magnetic Nanoparticle Modification
6.3. Gold Nanoparticles Modification
7. Applications of Nanobiocatalysis
7.1. Pharmaceutical Industry
7.2. Biomedical
7.2.1. Nano-Enzymes for Thrombolytic Therapy
7.2.2. Nano-Enzymes for Treatment of Oxidative Stress and Inflammation
7.2.3. Nano-Enzymes for Antibacterial Treatment
7.2.4. Drug Delivery Systems
7.3. Biotechnological
8. Research Needs and Future Directions
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| NBCs | Nanobiocatalysts |
| NER | Nanoscale enzyme reactor technique |
| NER | Nanoparticle Enzyme Reactor |
| MNPs | Magnetite nanoparticles |
| QDs | Quantum dots |
| NFs | Nanostructured fibers |
| APTES | (3-aminopropyl) triethoxysilane |
| GA | Glutaraldehyde |
| AuNPs | Gold nanoparticles |
| MMPs | Matrix metalloproteinases |
| Gox | Glucose oxidase |
| tPA | Tissue plasminogen activator |
| uPA | Urokinase-type plasminogen activator |
| SOD | Superoxide dismutase |
| CNS | Central nervous system |
| PBCA | Poly-butyl cyanoacrylate |
| MS | Mass spectrometry |
| GO | Graphene oxide |
| GSH-CdTeQD | Glutathione capped CdTe Quantum dots |
| CV | Cyclic voltammetry |
| EIS | Electrochemical impedance spectroscopy |
| SECM | Scanning electrochemical microscopy |
| ZS | Zetasizer Nano |
| PAN | Polyacrylonitrile |
| AgNPs | Silver nanoparticles |
| PLA2 | Phospholipase A2 |
| MPTMS | 3-mercaptopropyltrimethoxysilane |
| PLGA | Poly(lactic-co-glycolic acid) |
| PEG | Polyethylene glycol |
| 3-GPTMS | 3-Glycidyloxypropyl trimethoxysilane |
| SWCNTs | Single-walled carbon nanotubes |
| MWCNTs | Multi-walled carbon nanotubes |
| PGA | Penicillin G acylase |
| ABC | Accelerated blood clearance |
| PS | Polystyrene |
References
- Del Arco, J.; Alcántara, A.R.; Fernández-Lafuente, R.; Fernández-Lucas, J. Magnetic Micro-Macro Biocatalysts Applied to Industrial Bioprocesses. Bioresour. Technol. 2021, 322, 124547. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N. Chemical, Physical, and Biological Coordination: An Interplay between Materials and Enzymes as Potential Platforms for Immobilization. Coord. Chem. Rev. 2019, 388, 1–23. [Google Scholar] [CrossRef]
- Gkantzou, E.; Chatzikonstantinou, A.V.; Fotiadou, R.; Giannakopoulou, A.; Patila, M.; Stamatis, H. Trends in the Development of Innovative Nanobiocatalysts and Their Application in Biocatalytic Transformations. Biotechnol. Adv. 2021, 51, 107738. [Google Scholar] [CrossRef] [PubMed]
- Murugappan, G.; Sreeram, K.J. Nano-Biocatalyst: Bi-Functionalization of Protease and Amylase on Copper Oxide Nanoparticles. Colloids Surf. B Biointerfaces 2021, 197, 111386. [Google Scholar] [CrossRef] [PubMed]
- Misson, M.; Zhang, H.; Jin, B. Nanobiocatalyst Advancements and Bioprocessing Applications. J. R. Soc. Interface 2015, 12, 20140891. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An Overview of Technologies for Immobilization of Enzymes and Surface Analysis Techniques for Immobilized Enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef]
- Liu, D.-M.; Dong, C. Recent Advances in Nano-Carrier Immobilized Enzymes and Their Applications. Process Biochem. 2020, 92, 464–475. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Pelt, S. van Enzyme Immobilisation in Biocatalysis: Why, What and How. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef]
- Iyer, P.V.; Ananthanarayan, L. Enzyme Stability and Stabilization—Aqueous and Non-Aqueous Environment. Process Biochem. 2008, 43, 1019–1032. [Google Scholar] [CrossRef]
- Ribeiro, E.S.; de Farias, B.S.; Sant’Anna Cadaval Junior, T.R.; de Almeida Pinto, L.A.; Diaz, P.S. Chitosan–Based Nanofibers for Enzyme Immobilization. Int. J. Biol. Macromol. 2021, 183, 1959–1970. [Google Scholar] [CrossRef]
- Suresh, R.; Rajendran, S.; Khoo, K.S.; Soto-Moscoso, M. Enzyme Immobilized Nanomaterials: An Electrochemical Bio-Sensing and Biocatalytic Degradation Properties Toward Organic Pollutants. Top. Catal. 2023, 66, 691–706. [Google Scholar] [CrossRef]
- Wahab, R.A.; Elias, N.; Abdullah, F.; Ghoshal, S.K. On the Taught New Tricks of Enzymes Immobilization: An All-Inclusive Overview. React. Funct. Polym. 2020, 152, 104613. [Google Scholar] [CrossRef]
- Barbosa, O.; Torres, R.; Ortiz, C.; Berenguer-Murcia, Á.; Rodrigues, R.C.; Fernandez-Lafuente, R. Heterofunctional Supports in Enzyme Immobilization: From Traditional Immobilization Protocols to Opportunities in Tuning Enzyme Properties. Biomacromolecules 2013, 14, 2433–2462. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying Enzyme Activity and Selectivity by Immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef]
- Abdelhamid, M.A.A.; Meligy, A.M.A.; Yeo, K.B.; Lee, C.-S.; Pack, S.P. Silaffin-3-Derived Pentalysine Cluster as a New Fusion Tag for One-Step Immobilization and Purification of Recombinant Bacillus Subtilis Catalase on Bare Silica Particles. Int. J. Biol. Macromol. 2020, 159, 1103–1112. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Strategies for the One-Step Immobilization–Purification of Enzymes as Industrial Biocatalysts. Biotechnol. Adv. 2015, 33, 435–456. [Google Scholar] [CrossRef]
- Zhao, F.; Song, Q.; Wang, B.; Han, Y.; Zhou, Z. Purification and Immobilization of the Soluble and Insoluble Portions of Recombinant Lipase by Gram-Positive Enhancer Matrix (GEM) Particles. Int. J. Biol. Macromol. 2020, 145, 1099–1105. [Google Scholar] [CrossRef]
- Reshmy, R.; Philip, E.; Sirohi, R.; Tarafdar, A.; Arun, K.B.; Madhavan, A.; Binod, P.; Kumar Awasthi, M.; Varjani, S.; Szakacs, G.; et al. Nanobiocatalysts: Advancements and Applications in Enzyme Technology. Bioresour. Technol. 2021, 337, 125491. [Google Scholar] [CrossRef]
- Kim, H.S.; Hong, S.-G.; Woo, K.M.; Teijeiro Seijas, V.; Kim, S.; Lee, J.; Kim, J. Precipitation-Based Nanoscale Enzyme Reactor with Improved Loading, Stability, and Mass Transfer for Enzymatic CO2 Conversion and Utilization. ACS Catal. 2018, 8, 6526–6536. [Google Scholar] [CrossRef]
- Qamar, S.A.; Asgher, M.; Bilal, M. Immobilization of Alkaline Protease From Bacillus Brevis Using Ca-Alginate Entrapment Strategy for Improved Catalytic Stability, Silver Recovery, and Dehairing Potentialities. Catal. Lett. 2020, 150, 3572–3583. [Google Scholar] [CrossRef]
- Imam, H.T.; Marr, P.C.; Marr, A.C. Enzyme Entrapment, Biocatalyst Immobilization without Covalent Attachment. Green Chem. 2021, 23, 4980–5005. [Google Scholar] [CrossRef]
- Bilal, M.; Qamar, S.A.; Ashraf, S.S.; Rodríguez-Couto, S.; Iqbal, H.M.N. Robust Nanocarriers to Engineer Nanobiocatalysts for Bioprocessing Applications. Adv. Colloid Interface Sci. 2021, 293, 102438. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Nie, M.; Li, Y.; Zhu, H.; Shi, G. Design of Composite Nanosupports and Applications Thereof in Enzyme Immobilization: A Review. Colloids Surf. B Biointerfaces 2022, 217, 112602. [Google Scholar] [CrossRef] [PubMed]
- Reiss, R.; Ihssen, J.; Richter, M.; Eichhorn, E.; Schilling, B.; Thöny-Meyer, L. Laccase versus Laccase-like Multi-Copper Oxidase: A Comparative Study of Similar Enzymes with Diverse Substrate Spectra. PLoS ONE 2013, 8, e65633. [Google Scholar] [CrossRef] [PubMed]
- Chiadò, A.; Bosco, F.; Bardelli, M.; Simonelli, L.; Pedotti, M.; Marmo, L.; Varani, L. Rational Engineering of the Lccβ T. Versicolor Laccase for the Mediator-Less Oxidation of Large Polycyclic Aromatic Hydrocarbons. Comput. Struct. Biotechnol. J. 2021, 19, 2213–2222. [Google Scholar] [CrossRef]
- Wang, L.; Ding, X.; Huang, Q.; Hu, B.; Liang, L.; Wang, Q. Gllac7 Is Induced by Agricultural and Forestry Residues and Exhibits Allelic Expression Bias in Ganoderma Lucidum. Front. Microbiol. 2022, 13, 890686. [Google Scholar] [CrossRef]
- Li, Z.; Zeng, H.-Y.; Cao, X.-J.; Li, H.-B.; Long, Y.-W.; Feng, B.; Lv, S.-B. High-Sensitive Sensor for the Simultaneous Determination of Phenolics Based on Multi-Walled Carbon Nanotube/NiCoAl Hydrotalcite Electrode Material. Microchim Acta 2021, 188, 308. [Google Scholar] [CrossRef]
- dos Santos, J.C.S.; Barbosa, O.; Ortiz, C.; Berenguer-Murcia, A.; Rodrigues, R.C.; Fernandez-Lafuente, R. Importance of the Support Properties for Immobilization or Purification of Enzymes. ChemCatChem 2015, 7, 2413–2432. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, Z.; Liu, X.Y. Using Inorganic Nanomaterials to Endow Biocatalytic Systems with Unique Features. Trends Biotechnol. 2016, 34, 303–315. [Google Scholar] [CrossRef]
- Heller, A. Electrical Wiring of Redox Enzymes. Acc. Chem. Res. 1990, 23, 128–134. [Google Scholar] [CrossRef]
- Willner, I.; Basnar, B.; Willner, B. Nanoparticle-Enzyme Hybrid Systems for Nanobiotechnology. FEBS J. 2007, 274, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Zayats, M.; Willner, B.; Willner, I. Design of Amperometric Biosensors and Biofuel Cells by the Reconstitution of Electrically Contacted Enzyme Electrodes. Electroanalysis 2008, 20, 583–601. [Google Scholar] [CrossRef]
- Kohse, S.; Neubauer, A.; Pazidis, A.; Lochbrunner, S.; Kragl, U. Photoswitching of Enzyme Activity by Laser-Induced pH-Jump. J. Am. Chem. Soc. 2013, 135, 9407–9411. [Google Scholar] [CrossRef] [PubMed]
- Mayer, G.; Heckel, A. Biologically Active Molecules with a “Light Switch”. Angew. Chem. Int. Ed. Engl. 2006, 45, 4900–4921. [Google Scholar] [CrossRef] [PubMed]
- Zayats, M.; Baron, R.; Popov, I.; Willner, I. Biocatalytic Growth of Au Nanoparticles: From Mechanistic Aspects to Biosensors Design. Nano Lett. 2005, 5, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Shalini, P.; Deepanraj, B.; Vijayalakshmi, S.; Ranjitha, J. Synthesis and Characterisation of Lipase Immobilised Magnetic Nanoparticles and Its Role as a Catalyst in Biodiesel Production. Mater. Today Proc. 2023, 80, 2725–2730. [Google Scholar] [CrossRef]
- Yu, D.; Li, Z.; Zhou, X.; Wang, W.; Wang, L.; Liu, T.; Du, J. Study on the Modification of Magnetic Graphene Oxide and the Effect of Immobilized Lipase. Int. J. Biol. Macromol. 2022, 216, 498–509. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, Q.; Gu, P.; Ma, L.; Wang, Y. Engineering of a Novel, Magnetic, Bi-Functional, Enzymatic Nanobiocatalyst for the Highly Efficient Synthesis of Enantiopure (R)-3-Quinuclidinol. Catalysts 2021, 11, 1126. [Google Scholar] [CrossRef]
- Rashid, S.S.; Mustafa, A.H.; Ab Rahim, M.H. Ferromagnetic Nanoparticles Synthesis and Functionalization for Laccase Enzyme Immobilization. Mater. Today Proc. 2022, 48, 916–919. [Google Scholar] [CrossRef]
- Lozano-López, D.; Galván-Valencia, M.; Rojas-de Soto, I.; Escalona-Villalpando, R.A.; Ledesma-García, J.; Durón-Torres, S. Immobilization of Glucose Oxidase on Glutathione Capped CdTe Quantum Dots for Bioenergy Generation. Catalysts 2022, 12, 1659. [Google Scholar] [CrossRef]
- Ünlüer, Ö.B.; Ecevit, K.; Diltemiz, S.E. Carbonic Anhydrase Carrying Electrospun Nanofibers for Biocatalysis Applications. Protein Pept. Lett. 2021, 28, 520–532. [Google Scholar] [CrossRef]
- Khafaga, D.S.R.; Ewies, E.F. Drug Delivery Systems Designed to Maximize the Therapeutic Efficacy of Herbal Medication: A Review Article. Egypt. J. Chem. 2023, 66, 477–485. [Google Scholar] [CrossRef]
- San, B.H.; Kim, S.; Moh, S.H.; Lee, H.; Jung, D.-Y.; Kim, K.K. Platinum Nanoparticles Encapsulated by Aminopeptidase: A Multifunctional Bioinorganic Nanohybrid Catalyst. Angew. Chem. Int. Ed. Engl. 2011, 50, 11924–11929. [Google Scholar] [CrossRef]
- Tan, W.Y.; Gopinath, S.C.B.; Anbu, P.; Yaakub, A.R.W.; Subramaniam, S.; Chen, Y.; Sasidharan, S. Bio-Enzyme Hybrid with Nanomaterials: A Potential Cargo as Sustainable Biocatalyst. Sustainability 2023, 15, 7511. [Google Scholar] [CrossRef]
- Ipe, B.I.; Lehnig, M.; Niemeyer, C.M. On the Generation of Free Radical Species from Quantum Dots. Small 2005, 1, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Roy, A.; Bharadvaja, N. Qualitative Analysis of Phytocompounds and Synthesis of Silver Nanoparticles from Centella Asiatica. Innov. Tech. Agric. 2017, 1, 8. [Google Scholar]
- Nath, D.; Banerjee, P. Green Nanotechnology – A New Hope for Medical Biology. Environ. Toxicol. Pharmacol. 2013, 36, 997–1014. [Google Scholar] [CrossRef]
- Bharadvaja, N.; Roy, A. Silver Nanoparticles Synthesis from a Pharmaceutically Important Medicinal Plant Plumbago Zeylanica. MOJ Bioequivalence Bioavailab. 2017, 3, 118–121. [Google Scholar] [CrossRef]
- Dsr, K.; Am, E.-K.; Ra, E.M.; Hk, A. Green Synthesis of Nano-Based Drug Delivery Systems Developed for Hepatocellular Carcinoma Treatment: A Review. Mol. Biol. Rep. 2023. [CrossRef]
- Liang, X.-J.; Kumar, A.; Shi, D.; Cui, D. Nanostructures for Medicine and Pharmaceuticals. J. Nanomater. 2012, 2012, e921897. [Google Scholar] [CrossRef]
- Auría-Soro, C.; Nesma, T.; Juanes-Velasco, P.; Landeira-Viñuela, A.; Fidalgo-Gomez, H.; Acebes-Fernandez, V.; Gongora, R.; Almendral Parra, M.J.; Manzano-Roman, R.; Fuentes, M. Interactions of Nanoparticles and Biosystems: Microenvironment of Nanoparticles and Biomolecules in Nanomedicine. Nanomaterials 2019, 9, 1365. [Google Scholar] [CrossRef] [PubMed]
- Guilger-Casagrande, M.; de Lima, R. Synthesis of Silver Nanoparticles Mediated by Fungi: A Review. Front. Bioeng. Biotechnol. 2019, 7, 287. [Google Scholar] [CrossRef] [PubMed]
- Huynh, K.-H.; Pham, X.-H.; Kim, J.; Lee, S.H.; Chang, H.; Rho, W.-Y.; Jun, B.-H. Synthesis, Properties, and Biological Applications of Metallic Alloy Nanoparticles. Int. J. Mol. Sci. 2020, 21, 5174. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Senapati, S.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular Biosynthesis of Monodisperse Gold Nanoparticles by a Novel Extremophilic Actinomycete, Thermomonospora Sp. Langmuir 2003, 19, 3550–3553. [Google Scholar] [CrossRef]
- He, S.; Guo, Z.; Zhang, Y.; Zhang, S.; Wang, J.; Gu, N. Biosynthesis of Gold Nanoparticles Using the Bacteria Rhodopseudomonas Capsulata. Mater. Lett. 2007, 61, 3984–3987. [Google Scholar] [CrossRef]
- Parikh, R.Y.; Singh, S.; Prasad, B.L.V.; Patole, M.S.; Sastry, M.; Shouche, Y.S. Extracellular Synthesis of Crystalline Silver Nanoparticles and Molecular Evidence of Silver Resistance from Morganella Sp.: Towards Understanding Biochemical Synthesis Mechanism. Chembiochem 2008, 9, 1415–1422. [Google Scholar] [CrossRef]
- Reddy, A.S.; Chen, C.-Y.; Chen, C.-C.; Jean, J.-S.; Chen, H.-R.; Tseng, M.-J.; Fan, C.-W.; Wang, J.-C. Biological Synthesis of Gold and Silver Nanoparticles Mediated by the Bacteria Bacillus Subtilis. J. Nanosci. Nanotechnol. 2010, 10, 6567–6574. [Google Scholar] [CrossRef]
- Bruna, N.; Collao, B.; Tello, A.; Caravantes, P.; Díaz-Silva, N.; Monrás, J.P.; Órdenes-Aenishanslins, N.; Flores, M.; Espinoza-Gonzalez, R.; Bravo, D.; et al. Synthesis of Salt-Stable Fluorescent Nanoparticles (Quantum Dots) by Polyextremophile Halophilic Bacteria. Sci. Rep. 2019, 9, 1953. [Google Scholar] [CrossRef]
- Mukherjee, P.; Ahmad, A.; Mandal, D.; Senapati, S.; Sainkar, S.R.; Khan, M.I.; Parishcha, R.; Ajaykumar, P.V.; Alam, M.; Kumar, R.; et al. Fungus-Mediated Synthesis of Silver Nanoparticles and Their Immobilization in the Mycelial Matrix: A Novel Biological Approach to Nanoparticle Synthesis. Nano Lett. 2001, 1, 515–519. [Google Scholar] [CrossRef]
- Rashwan, D.S.; Abd El Hamed, M.M.; El-deen, M.D.; Afify, M.M.; Mohamed, M.H.; Mohamed, A.E.R.F.; Nagy, R.A.; Elhakim, H.K.A. Green Synthesis of Zinc Oxide Nanocomposite Using Fusarium Oxysporum and Evaluation of the Anticancer Effect on Hepatocellular Carcinoma. Egypt. J. Chem. 2022, 65, 197–207. [Google Scholar] [CrossRef]
- Nagy, R.A.A.; Mohamed, M.H.; Elhakim, H.K.A.; Abd El-Maksoud, M.D.E.; Afify, M.; Mohamed, A.E.R.F.; Eid, M.M.; Khafaga, D.S.R. Anticancer Effect of Sorafenib-Loaded Iron Oxide Nanoparticles and Bee Venom on Some Genes Expression in Hepatocellular Carcinoma. Egypt. J. Chem. 2022, 65, 1477–1487. [Google Scholar] [CrossRef]
- Bhainsa, K.C.; D’Souza, S.F. Extracellular Biosynthesis of Silver Nanoparticles Using the Fungus Aspergillus Fumigatus. Colloids Surf. B Biointerfaces 2006, 47, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Vahabi, K.; Mansoori, G.A.; Karimi, S. Biosynthesis of Silver Nanoparticles by Fungus Trichoderma Reesei (A Route for Large-Scale Production of AgNPs). Insci. J. 2011, 1, 65–79. [Google Scholar] [CrossRef]
- Mata, Y.N.; Torres, E.; Blázquez, M.L.; Ballester, A.; González, F.; Muñoz, J.A. Gold(III) Biosorption and Bioreduction with the Brown Alga Fucus Vesiculosus. J. Hazard. Mater. 2009, 166, 612–618. [Google Scholar] [CrossRef]
- Shakibaie, M.; Forootanfar, H.; Mollazadeh-Moghaddam, K.; Bagherzadeh, Z.; Nafissi-Varcheh, N.; Shahverdi, A.R.; Faramarzi, M.A. Green Synthesis of Gold Nanoparticles by the Marine Microalga Tetraselmis Suecica. Biotechnol. Appl. Biochem. 2010, 57, 71–75. [Google Scholar] [CrossRef]
- Barwal, I.; Ranjan, P.; Kateriya, S.; Yadav, S.C. Cellular Oxido-Reductive Proteins of Chlamydomonas Reinhardtii Control the Biosynthesis of Silver Nanoparticles. J. Nanobiotechnology 2011, 9, 56. [Google Scholar] [CrossRef]
- Sanaeimehr, Z.; Javadi, I.; Namvar, F. Antiangiogenic and Antiapoptotic Effects of Green-Synthesized Zinc Oxide Nanoparticles Using Sargassum Muticum Algae Extraction. Cancer Nanotechnol. 2018, 9, 3. [Google Scholar] [CrossRef]
- Fatima, R.; Priya, M.; Indurthi, L.; Radhakrishnan, V.; Sudhakaran, R. Biosynthesis of Silver Nanoparticles Using Red Algae Portieria Hornemannii and Its Antibacterial Activity against Fish Pathogens. Microb. Pathog. 2020, 138, 103780. [Google Scholar] [CrossRef]
- Nair, B.; Pradeep, T. Coalescence of Nanoclusters and Formation of Submicron Crystallites Assisted by Lactobacillus Strains. Cryst. Growth Des. 2002, 2, 293–298. [Google Scholar] [CrossRef]
- Dorcheh, S.K.; Vahabi, K. Biosynthesis of Nanoparticles by Fungi: Large-Scale Production. In Fungal Metabolites; Mérillon, J.-M., Ramawat, K.G., Eds.; Reference Series in Phytochemistry; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–20. ISBN 978-3-319-19456-1. [Google Scholar]
- Molnár, Z.; Bódai, V.; Szakacs, G.; Erdélyi, B.; Fogarassy, Z.; Sáfrán, G.; Varga, T.; Kónya, Z.; Tóth-Szeles, E.; Szűcs, R.; et al. Green Synthesis of Gold Nanoparticles by Thermophilic Filamentous Fungi. Sci. Rep. 2018, 8, 3943. [Google Scholar] [CrossRef] [PubMed]
- Gudikandula, K.; Vadapally, P.; Singara Charya, M.A. Biogenic Synthesis of Silver Nanoparticles from White Rot Fungi: Their Characterization and Antibacterial Studies. OpenNano 2017, 2, 64–78. [Google Scholar] [CrossRef]
- Thajuddin, N.; Dhanasekaran, D. Algae: Organisms for Imminent Biotechnology; InTech: London, UK, 2016; ISBN 978-953-51-2431-3. [Google Scholar] [CrossRef]
- Singaravelu, G.; Arockiamary, J.S.; Kumar, V.G.; Govindaraju, K. A Novel Extracellular Synthesis of Monodisperse Gold Nanoparticles Using Marine Alga, Sargassum Wightii Greville. Colloids Surf. B Biointerfaces 2007, 57, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, X.; Shen, D.; Wu, F.; Pleixats, R.; Pan, J. Functionalized Silica Nanoparticles: Classification, Synthetic Approaches and Recent Advances in Adsorption Applications. Nanoscale 2021, 13, 15998–16016. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Feng, Y.; Wang, G.; Wang, Z.; Bilal, M.; Lv, H.; Jia, S.; Cui, J. Production and Use of Immobilized Lipases in/on Nanomaterials: A Review from the Waste to Biodiesel Production. Int. J. Biol. Macromol. 2020, 152, 207–222. [Google Scholar] [CrossRef]
- Zhao, K.; Kang, S.-X.; Yang, Y.-Y.; Yu, D.-G. Electrospun Functional Nanofiber Membrane for Antibiotic Removal in Water: Review. Polymers 2021, 13, 226. [Google Scholar] [CrossRef]
- Xu, J.; Sun, J.; Wang, Y.; Sheng, J.; Wang, F.; Sun, M. Application of Iron Magnetic Nanoparticles in Protein Immobilization. Molecules 2014, 19, 11465–11486. [Google Scholar] [CrossRef]
- Knothe, G. Analyzing Biodiesel: Standards and Other Methods. J. Am. Oil Chem. Soc. 2006, 83, 823–833. [Google Scholar] [CrossRef]
- Kobayashi, H.; Matsunaga, T. Amino-Silane Modified Superparamagnetic Particles with Surface-Immobilized Enzyme. J. Colloid Interface Sci. 1991, 141, 505–511. [Google Scholar] [CrossRef]
- Thangaraj, B.; Jia, Z.; Dai, L.; Liu, D.; Du, W. Effect of Silica Coating on Fe3O4 Magnetic Nanoparticles for Lipase Immobilization and Their Application for Biodiesel Production. Arab. J. Chem. 2019, 12, 4694–4706. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A Review of Synthesis Methods, Properties, Recent Progress, and Challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Wan, D.; Yan, C.; Zhang, Q. Facile and Rapid Synthesis of Hollow Magnetic Mesoporous Polydopamine Nanoflowers with Tunable Pore Structures for Lipase Immobilization: Green Production of Biodiesel. Ind. Eng. Chem. Res. 2019, 58, 16358–16369. [Google Scholar] [CrossRef]
- Dai, Y.; Liu, C.C. Detection of 17 β-Estradiol in Environmental Samples and for Health Care Using a Single-Use, Cost-Effective Biosensor Based on Differential Pulse Voltammetry (DPV). Biosensors 2017, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Alshanberi, A.M.; Ansari*, S.A. Validation and Optimization of Polyvinyl Alcohol-Functionalized Gold Nanoparticles for Producing Lactose-Free Dairy Products. Orient. J. Chem. 2021, 37, 643–647. [Google Scholar] [CrossRef]
- Iwashita, K.; Shiraki, K.; Ishii, R.; Tanaka, T.; Hirano, A. Arginine Suppresses the Adsorption of Lysozyme onto Single-Wall Carbon Nanotubes. Chem. Lett. 2016, 45, 952–954. [Google Scholar] [CrossRef]
- Chen, M.; Zeng, G.; Xu, P.; Lai, C.; Tang, L. How Do Enzymes “Meet” Nanoparticles and Nanomaterials? Trends Biochem. Sci. 2017, 42, 914–930. [Google Scholar] [CrossRef]
- Johnson, B.J.; Russ Algar, W.; Malanoski, A.P.; Ancona, M.G.; Medintz, I.L. Understanding Enzymatic Acceleration at Nanoparticle Interfaces: Approaches and Challenges. Nano Today 2014, 9, 102–131. [Google Scholar] [CrossRef]
- Du, P.; Zhao, J.; Mashayekhi, H.; Xing, B. Adsorption of Bovine Serum Albumin and Lysozyme on Functionalized Carbon Nanotubes. J. Phys. Chem. C 2014, 118, 22249–22257. [Google Scholar] [CrossRef]
- Pan, Y.; Neupane, S.; Farmakes, J.; Bridges, M.; Froberg, J.; Rao, J.; Qian, S.Y.; Liu, G.; Choi, Y.; Yang, Z. Probing the Structural Basis and Adsorption Mechanism of an Enzyme on Nano-Sized Protein Carriers. Nanoscale 2017, 9, 3512–3523. [Google Scholar] [CrossRef]
- Li, X.; Tian, L.; Ali, Z.; Wang, W.; Zhang, Q. Design of Flexible Dendrimer-Grafted Flower-like Magnetic Microcarriers for Penicillin G Acylase Immobilization. J. Mater. Sci. 2018, 53, 937–947. [Google Scholar] [CrossRef]
- Pérez, E.; Sánchez-Murcia, P.A.; Jordaan, J.; Blanco, M.D.; Mancheño, J.M.; Gago, F.; Fernández-Lucas, J. Enzymatic Synthesis of Therapeutic Nucleosides Using a Highly Versatile Purine Nucleoside 2’-DeoxyribosylTransferase from Trypanosoma Brucei. ChemCatChem 2018, 10, 4406–4416. [Google Scholar] [CrossRef]
- Arco, J.D.; Pérez, E.; Naitow, H.; Matsuura, Y.; Kunishima, N.; Fernández-Lucas, J. Structural and Functional Characterization of Thermostable Biocatalysts for the Synthesis of 6-Aminopurine Nucleoside-5′-Monophospate Analogues. Bioresour. Technol. 2019, 276, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Vellard, M. The Enzyme as Drug: Application of Enzymes as Pharmaceuticals. Curr. Opin. Biotechnol. 2003, 14, 444–450. [Google Scholar] [CrossRef]
- Hood, E.D.; Chorny, M.; Greineder, C.F.; S Alferiev, I.; Levy, R.J.; Muzykantov, V.R. Endothelial Targeting of Nanocarriers Loaded with Antioxidant Enzymes for Protection against Vascular Oxidative Stress and Inflammation. Biomaterials 2014, 35, 3708–3715. [Google Scholar] [CrossRef] [PubMed]
- Dziubla, T.D.; Shuvaev, V.V.; Hong, N.K.; Hawkins, B.J.; Madesh, M.; Takano, H.; Simone, E.; Nakada, M.T.; Fisher, A.; Albelda, S.M.; et al. Endothelial Targeting of Semi-Permeable Polymer Nanocarriers for Enzyme Therapies. Biomaterials 2008, 29, 215–227. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Chen, C.-T.; Hung, Y.; Chou, C.-M.; Liu, T.-P.; Liang, M.-R.; Chen, C.-T.; Mou, C.-Y. A New Strategy for Intracellular Delivery of Enzyme Using Mesoporous Silica Nanoparticles: Superoxide Dismutase. J. Am. Chem. Soc. 2013, 135, 1516–1523. [Google Scholar] [CrossRef]
- Law, B.; Weissleder, R.; Tung, C.-H. Peptide-Based Biomaterials for Protease-Enhanced Drug Delivery. Biomacromolecules 2006, 7, 1261–1265. [Google Scholar] [CrossRef]
- Aili, D.; Mager, M.; Roche, D.; Stevens, M.M. Hybrid Nanoparticle−Liposome Detection of Phospholipase Activity. Nano Lett. 2011, 11, 1401–1405. [Google Scholar] [CrossRef]
- Hu, Z.; Zhao, L.; Zhang, H.; Zhang, Y.; Wu, R.; Zou, H. The On-Bead Digestion of Protein Corona on Nanoparticles by Trypsin Immobilized on the Magnetic Nanoparticle. J. Chromatogr. A 2014, 1334, 55–63. [Google Scholar] [CrossRef]
- Illanes, A.; Cauerhff, A.; Wilson, L.; Castro, G.R. Recent Trends in Biocatalysis Engineering. Bioresour. Technol. 2012, 115, 48–57. [Google Scholar] [CrossRef]
- Madhavan, A.; Sindhu, R.; Binod, P.; Sukumaran, R.K.; Pandey, A. Strategies for Design of Improved Biocatalysts for Industrial Applications. Bioresour. Technol. 2017, 245, 1304–1313. [Google Scholar] [CrossRef]
- Torchilin, V.P. Multifunctional Nanocarriers. Adv. Drug Deliv. Rev. 2012, 64, 302–315. [Google Scholar] [CrossRef]
- Driscoll, C.F.; Morris, R.M.; Senyei, A.E.; Widder, K.J.; Heller, G.S. Magnetic Targeting of Microspheres in Blood Flow. Microvasc. Res. 1984, 27, 353–369. [Google Scholar] [CrossRef] [PubMed]
- De Strooper, B.; Vassar, R.; Golde, T. The Secretases: Enzymes with Therapeutic Potential in Alzheimer Disease. Nat. Rev. Neurol. 2010, 6, 99–107. [Google Scholar] [CrossRef]
- Vertegel, A.A.; Reukov, V.; Maximov, V. Enzyme-Nanoparticle Conjugates for Biomedical Applications. Methods Mol. Biol. 2011, 679, 165–182. [Google Scholar] [CrossRef]
- Razzaghi, M.; Homaei, A.; Vianello, F.; Azad, T.; Sharma, T.; Nadda, A.K.; Stevanato, R.; Bilal, M.; Iqbal, H.M.N. Industrial Applications of Immobilized Nano-Biocatalysts. Bioprocess Biosyst. Eng. 2022, 45, 237–256. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Chatha, M.A.; Ejaz, W.; Janjua, H.A.; Hussain, I. Lysozyme-Coated Silver Nanoparticles for Differentiating Bacterial Strains on the Basis of Antibacterial Activity. Nanoscale Res. Lett. 2014, 9, 565. [Google Scholar] [CrossRef]
- Hu, Q.; Katti, P.S.; Gu, Z. Enzyme-Responsive Nanomaterials for Controlled Drug Delivery. Nanoscale 2014, 6, 12273–12286. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-Responsive Nanocarriers for Drug Delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Kang, J.-H.; Asai, D.; Kim, J.-H.; Mori, T.; Toita, R.; Tomiyama, T.; Asami, Y.; Oishi, J.; Sato, Y.T.; Niidome, T.; et al. Design of Polymeric Carriers for Cancer-Specific Gene Targeting: Utilization of Abnormal Protein Kinase Cα Activation in Cancer Cells. J. Am. Chem. Soc. 2008, 130, 14906–14907. [Google Scholar] [CrossRef]
- Nguyen, M.M.; Carlini, A.S.; Chien, M.-P.; Sonnenberg, S.; Luo, C.; Braden, R.L.; Osborn, K.G.; Li, Y.; Gianneschi, N.C.; Christman, K.L. Enzyme-Responsive Nanoparticles for Targeted Accumulation and Prolonged Retention in Heart Tissue after Myocardial Infarction. Adv. Mater. 2015, 27, 5547–5552. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Mo, R.; Bellotti, A.; Zhou, J.; Gu, Z. Gel–Liposome-Mediated Co-Delivery of Anticancer Membrane-Associated Proteins and Small-Molecule Drugs for Enhanced Therapeutic Efficacy. Adv. Funct. Mater. 2014, 24, 2295–2304. [Google Scholar] [CrossRef]
- Gardimalla, H.M.R.; Mandal, D.; Stevens, P.D.; Yen, M.; Gao, Y. Superparamagnetic Nanoparticle-Supported Enzymatic Resolution of Racemic Carboxylates. Chem. Commun. 2005, 4432–4434. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.A.; Mauchley, D.; Li, H.; Meng, X.; Nemenoff, R.A.; Fullerton, D.A.; Weyant, M.J. Knockdown of Secretory Phospholipase A2 IIa Reduces Lung Cancer Growth in Vitro and in Vivo. J. Thorac. Cardiovasc. Surg. 2012, 144, 1185–1191. [Google Scholar] [CrossRef]
- Andresen, T.L.; Davidsen, J.; Begtrup, M.; Mouritsen, O.G.; Jørgensen, K. Enzymatic Release of Antitumor Ether Lipids by Specific Phospholipase A2 Activation of Liposome-Forming Prodrugs. J. Med. Chem. 2004, 47, 1694–1703. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, Y.; Lu, D.; Ge, J.; Liu, Z.; Zare, R.N. Functional Protein–Organic/Inorganic Hybrid Nanomaterials. WIREs Nanomed. Nanobiotechnology 2013, 5, 320–328. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Singh, V.; Kumar, P. Carboxymethyl Tamarind Gum–Silica Nanohybrids for Effective Immobilization of Amylase. J. Mol. Catal. B Enzym. 2011, 70, 67–73. [Google Scholar] [CrossRef]
- Ferguson, R.M.; Minard, K.R.; Krishnan, K.M. Optimization of Nanoparticle Core Size for Magnetic Particle Imaging. J. Magn. Magn. Mater. 2009, 321, 1548–1551. [Google Scholar] [CrossRef]
- Harnoy, A.J.; Rosenbaum, I.; Tirosh, E.; Ebenstein, Y.; Shaharabani, R.; Beck, R.; Amir, R.J. Enzyme-Responsive Amphiphilic PEG-Dendron Hybrids and Their Assembly into Smart Micellar Nanocarriers. J. Am. Chem. Soc. 2014, 136, 7531–7534. [Google Scholar] [CrossRef]
- Napoli, A.; Boerakker, M.J.; Tirelli, N.; Nolte, R.J.M.; Sommerdijk, N.A.J.M.; Hubbell, J.A. Glucose-Oxidase Based Self-Destructing Polymeric Vesicles. Langmuir 2004, 20, 3487–3491. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Yan, M.; Hu, B.; Joo, K.-I.; Biswas, A.; Huang, Y.; Lu, Y.; Wang, P.; Tang, Y. Protein Nanocapsule Weaved with Enzymatically Degradable Polymeric Network. Nano Lett. 2009, 9, 4533–4538. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; Khan, A. Enzyme Sensitive Synthetic Polymer Micelles Based on the Azobenzene Motif. J. Am. Chem. Soc. 2013, 135, 14056–14059. [Google Scholar] [CrossRef]
- Panyam, J.; Sahoo, S.K.; Prabha, S.; Bargar, T.; Labhasetwar, V. Fluorescence and Electron Microscopy Probes for Cellular and Tissue Uptake of Poly(d,l-Lactide-Co-Glycolide) Nanoparticles. Int. J. Pharm. 2003, 262, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Vila-Jato, J.L.; Alonso, M.J. Evaluation of Cationic Polymer-Coated Nanocapsules as Ocular Drug Carriers. Int. J. Pharm. 1997, 153, 41–50. [Google Scholar] [CrossRef]
- Singh, R.; Lillard, J.W. Nanoparticle-Based Targeted Drug Delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Long-Circulating and Target-Specific Nanoparticles: Theory to Practice. Pharmacol. Rev. 2001, 53, 283–318. [Google Scholar]
- Olivier, J.-C. Drug Transport to Brain with Targeted Nanoparticles. Neurotherapeutics 2005, 2, 108–119. [Google Scholar] [CrossRef]
- Ansari, S.A.; Husain, Q. Potential Applications of Enzymes Immobilized on/in Nano Materials: A Review. Biotechnol. Adv. 2012, 30, 512–523. [Google Scholar] [CrossRef]
- Muteeb, G. Nanotechnology—A Light of Hope for Combating Antibiotic Resistance. Microorganisms 2023, 11, 1489. [Google Scholar] [CrossRef]
- Sigurdardóttir, S.B.; Lehmann, J.; Ovtar, S.; Grivel, J.-C.; Negra, M.D.; Kaiser, A.; Pinelo, M. Enzyme Immobilization on Inorganic Surfaces for Membrane Reactor Applications: Mass Transfer Challenges, Enzyme Leakage and Reuse of Materials. Adv. Synth. Catal. 2018, 360, 2578–2607. [Google Scholar] [CrossRef]
- Aebersold, R.; Mann, M. Mass Spectrometry-Based Proteomics. Nature 2003, 422, 198–207. [Google Scholar] [CrossRef] [PubMed]






| Types of Nanomaterials | Enzyme | Nanomaterial Preparation Method | Nanomaterial Characterization Techniques | Application | Ref. |
|---|---|---|---|---|---|
| Fe3O4 | Lipase | Chemical | XRD, SEM and FTIR | Biodiesel production from algal biomass | [36] |
| Magnetic graphene oxide | Candida rugosa lipase | Chemical | Autosorb-iQ automatic specific surface, pore size distribution Analyzer, XRD, vibrating sample magnetometer, Malvern potentiometer (NANO ZS90), SEM, TEM, and FTIR | ----- | [37] |
| Ni2+-NTA-boosted magnetic porous silica nanoparticles | Bi-functional enzyme (MLG), which consists of 3-Quinuclidinone reductase and glucose dehydrogenase. | Chemical | SEM, TEM, XRD, FTIR, and Thermal Gravimetric analysis | Biotransformation of 3-quinuclidinone to (R)-3-quinuclidinol | [38] |
| Fe3O4 ferromagnetic | Laccase | Chemical | TEM, FTIR, and XRD | ----- | [39] |
| Glutathione capped CdTe quantum dots (GSH-CdTeQD) | Glucose oxidase | Chemical | CV, EIS, SECM, SEM, FTIR, TEM, XRD, UV-Vis, and ZS | Developing fuel cells | [40] |
| Polyacrylonitrile (PAN) nanofibers | Carbonic anhydrase | Electro spun nanofibers | SEM, FTIR, and thermal stability measurements | Interconversion between carbon dioxide and water and the dissociated ions of carbonic acid | [41] |
| Green Synthesis Method | Nanomaterial | Organism | Ref. |
|---|---|---|---|
| Bacterial Synthesis | Gold | Rhodococcus species | [55] |
| Gold | Rhodopseudomonas casulata | [56] | |
| Silver | Morganella sp. | [57] | |
| Silver and gold | Bacillus subtilis | [58] | |
| CdS fluorescent | Halobacillus sp. | [59] | |
| Fungal Synthesis | Gold | Verticillium sp. | [60] |
| ZnO@SPION@Ag | Fusarium oxysporum | [61] | |
| SPION@Ag@Cs | Fusarium oxysporum | [62] | |
| Silver | Aspergillus fumigatus | [63] | |
| Silver | Trichoderma reesei | [64] | |
| Algal Synthesis | Gold | Fucus vesiculosus | [65] |
| Gold | Tetraselmis suecica | [66] | |
| Silver | Chlamydomonas reinhardtii | [67] | |
| Zinc oxide | Sargassum muticum | [68] | |
| Silver | Portieria hornemannii | [69] |
| Application | Nanomaterial | Enzyme | Characterization | Ref. |
|---|---|---|---|---|
| Pharmaceutical industry | Dendrimer-grafted flower-like Fe3O4@SiO2/PAMAM microcarriers | Penicillin G acylase | SEM, TEM, VSM and XRD | [22,92] |
| Glutaraldehyde-activated magnetic microspheres | Purine nucleoside 2′-deoxyribosyltransferase from Trypanosoma brucei | SEM and DLS | [93] | |
| Glutaraldehyde-activated MagReSyn®Amine magnetic iron oxide porous microparticles | Adenine phosphoribosyltransferase 2 from Thermus thermophilus HB8 | ----- | [94] | |
| Biomedical | Magnetic, mesoporous, polymeric and liposomes | tPA, streptokinase, and uPA | ----- | [95] |
| Protective antioxidant carriers | Catalase and superoxide dismutase | Zeta potential and hydrodynamic diameter | [96] | |
| Co-polymers of polyethylene glycol and poly-lactic/poly-glycolic acid | Catalase, peroxidase, and xanthine oxidase | ----- | [97] | |
| Mesoporous silica | Superoxide dismutase | TEM and zeta potential | [98] | |
| Peptide-Based Biomaterials | Protease | ----- | [99] | |
| Liposome | Phospholipase | Zeta potential | [100] | |
| Biotechnological | Magnetic Fe3O4 nanoparticles modified graphene oxide nanocomposites | Trypsin reactor | TEM and SEM | [101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khafaga, D.S.R.; Radwan, M.G.; Muteeb, G.; Aatif, M.; Farhan, M. Green Synthesis of Biocatalysts Based on Nanocarriers Promises an Effective Role in Pharmaceutical and Biomedical Fields. Catalysts 2023, 13, 1448. https://doi.org/10.3390/catal13111448
Khafaga DSR, Radwan MG, Muteeb G, Aatif M, Farhan M. Green Synthesis of Biocatalysts Based on Nanocarriers Promises an Effective Role in Pharmaceutical and Biomedical Fields. Catalysts. 2023; 13(11):1448. https://doi.org/10.3390/catal13111448
Chicago/Turabian StyleKhafaga, Doaa S. R., Mohamed G. Radwan, Ghazala Muteeb, Mohammad Aatif, and Mohd Farhan. 2023. "Green Synthesis of Biocatalysts Based on Nanocarriers Promises an Effective Role in Pharmaceutical and Biomedical Fields" Catalysts 13, no. 11: 1448. https://doi.org/10.3390/catal13111448