In Situ X-ray Diffraction as a Basic Tool to Study Oxide and Metal Oxide Catalysts
Abstract
:1. Introduction
2. Techniques and Instruments
2.1. Diffraction Techniques
- -
- The bulk structural research—This is quite useful for getting general information on the bulk catalyst/support (phase, structure, and crystallinity), including the changes caused by the sample environment (reaction, temperature, etc.) during synthesis, activation, or reaction studies;
- -
- More sophisticated approaches include when the information on the surface structure/composition is either obtained indirectly by combining the XRD data with the data obtained via different surface- or element-sensitive techniques (XPS [4], XAS [5], IR-/UV spectroscopy [6,7], STEM-EDX [8], SOR [9] etc.) or directly using grazing incidence diffraction optionally with microfocused beams (generally requires a synchrotron and mostly limited by a model atomically flat systems) [10,11].
2.2. X-ray Photon Sources
- -
- Large-scale facilities based on either the linear accelerators—free electron laser facilities such as XFEL (https://www.xfel.eu/ (accessed on 2 November 2023)), SLAC (https://lcls.slac.stanford.edu/ (accessed on 2 November 2023)), etc.—or circular particle accelerators, i.e., synchrotron facilities such as MAX VI (https://www.maxiv.lu.se/ (accessed on 2 November 2023)), ESRF (https://www.esrf.fr/ (accessed on 2 November 2023)), Diamond (https://www.diamond.ac.uk/ (accessed on 2 November 2023)), etc., where the radiation is emitted by accelerating particles (usually electrons);
- -
- Tabletop X-ray sources mainly use the method to generate X-rays by the interaction between accelerated electrons and matter.
2.3. Detectors
2.4. Cells and Reactors
2.5. Reaction Products Analysis
2.6. X-ray Diffraction Data Analysis
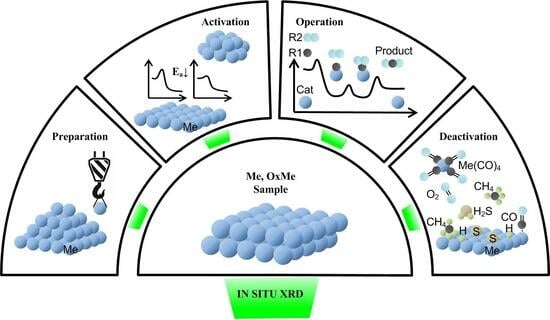
3. Application of In Situ XRD for the Characterization of Different Steps of Preparation, Activation, Operation, and Deactivation
3.1. Study of the Preparation Procedure
3.2. Study of the Process of Activation of Catalysts and Reduction of Model Oxides
3.3. Study of the Catalyst under Reaction Conditions (Operando)
3.4. Study of the Process of the Deactivation of Catalysts


4. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mosesman, M.A. In Situ X-ray Diffraction Studies of Heterogeneous Reactions. J. Am. Chem. Soc. 1951, 73, 5635–5639. [Google Scholar] [CrossRef]
- Bañares, M.A.; Wachs, I.E. Molecular Structures of Supported Metal Oxide Catalysts under Different Environments: Molecular Structures of Supported Metal Oxide Catalysts. J. Raman Spectrosc. 2002, 33, 359–380. [Google Scholar] [CrossRef]
- Hull, A.W. A New Method of X-ray Crystal Analysis. Phys. Rev. 1917, 10, 661–696. [Google Scholar] [CrossRef]
- Velasco-Vélez, J.J.; Teschner, D.; Girgsdies, F.; Hävecker, M.; Streibel, V.; Willinger, M.G.; Cao, J.; Lamoth, M.; Frei, E.; Wang, R.; et al. The Role of Adsorbed and Subsurface Carbon Species for the Selective Alkyne Hydrogenation over a Pd-Black Catalyst: An Operando Study of Bulk and Surface. Top. Catal. 2018, 61, 2052–2061. [Google Scholar] [CrossRef]
- Timoshenko, J.; Roldan Cuenya, B. Electrocatalyst Characterization by X-ray Absorption Spectroscopy. Chem. Rev. 2021, 121, 882–961. [Google Scholar] [CrossRef] [PubMed]
- Anic, K.; Wolfbeisser, A.; Li, H.; Rameshan, C.; Föttinger, K.; Bernardi, J.; Rupprechter, G. Surface Spectroscopy on UHV-Grown and Technological Ni–ZrO2 Reforming Catalysts: From UHV to Operando Conditions. Top. Catal. 2016, 59, 1614–1627. [Google Scholar] [CrossRef]
- Mekhemer, G.A.H.; Mohamed, H.A.A.; Bumajdad, A.; Zaki, M.I. Lattice-Charge Imbalance and Redox Catalysis over Perovskite-Type Ferrite- and Manganite-Based Mixed Oxides as Studied by XRD, FTIR, UV–Vis DRS, and XPS. Sci. Rep. 2023, 13, 7453. [Google Scholar] [CrossRef]
- Eijsbouts, S.; Van Den Oetelaar, L.C.A.; Rayner, M.; Govaers, H.; Boonen, T. Combined HR TEM and STEM-EDX Evaluation—The Key to Better Understanding of the Co-Mo Sulfide Active Phase in Real-Life Co-Mo-P/Al2O3 Catalysts. J. Catal. 2021, 403, 56–73. [Google Scholar] [CrossRef]
- Albertin, S.; Gustafson, J.; Zhou, J.; Pfaff, S.; Shipilin, M.; Blomberg, S.; Merte, L.R.; Gutowski, O.; Dippel, A.-C.; Zetterberg, J.; et al. Surface Optical Reflectance Combined with X-ray Techniques during Gas-Surface Interactions. J. Phys. Appl. Phys. 2020, 53, 224001. [Google Scholar] [CrossRef]
- Hejral, U.; Shipilin, M.; Gustafson, J.; Stierle, A.; Lundgren, E. High Energy Surface X-ray Diffraction Applied to Model Catalyst Surfaces at Work. J. Phys. Condens. Matter 2021, 33, 073001. [Google Scholar] [CrossRef]
- Merte, L.R.; Olsson, P.A.T.; Shipilin, M.; Gustafson, J.; Bertram, F.; Zhang, C.; Grönbeck, H.; Lundgren, E. Structure of Two-Dimensional Fe3O4. J. Chem. Phys. 2020, 152, 114705. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, N.K.; Müller, C.R.; Abdala, P.M. Deciphering the Structure of Heterogeneous Catalysts across Scales Using Pair Distribution Function Analysis. Trends Chem. 2022, 4, 807–821. [Google Scholar] [CrossRef]
- Terrill, N.J.; Dent, A.J.; Dobson, B.; Beale, A.M.; Allen, L.; Bras, W. Past, Present and Future—Sample Environments for Materials Research Studies in Scattering and Spectroscopy; a UK Perspective. J. Phys. Condens. Matter 2021, 33, 483002. [Google Scholar] [CrossRef]
- Cheng, W.; Chen, Z.; Wu, X.; Wu, Z.; Wang, X.; Zhao, M.; Liu, H.; Jia, H.; Wang, C.; Wang, X.; et al. Anomalous Small-Angle X-ray Scattering and Its Application in the Dynamic Reconstruction of Electrochemical CO2 Reduction Catalysts. Symmetry 2023, 15, 1034. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, Y. Defect Engineering of Nanomaterials for Catalysis. Nanomaterials 2023, 13, 1116. [Google Scholar] [CrossRef]
- Pinto, F.M.; Suzuki, V.Y.; Silva, R.C.; La Porta, F.A. Oxygen Defects and Surface Chemistry of Reducible Oxides. Front. Mater. 2019, 6, 260. [Google Scholar] [CrossRef]
- Masliuk, L.; Heggen, M.; Noack, J.; Girgsdies, F.; Trunschke, A.; Hermann, K.E.; Willinger, M.G.; Schlögl, R.; Lunkenbein, T. Structural Complexity in Heterogeneous Catalysis: Cataloging Local Nanostructures. J. Phys. Chem. C 2017, 121, 24093–24103. [Google Scholar] [CrossRef]
- Zieliński, M.; Kaszkur, Z.; Juszczyk, W.; Sobczak, J. In Situ Diffraction Monitoring of Nanocrystals Structure Evolving during Catalytic Reaction at Their Surface. Sci. Rep. 2023, 13, 1469. [Google Scholar] [CrossRef]
- Nezhad, P.D.K.; Bekheet, M.F.; Bonmassar, N.; Schlicker, L.; Gili, A.; Kamutzki, F.; Gurlo, A.; Doran, A.; Gao, Y.; Heggen, M.; et al. Mechanistic In Situ Insights into the Formation, Structural and Catalytic Aspects of the La2NiO4 Intermediate Phase in the Dry Reforming of Methane over Ni-Based Perovskite Catalysts. Appl. Catal. Gen. 2021, 612, 117984. [Google Scholar] [CrossRef]
- Mutschler, R.; Moioli, E.; Zhao, K.; Lombardo, L.; Oveisi, E.; Porta, A.; Falbo, L.; Visconti, C.G.; Lietti, L.; Züttel, A. Imaging Catalysis: Operando Investigation of the CO2 Hydrogenation Reaction Dynamics by Means of Infrared Thermography. ACS Catal. 2020, 10, 1721–1730. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Q.; Xie, L.; Liu, Y.; Li, D.; Feng, J.; Duan, X. Thermal Effect Optimization Endows a Selective and Stable PdCu Single Atom Alloy Catalyst for Acetylene Hydrogenation. AIChE J. 2023, 69, e18042. [Google Scholar] [CrossRef]
- Rochet, A.; Suzana, A.F.; Passos, A.R.; Kalile, T.; Berenguer, F.; Santilli, C.V.; Pulcinelli, S.H.; Meneau, F. In Situ Reactor to Image Catalysts at Work in Three-Dimensions by Bragg Coherent X-ray Diffraction. Catal. Today 2019, 336, 169–173. [Google Scholar] [CrossRef]
- Becher, J.; Weber, S.; Ferreira Sanchez, D.; Doronkin, D.E.; Garrevoet, J.; Falkenberg, G.; Motta Meira, D.; Pascarelli, S.; Grunwaldt, J.-D.; Sheppard, T.L. Sample Environment for Operando Hard X-ray Tomography—An Enabling Technology for Multimodal Characterization in Heterogeneous Catalysis. Catalysts 2021, 11, 459. [Google Scholar] [CrossRef]
- Wollak, B.; Espinoza, D.; Dippel, A.-C.; Sturm, M.; Vrljic, F.; Gutowski, O.; Nielsen, I.G.; Sheppard, T.L.; Korup, O.; Horn, R. Catalytic Reactor for Spatially Resolved Structure–Activity Profiling Using High-Energy X-ray Diffraction. J. Synchrotron Radiat. 2023, 30, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, A.C. The Sensitivity of Focusing, Parallel Beam and Mixed Optics to Alignment Errors in XRD Residual Stress Measurements. Mater. Sci. Forum 2005, 490–491, 131–136. [Google Scholar] [CrossRef]
- Zhao, P.; Lu, L.; Liu, X.; De La Torre, A.; Cheng, X. Error Analysis and Correction for Quantitative Phase Analysis Based on Rietveld-Internal Standard Method: Whether the Minor Phases Can Be Ignored? Crystals 2018, 8, 110. [Google Scholar] [CrossRef]
- Moss, A.B.; Garg, S.; Mirolo, M.; Giron Rodriguez, C.A.; Ilvonen, R.; Chorkendorff, I.; Drnec, J.; Seger, B. In Operando Investigations of Oscillatory Water and Carbonate Effects in MEA-Based CO2 Electrolysis Devices. Joule 2023, 7, 350–365. [Google Scholar] [CrossRef]
- Cats, K.H.; Weckhuysen, B.M. Combined Operando X-ray Diffraction/Raman Spectroscopy of Catalytic Solids in the Laboratory: The Co/TiO2 Fischer-Tropsch Synthesis Catalyst Showcase. ChemCatChem 2016, 8, 1531–1542. [Google Scholar] [CrossRef]
- Sá, J.; Szlachetko, J. Heterogeneous Catalysis Experiments at XFELs. Are We Close to Producing a Catalysis Movie? Catal. Lett. 2014, 144, 197–203. [Google Scholar] [CrossRef]
- Galler, A.; Gawelda, W.; Biednov, M.; Bomer, C.; Britz, A.; Brockhauser, S.; Choi, T.-K.; Diez, M.; Frankenberger, P.; French, M.; et al. Scientific Instrument Femtosecond X-ray Experiments (FXE): Instrumentation and Baseline Experimental Capabilities. J. Synchrotron Radiat. 2019, 26, 1432–1447. [Google Scholar] [CrossRef]
- Wiedorn, M.O.; Awel, S.; Morgan, A.J.; Ayyer, K.; Gevorkov, Y.; Fleckenstein, H.; Roth, N.; Adriano, L.; Bean, R.; Beyerlein, K.R.; et al. Rapid Sample Delivery for Megahertz Serial Crystallography at X-ray FELs. IUCrJ 2018, 5, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, P.; Benabderrahmane, C.; Berkvens, P.; Biasci, J.C.; Borowiec, P.; Bouteille, J.-F.; Brochard, T.; Brookes, N.B.; Carmignani, N.; Carver, L.R.; et al. The Extremely Brilliant Source Storage Ring of the European Synchrotron Radiation Facility. Commun. Phys. 2023, 6, 82. [Google Scholar] [CrossRef] [PubMed]
- Lo, B.T.W.; Ye, L.; Tsang, S.C.E. The Contribution of Synchrotron X-ray Powder Diffraction to Modern Zeolite Applications: A Mini-Review and Prospects. Chem 2018, 4, 1778–1808. [Google Scholar] [CrossRef]
- Newton, M. Time Resolved Operando X-ray Techniques in Catalysis, a Case Study: CO Oxidation by O2 over Pt Surfaces and Alumina Supported Pt Catalysts. Catalysts 2017, 7, 58. [Google Scholar] [CrossRef]
- Behling, R. Modern Diagnostic X-ray Sources: Technology, Manufacturing, Reliability, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2021; ISBN 978-1-00-309540-8. [Google Scholar]
- Yamada, H. The Smallest Electron Storage Ring for High-Intensity Far-Infrared and Hard X-ray Productions. J. Synchrotron Radiat. 1998, 5, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.G.; Lanceros-Mendez, S. Review on X-ray Detectors Based on Scintillators and CMOS Technology. Recent Pat. Electr. Eng. 2011, 4, 16–41. [Google Scholar] [CrossRef]
- Kasap, S.; Frey, J.B.; Belev, G.; Tousignant, O.; Mani, H.; Greenspan, J.; Laperriere, L.; Bubon, O.; Reznik, A.; DeCrescenzo, G.; et al. Amorphous and Polycrystalline Photoconductors for Direct Conversion Flat Panel X-ray Image Sensors. Sensors 2011, 11, 5112–5157. [Google Scholar] [CrossRef]
- Rosenfeld, A.; Silari, M.; Campbell, M. The Editorial. Radiat. Meas. 2020, 139, 106483. [Google Scholar] [CrossRef]
- Aulchenko, V.M.; Evdokov, O.V.; Kutovenko, V.D.; Pirogov, B.Y.; Sharafutdinov, M.R.; Titov, V.M.; Tolochko, B.P.; Vasiljev, A.V.; Zhogin, I.A.; Zhulanov, V.V. One-Coordinate X-ray Detector OD-3M. Nucl. Instrum. Methods Phys. Res. Sect. A 2009, 603, 76–79. [Google Scholar] [CrossRef]
- Ermrich, M.; Hahn, F.; Wölfel, E.R. Use of Imaging Plates in X-ray Analysis. Textures Microstruct. 1997, 29, 89–101. [Google Scholar] [CrossRef]
- Rocha, J.G.; Goncalves, L.M.; Lanceros-Mendez, S. Flexible X-ray Detector Based on the Seebeck Effect. In Proceedings of the TRANSDUCERS 2009–2009 International Solid-State Sensors, Actuators and Microsystems Conference, Denver, CO, USA, 21–25 June 2009; IEEE: Denver, CO, USA, 2009; pp. 1967–1970. [Google Scholar]
- Allé, P.; Wenger, E.; Dahaoui, S.; Schaniel, D.; Lecomte, C. Comparison of CCD, CMOS and Hybrid Pixel X-ray Detectors: Detection Principle and Data Quality. Phys. Scr. 2016, 91, 063001. [Google Scholar] [CrossRef]
- Hodeau, J.-L.; Bordet, P.; Anne, M.; Prat, A.; Fitch, A.N.; Dooryhee, E.; Vaughan, G.; Freund, A.K. Nine-Crystal Multianalyzer Stage for High-Resolution Powder Diffraction between 6 keV and 40 keV, Proceedings of the SPIE’s International Symposium on Optical Science, Engineering, and Instrumentation, 19–24 July 1998, San Diego, CA, USA; Macrander, A.T., Freund, A.K., Ishikawa, T., Mills, D.M., Eds.; SPIE: San Diego, CA, USA, 1998; p. 353. [Google Scholar]
- Thompson, S.P.; Parker, J.E.; Potter, J.; Hill, T.P.; Birt, A.; Cobb, T.M.; Yuan, F.; Tang, C.C. Beamline I11 at Diamond: A New Instrument for High Resolution Powder Diffraction. Rev. Sci. Instrum. 2009, 80, 075107. [Google Scholar] [CrossRef] [PubMed]
- Drakopoulos, M.; Connolley, T.; Reinhard, C.; Atwood, R.; Magdysyuk, O.; Vo, N.; Hart, M.; Connor, L.; Humphreys, B.; Howell, G.; et al. I12: The Joint Engineering, Environment and Processing (JEEP) Beamline at Diamond Light Source. J. Synchrotron Radiat. 2015, 22, 828–838. [Google Scholar] [CrossRef]
- Förster, A.; Brandstetter, S.; Schulze-Briese, C. Transforming X-ray Detection with Hybrid Photon Counting Detectors. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 2019, 377, 20180241. [Google Scholar] [CrossRef] [PubMed]
- Brönnimann, C.; Trüb, P. Hybrid Pixel Photon Counting X-ray Detectors for Synchrotron Radiation. In Synchrotron Light Sources and Free-Electron Lasers; Jaeschke, E., Khan, S., Schneider, J.R., Hastings, J.B., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–29. ISBN 978-3-319-04507-8. [Google Scholar]
- Volkov, S.; Vonk, V.; Khorshidi, N.; Franz, D.; Kubicek, M.; Kilic, V.; Felici, R.; Huber, T.M.; Navickas, E.; Rupp, G.M.; et al. Operando X-ray Investigation of Electrode/Electrolyte Interfaces in Model Solid Oxide Fuel Cells. Chem. Mater. 2016, 28, 3727–3733. [Google Scholar] [CrossRef] [PubMed]
- Alabdulkarim, M.E.; Maxwell, W.D.; Thapliyal, V.; Maxwell, J.L. A Comprehensive Review of High-Pressure Laser-Induced Materials Processing, Part I: Laser-Heated Diamond Anvil Cells. J. Manuf. Mater. Process. 2022, 6, 111. [Google Scholar] [CrossRef]
- Saraev, A.A.; Vinokurov, Z.S.; Kaichev, V.V.; Shmakov, A.N.; Bukhtiyarov, V.I. The Origin of Self-Sustained Reaction-Rate Oscillations in the Oxidation of Methane over Nickel: An Operando XRD and Mass Spectrometry Study. Catal. Sci. Technol. 2017, 7, 1646–1649. [Google Scholar] [CrossRef]
- Wu, H.; Stacey, D. Small- and Wide-Angle X-ray Scattering (SAXS/WAXS) with Temperature-Controlled Stages Makes Phase Identification Faster than Ever. Microsc. Today 2021, 29, 30–36. [Google Scholar] [CrossRef]
- Saleta, M.E.; Eleotério, M.; Mesquita, A.; Mastelaro, V.R.; Granado, E. Atomic Pair Distribution Function at the Brazilian Synchrotron Light Laboratory: Application to the Pb1−xLaxZr0.40Ti0.60O3 Ferroelectric System. J. Synchrotron Radiat. 2017, 24, 1098–1104. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Nachtegaal, M.; Marchionni, V.; Quaroni, L.; Ferri, D. Adding Diffuse Reflectance Infrared Fourier Transform Spectroscopy Capability to Extended X-ray-Absorption Fine Structure in a New Cell to Study Solid Catalysts in Combination with a Modulation Approach. Rev. Sci. Instrum. 2014, 85, 074102. [Google Scholar] [CrossRef]
- Rothensteiner, M.; Jenni, J.; Emerich, H.; Bonk, A.; Vogt, U.F.; Van Bokhoven, J.A. In Situ Flow Cell for Combined X-ray Absorption Spectroscopy, X-ray Diffraction, and Mass Spectrometry at High Photon Energies under Solar Thermochemical Looping Conditions. Rev. Sci. Instrum. 2017, 88, 083116. [Google Scholar] [CrossRef]
- Baimpas, N.; Drakopoulos, M.; Connolley, T.; Song, X.; Pandazaras, C.; Korsunsky, A.M. A Feasibility Study of Dynamic Stress Analysis inside a Running Internal Combustion Engine Using Synchrotron X-ray Beams. J. Synchrotron Radiat. 2013, 20, 316–323. [Google Scholar] [CrossRef]
- Čajka, T.; Hajšlová, J.; Kazda, R.; Poustka, J. Challenges of Gas Chromatography-High-Resolution Time-of-Flight Mass Spectrometry for Simultaneous Analysis of Polybrominated Diphenyl Ethers and Other Halogenated Persistent Organic Pollutants in Environmental Samples. J. Sep. Sci. 2005, 28, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Hejazi, L.; Ebrahimi, D.; Hibbert, D.B.; Guilhaus, M. Compatibility of Electron Ionization and Soft Ionization Methods in Gas Chromatography/Orthogonal Time-of-Flight Mass Spectrometry: EI and Soft Ionization Methods in GC/Oa-TOFMS. Rapid Commun. Mass Spectrom. 2009, 23, 2181–2189. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhang, L.; Zhang, T.; Guo, H.; Hong, X.; Qi, F. Photoionization Studies on Various Quinones by an Infrared Laser Desorption/Tunable VUV Photoionization TOF Mass Spectrometry. J. Mass Spectrom. 2008, 43, 1701–1710. [Google Scholar] [CrossRef]
- Mukarakate, C.; Scheer, A.M.; Robichaud, D.J.; Jarvis, M.W.; David, D.E.; Ellison, G.B.; Nimlos, M.R.; Davis, M.F. Laser Ablation with Resonance-Enhanced Multiphoton Ionization Time-of-Flight Mass Spectrometry for Determining Aromatic Lignin Volatilization Products from Biomass. Rev. Sci. Instrum. 2011, 82, 033104. [Google Scholar] [CrossRef]
- Bicchi, C.; Brunelli, C.; Galli, M.; Sironi, A. Conventional Inner Diameter Short Capillary Columns: An Approach to Speeding up Gas Chromatographic Analysis of Medium Complexity Samples. J. Chromatogr. A 2001, 931, 129–140. [Google Scholar] [CrossRef] [PubMed]
- van Deursen, M.M.; Beens, J.; Janssen, H.-G.; Leclercq, P.A.; Cramers, C.A. Evaluation of Time-of-Flight Mass Spectrometric Detection for Fast Gas Chromatography. J. Chromatogr. A 2000, 878, 205–213. [Google Scholar] [CrossRef]
- Van Deursen, M.; Janssen, H.-G.; Beens, J.; Lipman, P.; Reinierkens, R.; Rutten, G.; Cramers, C. Fast Gas Chromatography Using Vacuum Outlet Conditions. J. Microcolumn Sep. 2000, 12, 613–622. [Google Scholar] [CrossRef]
- Tranchida, P.Q.; Zoccali, M.; Mondello, L. High-Speed GC-MS. In Hyphenations of Capillary Chromatography with Mass Spectrometry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 109–132. ISBN 978-0-12-809638-3. [Google Scholar]
- Gates-Rector, S.; Blanton, T. The Powder Diffraction File: A Quality Materials Characterization Database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef]
- Cranswick, L.M.D. Chapter 17. Computer Software for Powder Diffraction. In Powder Diffraction; Dinnebier, R.E., Billinge, S.J.L., Eds.; Royal Society of Chemistry: Cambridge, UK, 2008; pp. 494–570. ISBN 978-0-85404-231-9. [Google Scholar]
- Johny, J.; Prymak, O.; Kamp, M.; Calvo, F.; Kim, S.-H.; Tymoczko, A.; El-Zoka, A.; Rehbock, C.; Schürmann, U.; Gault, B.; et al. Multidimensional Thermally-Induced Transformation of Nest-Structured Complex Au-Fe Nanoalloys towards Equilibrium. Nano Res. 2022, 15, 581–592. [Google Scholar] [CrossRef]
- Larsson, A.; Abbondanza, G.; Rämisch, L.; Linpé, W.; Novikov, D.V.; Lundgren, E.; Harlow, G.S. In Situ Scanning X-ray Diffraction Reveals Strain Variations in Electrochemically Grown Nanowires. J. Phys. Appl. Phys. 2021, 54, 235301. [Google Scholar] [CrossRef]
- Słowik, G.; Gawryszuk-Rżysko, A.; Greluk, M.; Machocki, A. Estimation of Average Crystallites Size of Active Phase in Ceria-Supported Cobalt-Based Catalysts by Hydrogen Chemisorption vs TEM and XRD Methods. Catal. Lett. 2016, 146, 2173–2184. [Google Scholar] [CrossRef]
- Langford, J.I.; Wilson, A.J.C. Scherrer after Sixty Years: A Survey and Some New Results in the Determination of Crystallite Size. J. Appl. Crystallogr. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Williamson, G.K.; Hall, W.H. X-ray Line Broadening from Filed Aluminium and Wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Rietveld, H.M. A Profile Refinement Method for Nuclear and Magnetic Structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Davies, R.J. A New Batch-Processing Data-Reduction Application for X-ray Diffraction Data. J. Appl. Crystallogr. 2006, 39, 267–272. [Google Scholar] [CrossRef]
- Stinton, G.W.; Evans, J.S.O. Parametric Rietveld Refinement. J. Appl. Crystallogr. 2007, 40, 87–95. [Google Scholar] [CrossRef]
- Schuetzke, J.; Benedix, A.; Mikut, R.; Reischl, M. Enhancing Deep-Learning Training for Phase Identification in Powder X-ray Diffractograms. IUCrJ 2021, 8, 408–420. [Google Scholar] [CrossRef]
- Wang, H.; Xie, Y.; Li, D.; Deng, H.; Zhao, Y.; Xin, M.; Lin, J. Rapid Identification of X-ray Diffraction Patterns Based on Very Limited Data by Interpretable Convolutional Neural Networks. J. Chem. Inf. Model. 2020, 60, 2004–2011. [Google Scholar] [CrossRef]
- Oviedo, F.; Ren, Z.; Sun, S.; Settens, C.; Liu, Z.; Hartono, N.T.P.; Ramasamy, S.; DeCost, B.L.; Tian, S.I.P.; Romano, G.; et al. Fast and Interpretable Classification of Small X-ray Diffraction Datasets Using Data Augmentation and Deep Neural Networks. npj Comput. Mater. 2019, 5, 60. [Google Scholar] [CrossRef]
- Lee, J.-W.; Park, W.B.; Kim, M.; Pal Singh, S.; Pyo, M.; Sohn, K.-S. A Data-Driven XRD Analysis Protocol for Phase Identification and Phase-Fraction Prediction of Multiphase Inorganic Compounds. Inorg. Chem. Front. 2021, 8, 2492–2504. [Google Scholar] [CrossRef]
- Baylet, A.; Marécot, P.; Duprez, D.; Castellazzi, P.; Groppi, G.; Forzatti, P. In Situ Raman and In Situ XRD Analysis of PdO Reduction and Pd° Oxidation Supported on γ-Al2O3 Catalyst under Different Atmospheres. Phys. Chem. Chem. Phys. 2011, 13, 4607. [Google Scholar] [CrossRef]
- Kulbakov, A.A.; Kuriganova, A.B.; Allix, M.; Rakhmatullin, A.; Smirnova, N.V.; Maslova, O.A.; Leontyev, I.N. Non-Isothermal Decomposition of Platinum Acetylacetonate as a Cost-Efficient and Size-Controlled Synthesis of Pt/C Nanoparticles. Catal. Commun. 2018, 117, 14–18. [Google Scholar] [CrossRef]
- Zadesenets, A.V.; Garkul, I.A.; Filatov, E.Y.; Plyusnin, P.E.; Filippov, T.N.; Asanova, T.I.; Korolkov, I.V.; Baidina, I.A.; Asanov, I.P.; Korenev, S.V. Oxalato Complexes of Pd(II) with Co(II) and Ni(II) as Single-Source Precursors for Bimetallic Nanoalloys. J. Therm. Anal. Calorim. 2019, 138, 111–121. [Google Scholar] [CrossRef]
- Kardash, T.Y.; Plyasova, L.M.; Bondareva, V.M.; Andrushkevich, T.V.; Ishchenko, A.V.; Chesalov, Y.A.; Dovlitova, L.S. Effect of Thermal Treatment Conditions on the Phase Composition and Structural Characteristics of V-Mo-Nb-O Catalysts. Kinet. Catal. 2009, 50, 48–56. [Google Scholar] [CrossRef]
- Girgsdies, F.; Schlögl, R.; Trunschke, A. In-Situ X-ray Diffraction Study of Phase Crystallization from an Amorphous MoVTeNb Oxide Catalyst Precursor. Catal. Commun. 2012, 18, 60–62. [Google Scholar] [CrossRef]
- Kardash, T.Y.; Lazareva, E.V.; Svintsitskiy, D.A.; Kovalev, E.P.; Bondareva, V.M. Effect of Selenium Additives on the Physicochemical and Catalytic Properties of VMoTeNbO Catalysts in the Oxidative Dehydrogenation of Ethane. Kinet. Catal. 2019, 60, 355–365. [Google Scholar] [CrossRef]
- Norby, P.; Hanson, J.C. Hydrothermal Synthesis of the Microporous Aluminophosphate CoAPO-5; In Situ Time-Resolved Synchrotron X-ray Powder Diffraction Studies. Catal. Today 1998, 39, 301–309. [Google Scholar] [CrossRef]
- Norby, P. In-Situ XRD as a Tool to Understanding Zeolite Crystallization. Curr. Opin. Colloid Interface Sci. 2006, 11, 118–125. [Google Scholar] [CrossRef]
- Tsybulya, S.V. Structural Aspect of a Thermal Activation Effect in the MnOx/γ-Al2O3 System. Kinet. Catal. 2003, 44, 287–296. [Google Scholar] [CrossRef]
- Bulavchenko, O.A.; Afonasenko, T.N.; Tsyrul’nikov, P.G.; Tsybulya, S.V. Effect of Heat Treatment Conditions on the Structure and Catalytic Properties of MnO/Al2O3 in the Reaction of CO Oxidation. Appl. Catal. Gen. 2013, 459, 73–80. [Google Scholar] [CrossRef]
- Cherepanova, S.V.; Bulavchenko, O.A.; Gerasimov, E.Y.; Tsybulya, S.V. Low- and High-Temperature Oxidation of Mn1.5Al1.5O4 in Relation to Decomposition Mechanism and Microstructure. CrystEngComm 2016, 18, 3411–3421. [Google Scholar] [CrossRef]
- Bulavchenko, O.A.; Tsybulya, S.V.; Cherepanova, S.V.; Afonasenko, T.N.; Tsyrulnikov, P.G. High-Temperature X-ray Study of the Formation and Delamination of Manganese-Alumina Spinel Mn1.5Al1.5O4. J. Struct. Chem. 2009, 50, 474–478. [Google Scholar] [CrossRef]
- Bulavchenko, O.A.; Afonasenko, T.N.; Vinokurov, Z.S.; Pochtar, A.A.; Rogov, V.A.; Tsybulya, S.V. The Thermal Activation of MnOx-Al2O3 Catalysts: Effect of Gallium Doping. Mater. Chem. Phys. 2022, 291, 126715. [Google Scholar] [CrossRef]
- Sietsma, J.R.A.; Friedrich, H.; Broersma, A.; Versluijs-Helder, M.; Jos Van Dillen, A.; De Jongh, P.E.; De Jong, K.P. How Nitric Oxide Affects the Decomposition of Supported Nickel Nitrate to Arrive at Highly Dispersed Catalysts. J. Catal. 2008, 260, 227–235. [Google Scholar] [CrossRef]
- Munnik, P.; Wolters, M.; Gabrielsson, A.; Pollington, S.D.; Headdock, G.; Bitter, J.H.; De Jongh, P.E.; De Jong, K.P. Copper Nitrate Redispersion to Arrive at Highly Active Silica-Supported Copper Catalysts. J. Phys. Chem. C 2011, 115, 14698–14706. [Google Scholar] [CrossRef]
- Mao, X.; Xia, X.; Li, J.; Chen, C.; Gu, X.; Li, S.; Lan, Y.-P. Self-Assembly of Structured CeCO3OH and Its Decomposition in H2 for a Novel Tactic to Obtain CeO2−x with Excellent Photocatalytic Property. J. Alloys Compd. 2021, 870, 159424. [Google Scholar] [CrossRef]
- Bulavchenko, O.A.; Vinokurov, Z.S.; Nikolaeva, O.A.; Afonasenko, T.N.; Tsybulya, S.V. STUDY OF THERMAL CO-DECOMPOSITION OF MANGANESE AND CERIUM OXALATES IN AIR AND IN INERT MEDIA. J. Struct. Chem. 2021, 62, 467–480. [Google Scholar] [CrossRef]
- Mamede, A. Characterization of WOx/CeO2 Catalysts and Their Reactivity in the Isomerization of Hexane. J. Catal. 2004, 223, 1–12. [Google Scholar] [CrossRef]
- Holmgreen, E.M.; Yung, M.M.; Ozkan, U.S. Pd-Based Sulfated Zirconia Prepared by a Single Step Sol–Gel Procedure for Lean NOx Reduction. J. Mol. Catal. Chem. 2007, 270, 101–111. [Google Scholar] [CrossRef]
- Lagunova, V.I.; Filatov, E.Y.; Plyusnin, P.E.; Korenev, S.V. In Situ and Ex Situ Studies of Tetrammineplatinum(II) Chromate Thermolysis. Russ. J. Inorg. Chem. 2020, 65, 1566–1570. [Google Scholar] [CrossRef]
- Shen, X.-F.; Ding, Y.-S.; Hanson, J.C.; Aindow, M.; Suib, S.L. In Situ Synthesis of Mixed-Valent Manganese Oxide Nanocrystals: An In Situ Synchrotron X-ray Diffraction Study. J. Am. Chem. Soc. 2006, 128, 4570–4571. [Google Scholar] [CrossRef] [PubMed]
- Heidari, S.; Balaghi, S.E.; Sologubenko, A.S.; Patzke, G.R. Economic Manganese-Oxide-Based Anodes for Efficient Water Oxidation: Rapid Synthesis and In Situ Transmission Electron Microscopy Monitoring. ACS Catal. 2021, 11, 2511–2523. [Google Scholar] [CrossRef]
- Ruiz Puigdollers, A.; Schlexer, P.; Tosoni, S.; Pacchioni, G. Increasing Oxide Reducibility: The Role of Metal/Oxide Interfaces in the Formation of Oxygen Vacancies. ACS Catal. 2017, 7, 6493–6513. [Google Scholar] [CrossRef]
- Lukashuk, L.; Yigit, N.; Rameshan, R.; Kolar, E.; Teschner, D.; Hävecker, M.; Knop-Gericke, A.; Schlögl, R.; Föttinger, K.; Rupprechter, G. Operando Insights into CO Oxidation on Cobalt Oxide Catalysts by NAP-XPS, FTIR, and XRD. ACS Catal. 2018, 8, 8630–8641. [Google Scholar] [CrossRef]
- Dionigi, F.; Zeng, Z.; Sinev, I.; Merzdorf, T.; Deshpande, S.; Lopez, M.B.; Kunze, S.; Zegkinoglou, I.; Sarodnik, H.; Fan, D.; et al. In-Situ Structure and Catalytic Mechanism of NiFe and CoFe Layered Double Hydroxides during Oxygen Evolution. Nat. Commun. 2020, 11, 2522. [Google Scholar] [CrossRef] [PubMed]
- Rochet, A.; Moizan, V.; Pichon, C.; Diehl, F.; Berliet, A.; Briois, V. In Situ and Operando Structural Characterisation of a Fischer–Tropsch Supported Cobalt Catalyst. Catal. Today 2011, 171, 186–191. [Google Scholar] [CrossRef]
- Jalama, K.; Kabuba, J.; Xiong, H.; Jewell, L.L. Co/TiO2 Fischer–Tropsch Catalyst Activation by Synthesis Gas. Catal. Commun. 2012, 17, 154–159. [Google Scholar] [CrossRef]
- Khassin, A.A.; Simentsova, I.I.; Shmakov, A.N.; Shtertser, N.V.; Bulavchenko, O.A.; Cherepanova, S.V. Effect of Nitric Oxide on the Formation of Cobalt–Aluminum Oxide Structure from Layered Double Hydroxide and Its Further Transformation during Reductive Activation. Appl. Catal. Gen. 2016, 514, 114–125. [Google Scholar] [CrossRef]
- Popa, T.; Xu, G.; Barton, T.F.; Argyle, M.D. High Temperature Water Gas Shift Catalysts with Alumina. Appl. Catal. Gen. 2010, 379, 15–23. [Google Scholar] [CrossRef]
- Reddy, G.K.; Gunasekara, K.; Boolchand, P.; Smirniotis, P.G. Cr- and Ce-Doped Ferrite Catalysts for the High Temperature Water−Gas Shift Reaction: TPR and Mossbauer Spectroscopic Study. J. Phys. Chem. C 2011, 115, 920–930. [Google Scholar] [CrossRef]
- Kaichev, V.V.; Chesalov, Y.A.; Saraev, A.A.; Tsapina, A.M. A Mechanistic Study of Dehydrogenation of Propane over Vanadia–Titania Catalysts. J. Phys. Chem. C 2019, 123, 19668–19680. [Google Scholar] [CrossRef]
- Toko, K.; Ito, K.; Saito, H.; Hosono, Y.; Murakami, K.; Misaki, S.; Higo, T.; Ogo, S.; Tsuneki, H.; Maeda, S.; et al. Catalytic Dehydrogenation of Ethane over Doped Perovskite via the Mars–van Krevelen Mechanism. J. Phys. Chem. C 2020, 124, 10462–10469. [Google Scholar] [CrossRef]
- Pussacq, T.; Mentré, O.; Tessier, F.; Löfberg, A.; Huvé, M.; Guererro Caballero, J.; Colis, S.; Kabbour, H. Nanometric Nickel Exsolution in the Hexagonal Perovskite Ba8Ta6NiO24: Survey of the Structural, Magnetic and Catalytic Features. J. Alloys Compd. 2018, 766, 987–993. [Google Scholar] [CrossRef]
- Kabbour, H.; Gauthier, G.H.; Tessier, F.; Huvé, M.; Pussacq, T.; Roussel, P.; Hayward, M.A.; Moreno B, Z.L.; Marinova, M.; Colmont, M.; et al. Topochemical Reduction of YMnO3 into a Composite Structure. Inorg. Chem. 2017, 56, 8547–8553. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef]
- Sabat, K.C.; Paramguru, R.K.; Mishra, B.K. Reduction of Copper Oxide by Low-Temperature Hydrogen Plasma. Plasma Chem. Plasma Process. 2016, 36, 1111–1124. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Kim, J.Y.; Hanson, J.C.; Pérez, M.; Frenkel, A.I. Reduction of CuO in H2: In Situ Time-Resolved XRD Studies. Catal. Lett. 2003, 85, 247–254. [Google Scholar] [CrossRef]
- Kim, J.Y.; Rodriguez, J.A.; Hanson, J.C.; Frenkel, A.I.; Lee, P.L. Reduction of CuO and Cu2O with H2: H Embedding and Kinetic Effects in the Formation of Suboxides. J. Am. Chem. Soc. 2003, 125, 10684–10692. [Google Scholar] [CrossRef]
- Pike, J.; Chan, S.-W.; Zhang, F.; Wang, X.; Hanson, J. Formation of Stable Cu2O from Reduction of CuO Nanoparticles. Appl. Catal. Gen. 2006, 303, 273–277. [Google Scholar] [CrossRef]
- Unutulmazsoy, Y.; Cancellieri, C.; Lin, L.; Jeurgens, L.P.H. Reduction of Thermally Grown Single-Phase CuO and Cu2O Thin Films by In-Situ Time-Resolved XRD. Appl. Surf. Sci. 2022, 588, 152896. [Google Scholar] [CrossRef]
- Svintsitskiy, D.A.; Kardash, T.Y.; Stonkus, O.A.; Slavinskaya, E.M.; Stadnichenko, A.I.; Koscheev, S.V.; Chupakhin, A.P.; Boronin, A.I. In Situ XRD, XPS, TEM, and TPR Study of Highly Active in CO Oxidation CuO Nanopowders. J. Phys. Chem. C 2013, 117, 14588–14599. [Google Scholar] [CrossRef]
- Den Breejen, J.P.; Radstake, P.B.; Bezemer, G.L.; Bitter, J.H.; Frøseth, V.; Holmen, A.; De Jong, K.P. On the Origin of the Cobalt Particle Size Effects in Fischer−Tropsch Catalysis. J. Am. Chem. Soc. 2009, 131, 7197–7203. [Google Scholar] [CrossRef]
- Nematollahi, B.; Rezaei, M.; Lay, E.N. Preparation of Highly Active and Stable NiO–CeO2 Nanocatalysts for CO Selective Methanation. Int. J. Hydrogen Energy 2015, 40, 8539–8547. [Google Scholar] [CrossRef]
- Edwards, M.A.; Whittle, D.M.; Rhodes, C.; Ward, A.M.; Rohan, D.; Shannon, M.D.; Hutchings, G.J.; Kiely, C.J. Microstructural Studies of the Copper Promoted Iron Oxide/Chromia Water-Gas Shift Catalyst. Phys. Chem. Chem. Phys. 2002, 4, 3902–3908. [Google Scholar] [CrossRef]
- He, M.; Luo, M.; Fang, P. Characterization of CuO Species and Thermal Solid-Solid Interaction in CuO/CeO2-Al2O3 Catalyst by In-Situ XRD, Raman Spectroscopy and TPR. J. Rare Earths 2006, 24, 188–192. [Google Scholar] [CrossRef]
- Du, H.; Ma, X.; Yan, P.; Jiang, M.; Zhao, Z.; Zhang, Z.C. Catalytic Furfural Hydrogenation to Furfuryl Alcohol over Cu/SiO2 Catalysts: A Comparative Study of the Preparation Methods. Fuel Process. Technol. 2019, 193, 221–231. [Google Scholar] [CrossRef]
- Luo, M.-F.; Fang, P.; He, M.; Xie, Y.-L. In Situ XRD, Raman, and TPR Studies of CuO/Al2O3 Catalysts for CO Oxidation. J. Mol. Catal. Chem. 2005, 239, 243–248. [Google Scholar] [CrossRef]
- Plyasova, L.M.; Kardash, T.Y.; Svintsitskiy, D.A.; Paukshtis, E.A.; Shtertser, N.V.; Minyukova, T.P. The Interaction of Copper-Containing Spinels CuFe2O4 and CuFeCrO4 with Hydrogen. Mater. Res. Bull. 2019, 118, 110481. [Google Scholar] [CrossRef]
- Estrella, M.; Barrio, L.; Zhou, G.; Wang, X.; Wang, Q.; Wen, W.; Hanson, J.C.; Frenkel, A.I.; Rodriguez, J.A. In Situ Characterization of CuFe2O4 and Cu/Fe3O4 Water−Gas Shift Catalysts. J. Phys. Chem. C 2009, 113, 14411–14417. [Google Scholar] [CrossRef]
- Wang, X.; Rodriguez, J.A.; Hanson, J.C.; Gamarra, D.; Martínez-Arias, A.; Fernández-García, M. Unusual Physical and Chemical Properties of Cu in Ce1−xCuxO2 Oxides. J. Phys. Chem. B 2005, 109, 19595–19603. [Google Scholar] [CrossRef] [PubMed]
- Barbier, A.; Tuel, A.; Arcon, I.; Kodre, A.; Martin, G.A. Characterization and Catalytic Behavior of Co/SiO2 Catalysts: Influence of Dispersion in the Fischer–Tropsch Reaction. J. Catal. 2001, 200, 106–116. [Google Scholar] [CrossRef]
- Bezemer, G.L.; Bitter, J.H.; Kuipers, H.P.C.E.; Oosterbeek, H.; Holewijn, J.E.; Xu, X.; Kapteijn, F.; Van Dillen, A.J.; De Jong, K.P. Cobalt Particle Size Effects in the Fischer−Tropsch Reaction Studied with Carbon Nanofiber Supported Catalysts. J. Am. Chem. Soc. 2006, 128, 3956–3964. [Google Scholar] [CrossRef] [PubMed]
- Bezemer, G.; Radstake, P.; Koot, V.; Vandillen, A.; Geus, J.; Dejong, K. Preparation of Fischer–Tropsch Cobalt Catalysts Supported on Carbon Nanofibers and Silica Using Homogeneous Deposition-Precipitation. J. Catal. 2006, 237, 291–302. [Google Scholar] [CrossRef]
- Radstake, P.B.; Breejen, J.P.D.; Bezemer, G.L.; Bitter, J.H.; Jong, K.P.D.; Frøseth, V.; Holmen, A. On the Origin of the Cobalt Particle Size Effect in the Fischer-Tropsch Synthesis. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2007; Volume 167, pp. 85–90. ISBN 978-0-444-53078-3. [Google Scholar]
- Panda, A.; Kim, E.; Choi, Y.; Lee, J.; Venkateswarlu, S.; Yoon, M. Phase Controlled Synthesis of Pt Doped Co Nanoparticle Composites Using a Metal-Organic Framework for Fischer–Tropsch Catalysis. Catalysts 2019, 9, 156. [Google Scholar] [CrossRef]
- Liu, L.; Qin, C.; Yu, M.; Wang, Q.; Wang, J.; Hou, B.; Jia, L.; Li, D. Morphology Evolution of Hcp Cobalt Nanoparticles Induced by Ru Promoter. ChemCatChem 2020, 12, 2083–2090. [Google Scholar] [CrossRef]
- Gholami, Z.; Tišler, Z.; Rubáš, V. Recent Advances in Fischer-Tropsch Synthesis Using Cobalt-Based Catalysts: A Review on Supports, Promoters, and Reactors. Catal. Rev. 2021, 63, 512–595. [Google Scholar] [CrossRef]
- Guo, S.; Ma, Z.; Wang, J.; Hou, B.; Jia, L.; Wang, B.; Li, D. Exploring the Reasons for Zr-Improved Performance of Alumina Supported Cobalt Fischer-Tropsch Synthesis. J. Energy Inst. 2021, 96, 31–37. [Google Scholar] [CrossRef]
- Suo, Y.; Yao, Y.; Zhang, Y.; Xing, S.; Yuan, Z.-Y. Recent Advances in Cobalt-Based Fischer-Tropsch Synthesis Catalysts. J. Ind. Eng. Chem. 2022, 115, 92–119. [Google Scholar] [CrossRef]
- Bulavchenko, O.A.; Cherepanova, S.V.; Malakhov, V.V.; Dovlitova, L.S.; Ishchenko, A.V.; Tsybulya, S.V. In Situ XRD Study of Nanocrystalline Cobalt Oxide Reduction. Kinet. Catal. 2009, 50, 192–198. [Google Scholar] [CrossRef]
- Khodakov, A.Y.; Lynch, J.; Bazin, D.; Rebours, B.; Zanier, N.; Moisson, B.; Chaumette, P. Reducibility of Cobalt Species In Silica-Supported Fischer–Tropsch Catalysts. J. Catal. 1997, 168, 16–25. [Google Scholar] [CrossRef]
- Zhang, Z.-S.; Fu, X.-P.; Wang, W.-W.; Jin, Z.; Song, Q.-S.; Jia, C.-J. Promoted Porous Co3O4-Al2O3 Catalysts for Ammonia Decomposition. Sci. China Chem. 2018, 61, 1389–1398. [Google Scholar] [CrossRef]
- Cherepanova, S.; Bulavchenko, O.; Simentsova, I.; Gerasimov, E.; Khassin, A. Influence of Al Ions on the Reduction of Co3−xAlxO4: In Situ XRD Investigation. In Proceedings of the European Powder Diffraction Conference, Darmstadt, Germany, 27–30 August 2010; Oldenbourg Wissenschaftsverlag: München, Germany, 2011; pp. 331–336, ISBN 978-3-486-98940-3. [Google Scholar]
- Wang, J.; Wang, J.; Huang, X.; Chen, C.; Ma, Z.; Jia, L.; Hou, B.; Li, D. Co Al Spinel Oxide Modified Ordered Mesoporous Alumina Supported Cobalt-Based Catalysts for Fischer–Tropsch Synthesis. Int. J. Hydrogen Energy 2018, 43, 13122–13132. [Google Scholar] [CrossRef]
- Van Deelen, T.W.; Yoshida, H.; Oord, R.; Zečević, J.; Weckhuysen, B.M.; De Jong, K.P. Cobalt Nanocrystals on Carbon Nanotubes in the Fischer-Tropsch Synthesis: Impact of Support Oxidation. Appl. Catal. Gen. 2020, 593, 117441. [Google Scholar] [CrossRef]
- Bulavchenko, O.A.; Gerasimov, E.Y.; Afonasenko, T.N. Reduction of Double Manganese–Cobalt Oxides: In Situ XRD and TPR Study. Dalton Trans. 2018, 47, 17153–17159. [Google Scholar] [CrossRef]
- Bulavchenko, O.A.; Afonasenko, T.N.; Ivanchikova, A.V.; Murzin, V.Y.; Kremneva, A.M.; Saraev, A.A.; Kaichev, V.V.; Tsybulya, S.V. In Situ Study of Reduction of MnxCo3–xO4 Mixed Oxides: The Role of Manganese Content. Inorg. Chem. 2021, 60, 16518–16528. [Google Scholar] [CrossRef]
- Wang, J.; Chernavskii, P.A.; Khodakov, A.Y.; Wang, Y. Structure and Catalytic Performance of Alumina-Supported Copper–Cobalt Catalysts for Carbon Monoxide Hydrogenation. J. Catal. 2012, 286, 51–61. [Google Scholar] [CrossRef]
- Ciotonea, C.; Chirieac, A.; Dragoi, B.; Dhainaut, J.; Marinova, M.; Pronier, S.; Arii-Clacens, S.; Dacquin, J.-P.; Dumitriu, E.; Ungureanu, A.; et al. Playing on 3D Spatial Distribution of Cu-Co (Oxide) Nanoparticles in Inorganic Mesoporous Sieves: Impact on Catalytic Performance toward the Cinnamaldehyde Hydrogenation. Appl. Catal. Gen. 2021, 623, 118303. [Google Scholar] [CrossRef]
- Kungurova, O.A.; Shtertser, N.V.; Koemets, E.G.; Cherepanova, S.V.; Khassin, A.A. The Effect of Ruthenium Promotion of the Co/δ-Al2O3 Catalyst on the Hydrogen Reduction Kinetics of Cobalt. React. Kinet. Mech. Catal. 2017, 120, 501–525. [Google Scholar] [CrossRef]
- Phaahlamohlaka, T.N.; Dlamini, M.W.; Kumi, D.O.; Forbes, R.; Jewell, L.L.; Coville, N.J. Co inside Hollow Carbon Spheres as a Fischer-Tropsch Catalyst: Spillover Effects from Ru Placed inside and Outside the HCS. Appl. Catal. Gen. 2020, 599, 117617. [Google Scholar] [CrossRef]
- Andreev, A.S.; Kazakova, M.A.; Ishchenko, A.V.; Selyutin, A.G.; Lapina, O.B.; Kuznetsov, V.L.; d’Espinose De Lacaillerie, J.-B. Magnetic and Dielectric Properties of Carbon Nanotubes with Embedded Cobalt Nanoparticles. Carbon 2017, 114, 39–49. [Google Scholar] [CrossRef]
- Kazakova, M.A.; Andreev, A.S.; Selyutin, A.G.; Ishchenko, A.V.; Shuvaev, A.V.; Kuznetsov, V.L.; Lapina, O.B.; d’Espinose De Lacaillerie, J.-B. Co Metal Nanoparticles Deposition inside or Outside Multi-Walled Carbon Nanotubes via Facile Support Pretreatment. Appl. Surf. Sci. 2018, 456, 657–665. [Google Scholar] [CrossRef]
- Mohd Ridzuan, N.D.; Shaharun, M.S.; Anawar, M.A.; Ud-Din, I. Ni-Based Catalyst for Carbon Dioxide Methanation: A Review on Performance and Progress. Catalysts 2022, 12, 469. [Google Scholar] [CrossRef]
- Strucks, P.; Failing, L.; Kaluza, S. A Short Review on Ni-Catalyzed Methanation of CO2: Reaction Mechanism, Catalyst Deactivation, Dynamic Operation. Chem. Ing. Tech. 2021, 93, 1526–1536. [Google Scholar] [CrossRef]
- Matsumura, Y.; Nakamori, T. Steam Reforming of Methane over Nickel Catalysts at Low Reaction Temperature. Appl. Catal. Gen. 2004, 258, 107–114. [Google Scholar] [CrossRef]
- Vogt, C.; Kranenborg, J.; Monai, M.; Weckhuysen, B.M. Structure Sensitivity in Steam and Dry Methane Reforming over Nickel: Activity and Carbon Formation. ACS Catal. 2020, 10, 1428–1438. [Google Scholar] [CrossRef]
- Hüfner, S. Electronic Structure of NiO and Related 3d-Transition-Metal Compounds. Adv. Phys. 1994, 43, 183–356. [Google Scholar] [CrossRef]
- Noguera, C.; Mackrodt, W.C. Ab Initio Study of Ground and Excited States of NiO(100) Monolayer. J. Phys. Condens. Matter 2000, 12, 2163–2181. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Hanson, J.C.; Frenkel, A.I.; Kim, J.Y.; Pérez, M. Experimental and Theoretical Studies on the Reaction of H2 with NiO: Role of O Vacancies and Mechanism for Oxide Reduction. J. Am. Chem. Soc. 2002, 124, 346–354. [Google Scholar] [CrossRef]
- Borges, R.P.; Moura, L.G.; Kanitkar, S.; Spivey, J.J.; Noronha, F.B.; Hori, C.E. Hydrogen Production by Steam Reforming of Propane Using Supported Nickel over Ceria-Silica Catalysts. Catal. Today 2021, 381, 3–12. [Google Scholar] [CrossRef]
- Shimoda, N.; Yoshimura, R.; Nukui, T.; Satokawa, S. Alloying Effect of Nickel–Cobalt Based Binary Metal Catalysts Supported on α-Alumina for Ammonia Decomposition. J. Chem. Eng. Jpn. 2019, 52, 413–422. [Google Scholar] [CrossRef]
- Theofanidis, S.-A.; Pieterse, J.A.Z.; Poelman, H.; Longo, A.; Sabbe, M.K.; Virginie, M.; Detavernier, C.; Marin, G.B.; Galvita, V.V. Effect of Rh in Ni-Based Catalysts on Sulfur Impurities during Methane Reforming. Appl. Catal. B Environ. 2020, 267, 118691. [Google Scholar] [CrossRef]
- Fedorov, A.V.; Kukushkin, R.G.; Yeletsky, P.M.; Bulavchenko, O.A.; Chesalov, Y.A.; Yakovlev, V.A. Temperature-Programmed Reduction of Model CuO, NiO and Mixed CuO–NiO Catalysts with Hydrogen. J. Alloys Compd. 2020, 844, 156135. [Google Scholar] [CrossRef]
- Bykova, M.V.; Ermakov, D.Y.; Kaichev, V.V.; Bulavchenko, O.A.; Saraev, A.A.; Lebedev, M.Y.; Yakovlev, V.A. Ni-Based Sol–Gel Catalysts as Promising Systems for Crude Bio-Oil Upgrading: Guaiacol Hydrodeoxygenation Study. Appl. Catal. B Environ. 2012, 113–114, 296–307. [Google Scholar] [CrossRef]
- Kukushkin, R.G.; Bulavchenko, O.A.; Kaichev, V.V.; Yakovlev, V.A. Influence of Mo on Catalytic Activity of Ni-Based Catalysts in Hydrodeoxygenation of Esters. Appl. Catal. B Environ. 2015, 163, 531–538. [Google Scholar] [CrossRef]
- Alekseeva, M.V.; Otyuskaya, D.S.; Rekhtina, M.A.; Bulavchenko, O.A.; Stonkus, O.A.; Kaichev, V.V.; Zavarukhin, S.G.; Thybaut, J.W.; Alexiadis, V.; Venderbosch, R.H.; et al. NiCuMo-SiO2 Catalyst for Pyrolysis Oil Upgrading: Model Acidic Treatment Study. Appl. Catal. Gen. 2019, 573, 1–12. [Google Scholar] [CrossRef]
- Alekseeva, M.V.; Gulyaeva, Y.K.; Bulavchenko, O.A.; Saraev, A.A.; Kremneva, A.M.; Stepanenko, S.A.; Koskin, A.P.; Kaichev, V.V.; Yakovlev, V.A. Promoting Effect of Zn in High-Loading Zn/Ni-SiO2 Catalysts for Selective Hydrogen Evolution from Methylcyclohexane. Dalton Trans. 2022, 51, 6068–6085. [Google Scholar] [CrossRef]
- Gulyaeva, Y.; Alekseeva, M.; Bulavchenko, O.; Kremneva, A.; Saraev, A.; Gerasimov, E.; Selishcheva, S.; Kaichev, V.; Yakovlev, V. Ni–Cu High-Loaded Sol–Gel Catalysts for Dehydrogenation of Liquid Organic Hydrides: Insights into Structural Features and Relationship with Catalytic Activity. Nanomaterials 2021, 11, 2017. [Google Scholar] [CrossRef]
- Bulavchenko, O.A.; Smirnov, A.A.; Khromova, S.A.; Vinokurov, Z.S.; Ishchenko, A.V.; Yakovlev, V.A.; Tsybulya, S.V. In Situ Powder X-ray Diffraction Study of the Process of NiMoO4–SiO2 Reduction with Hydrogen. J. Struct. Chem. 2016, 57, 955–961. [Google Scholar] [CrossRef]
- Tichit, D.; Medina, F.; Coq, B.; Dutartre, R. Activation under Oxidizing and Reducing Atmospheres of Ni-Containing Layered Double Hydroxides. Appl. Catal. Gen. 1997, 159, 241–258. [Google Scholar] [CrossRef]
- Ferreira, R.A.R.; Ávila-Neto, C.N.; Noronha, F.B.; Hori, C.E. Study of LPG Steam Reform Using Ni/Mg/Al Hydrotalcite-Type Precursors. Int. J. Hydrogen Energy 2019, 44, 24471–24484. [Google Scholar] [CrossRef]
- Baraka, S.; Bouearan, K.; Caner, L.; Fontaine, C.; Epron, F.; Brahmi, R.; Bion, N. Catalytic Performances of Natural Ni-Bearing Clay Minerals for Production of Syngas from Dry Reforming of Methane. J. CO2 Util. 2021, 52, 101696. [Google Scholar] [CrossRef]
- Da Silva, B.C.; Bastos, P.H.C.; Junior, R.B.S.; Checca, N.R.; Costa, D.S.; Fréty, R.; Brandão, S.T. Oxy-CO2 Reforming of CH4 on Ni-Based Catalysts: Evaluation of Cerium and Aluminum Addition on the Structure and Properties of the Reduced Materials. Catal. Today 2021, 381, 50–64. [Google Scholar] [CrossRef]
- Deka, D.J.; Kim, J.; Gunduz, S.; Aouine, M.; Millet, J.-M.M.; Co, A.C.; Ozkan, U.S. Investigation of Hetero-Phases Grown via in-Situ Exsolution on a Ni-Doped (La,Sr)FeO3 Cathode and the Resultant Activity Enhancement in CO2 Reduction. Appl. Catal. B Environ. 2021, 286, 119917. [Google Scholar] [CrossRef]
- Marinho, A.L.A.; Rabelo-Neto, R.C.; Epron, F.; Bion, N.; Toniolo, F.S.; Noronha, F.B. Embedded Ni Nanoparticles in CeZrO2 as Stable Catalyst for Dry Reforming of Methane. Appl. Catal. B Environ. 2020, 268, 118387. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Z.; Chen, X.; Rui, N.; Betancourt, L.E.; Lin, L.; Xu, W.; Sun, C.; Abeykoon, A.M.M.; Rodriguez, J.A.; et al. Effects of Zr Doping into Ceria for the Dry Reforming of Methane over Ni/CeZrO2 Catalysts: In Situ Studies with XRD, XAFS, and AP-XPS. ACS Catal. 2020, 10, 3274–3284. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Hanson, J.C.; Stacchiola, D.; Senanayake, S.D. In Situ/Operando Studies for the Production of Hydrogen through the Water-Gas Shift on Metal Oxide Catalysts. Phys. Chem. Chem. Phys. 2013, 15, 12004. [Google Scholar] [CrossRef]
- Zhu, M.; Rocha, T.C.R.; Lunkenbein, T.; Knop-Gericke, A.; Schlögl, R.; Wachs, I.E. Promotion Mechanisms of Iron Oxide-Based High Temperature Water–Gas Shift Catalysts by Chromium and Copper. ACS Catal. 2016, 6, 4455–4464. [Google Scholar] [CrossRef]
- Li, S.; Krishnamoorthy, S.; Li, A.; Meitzner, G.D.; Iglesia, E. Promoted Iron-Based Catalysts for the Fischer–Tropsch Synthesis: Design, Synthesis, Site Densities, and Catalytic Properties. J. Catal. 2002, 206, 202–217. [Google Scholar] [CrossRef]
- Schoch, R.; Huang, H.; Schünemann, V.; Bauer, M. A New Iron-Based Carbon Monoxide Oxidation Catalyst: Structure-Activity Correlation. ChemPhysChem 2014, 15, 3768–3775. [Google Scholar] [CrossRef] [PubMed]
- Bukhtiyarova, G.A.; Bukhtiyarov, V.I.; Sakaeva, N.S.; Kaichev, V.V.; Zolotovskii, B.P. XPS Study of the Silica-Supported Fe-Containing Catalysts for Deep or Partial H2S Oxidation. J. Mol. Catal. Chem. 2000, 158, 251–255. [Google Scholar] [CrossRef]
- Arabczyk, W.; Jasińska, I.; Lubkowski, K. The Surface Properties of Iron Catalyst for Ammonia Synthesis. React. Kinet. Catal. Lett. 2004, 83, 385–392. [Google Scholar] [CrossRef]
- Jozwiak, W.K.; Kaczmarek, E.; Maniecki, T.P.; Ignaczak, W.; Maniukiewicz, W. Reduction Behavior of Iron Oxides in Hydrogen and Carbon Monoxide Atmospheres. Appl. Catal. Gen. 2007, 326, 17–27. [Google Scholar] [CrossRef]
- Nielsen, M.R.; Moss, A.B.; Bjørnlund, A.S.; Liu, X.; Knop-Gericke, A.; Klyushin, A.Y.; Grunwaldt, J.-D.; Sheppard, T.L.; Doronkin, D.E.; Zimina, A.; et al. Reduction and Carburization of Iron Oxides for Fischer–Tropsch Synthesis. J. Energy Chem. 2020, 51, 48–61. [Google Scholar] [CrossRef]
- Zheng, Y.-F.; Liu, H.-Z.; Liu, Z.-J.; Li, X.-N. In Situ X-ray Diffraction Study of Reduction Processes of Fe3O4-and Fe1−xO-Based Ammonia-Synthesis Catalysts. J. Solid State Chem. 2009, 182, 2385–2391. [Google Scholar] [CrossRef]
- Meshkani, F.; Rezaei, M. Preparation of Nanocrystalline Metal (Cr, Al, Mn, Ce, Ni, Co and Cu) Modified Ferrite Catalysts for the High Temperature Water Gas Shift Reaction. Renew. Energy 2015, 74, 588–598. [Google Scholar] [CrossRef]
- Venugopal, A. Low Temperature Reductive Pretreatment of Au/Fe2O3 Catalysts, TPR/TPO Studies and Behaviour in the Water–Gas Shift Reaction. Appl. Catal. Gen. 2004, 258, 241–249. [Google Scholar] [CrossRef]
- Zhao, Z.; Tang, H.; Guo, Z. Effects of CaO on Precipitation Morphology of Metallic Iron in Reduction of Iron Oxides Under CO Atmosphere. J. Iron Steel Res. Int. 2013, 20, 16–24. [Google Scholar] [CrossRef]
- Shi, B.; Zhang, Z.; Zha, B.; Liu, D. Structure Evolution of Spinel Fe-MII (M = Mn, Fe, Co, Ni) Ferrite in CO Hydrogeneration. Mol. Catal. 2018, 456, 31–37. [Google Scholar] [CrossRef]
- Niu, L.; Liu, X.; Wen, X.; Yang, Y.; Xu, J.; Li, Y. Effect of Potassium Promoter on Phase Transformation during H2 Pretreatment of a Fe2O3 Fischer Tropsch Synthesis Catalyst Precursor. Catal. Today 2020, 343, 101–111. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.; Yang, C.; Li, X.; Sun, J.; Wang, H.; Gao, P.; Sun, Y. The Rare Earth Elements Modified FeK/Al2O3 Catalysts for Direct CO2 Hydrogenation to Liquid Hydrocarbons. Catal. Today 2020, 356, 613–621. [Google Scholar] [CrossRef]
- Yang, Z.; Luo, M.; Liu, Q.; Shi, B. In Situ XRD and Raman Investigation of the Activation Process over K–Cu–Fe/SiO2 Catalyst for Fischer–Tropsch Synthesis Reaction. Catal. Lett. 2020, 150, 2437–2445. [Google Scholar] [CrossRef]
- Bulavchenko, O.A.; Vinokurov, Z.S.; Saraev, A.A.; Tsapina, A.M.; Trigub, A.L.; Gerasimov, E.Y.; Gladky, A.Y.; Fedorov, A.V.; Yakovlev, V.A.; Kaichev, V.V. The Influence of Cu and Al Additives on Reduction of Iron(III) Oxide: In Situ XRD and XANES Study. Inorg. Chem. 2019, 58, 4842–4850. [Google Scholar] [CrossRef]
- Saraev, A.A.; Tsapina, A.M.; Fedorov, A.V.; Trigub, A.L.; Bulavchenko, O.A.; Vinokurov, Z.S.; Zubavichus, Y.V.; Kaichev, V.V. CuFeAl-Composite Catalysts of Oxidation of Gasification Products of Solid Fuels: In Situ XAS and XRD Study. Radiat. Phys. Chem. 2020, 175, 108071. [Google Scholar] [CrossRef]
- Grünbacher, M.; Schlicker, L.; Bekheet, M.F.; Gurlo, A.; Klötzer, B.; Penner, S. H 2 Reduction of Gd- and Sm-Doped Ceria Compared to Pure CeO2 at High Temperatures: Effect on Structure, Oxygen Nonstoichiometry, Hydrogen Solubility and Hydroxyl Chemistry. Phys. Chem. Chem. Phys. 2018, 20, 22099–22113. [Google Scholar] [CrossRef]
- Bekheet, M.F.; Grünbacher, M.; Schlicker, L.; Gili, A.; Doran, A.; Epping, J.D.; Gurlo, A.; Klötzer, B.; Penner, S. On the Structural Stability of Crystalline Ceria Phases in Undoped and Acceptor-Doped Ceria Materials under In Situ Reduction Conditions. CrystEngComm 2019, 21, 145–154. [Google Scholar] [CrossRef]
- Ozawa, M.; Loong, C.-K. In Situ X-ray and Neutron Powder Diffraction Studies of Redox Behavior in CeO2-Containing Oxide Catalysts. Catal. Today 1999, 50, 329–342. [Google Scholar] [CrossRef]
- Taira, K.; Murao, R. High Dispersion of CeO2 on CeO2/MgO Prepared under Dry Conditions and Its Improved Redox Properties. Energies 2021, 14, 7922. [Google Scholar] [CrossRef]
- Martínez-Munuera, J.C.; Giménez-Mañogil, J.; Castoldi, L.; Lietti, L.; García-García, A. Ceria-Based Catalysts for NOx Removal in NSR Processes: A Fundamental Study of the Catalyst Modifications Explored by In Situ Techniques. Appl. Surf. Sci. 2020, 529, 147019. [Google Scholar] [CrossRef]
- Pakharukova, V.P.; Potemkin, D.I.; Stonkus, O.A.; Kharchenko, N.A.; Saraev, A.A.; Gorlova, A.M. Investigation of the Structure and Interface Features of Ni/Ce1−xZrxO2 Catalysts for CO and CO2 Methanation. J. Phys. Chem. C 2021, 125, 20538–20550. [Google Scholar] [CrossRef]
- Zhang, F.; Yao, S.; Liu, Z.; Gutiérrez, R.A.; Vovchok, D.; Cen, J.; Xu, W.; Ramírez, P.J.; Kim, T.; Senanayake, S.D.; et al. Reaction of Methane with MOx/CeO2 (M = Fe, Ni, and Cu) Catalysts: In Situ Studies with Time-Resolved X-ray Diffraction. J. Phys. Chem. C 2018, 122, 28739–28747. [Google Scholar] [CrossRef]
- Wang, X.; Rodriguez, J.A.; Hanson, J.C.; Gamarra, D.; Martínez-Arias, A.; Fernández-García, M. In Situ Studies of the Active Sites for the Water Gas Shift Reaction over Cu−CeO2 Catalysts: Complex Interaction between Metallic Copper and Oxygen Vacancies of Ceria. J. Phys. Chem. B 2006, 110, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Bulavchenko, O.A.; Afonasenko, T.N.; Osipov, A.R.; Pochtar, A.A.; Saraev, A.A.; Gerasimov, E.Y. The Formation of Mn-Ce Oxide Catalysts for CO Oxidation by Oxalate Route: The Role of Annealing Conditions. Catal. Lett. 2021, 151, 2906–2918. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Rui, N.; Zhang, F.; Senanayake, S.D. In Situ Studies of Methane Activation Using Synchrotron-Based Techniques: Guiding the Conversion of C–H Bonds. ACS Catal. 2022, 12, 5470–5488. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Z.; Zhang, S.; Akter, N.; Palomino, R.M.; Vovchok, D.; Orozco, I.; Salazar, D.; Rodriguez, J.A.; Llorca, J.; et al. In Situ Elucidation of the Active State of Co–CeOx Catalysts in the Dry Reforming of Methane: The Important Role of the Reducible Oxide Support and Interactions with Cobalt. ACS Catal. 2018, 8, 3550–3560. [Google Scholar] [CrossRef]
- Wang, H.; Srinath, N.V.; Poelman, H.; Detavernier, C.; Li, P.; Marin, G.B.; Galvita, V.V. Hierarchical Fe-Modified MgAl2O4 as a Ni-Catalyst Support for Methane Dry Reforming. Catal. Sci. Technol. 2020, 10, 6987–7001. [Google Scholar] [CrossRef]
- Marin, C.M.; Popczun, E.J.; Nguyen-Phan, T.-D.; Tafen, D.N.; Alfonso, D.; Waluyo, I.; Hunt, A.; Kauffman, D.R. Designing Perovskite Catalysts for Controlled Active-Site Exsolution in the Microwave Dry Reforming of Methane. Appl. Catal. B Environ. 2021, 284, 119711. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.; Rui, N.; Li, X.; Lin, L.; Betancourt, L.E.; Su, D.; Xu, W.; Cen, J.; Attenkofer, K.; et al. Highly Active Ceria-Supported Ru Catalyst for the Dry Reforming of Methane: In Situ Identification of Ruδ+–Ce3+ Interactions for Enhanced Conversion. ACS Catal. 2019, 9, 3349–3359. [Google Scholar] [CrossRef]
- Bekheet, M.F.; Delir Kheyrollahi Nezhad, P.; Bonmassar, N.; Schlicker, L.; Gili, A.; Praetz, S.; Gurlo, A.; Doran, A.; Gao, Y.; Heggen, M.; et al. Steering the Methane Dry Reforming Reactivity of Ni/La2O3 Catalysts by Controlled In Situ Decomposition of Doped La2NiO4 Precursor Structures. ACS Catal. 2021, 11, 43–59. [Google Scholar] [CrossRef]
- Liu, Z.; Yao, S.; Johnston-Peck, A.; Xu, W.; Rodriguez, J.A.; Senanayake, S.D. Methanol Steam Reforming over Ni-CeO2 Model and Powder Catalysts: Pathways to High Stability and Selectivity for H2/CO2 Production. Catal. Today 2018, 311, 74–80. [Google Scholar] [CrossRef]
- Zhou, G.; Barrio, L.; Agnoli, S.; Senanayake, S.D.; Evans, J.; Kubacka, A.; Estrella, M.; Hanson, J.C.; Martínez-Arias, A.; Fernández-García, M.; et al. High Activity of Ce1−xNixO2−y for H2 Production through Ethanol Steam Reforming: Tuning Catalytic Performance through Metal-Oxide Interactions. Angew. Chem. Int. Ed. 2010, 49, 9680–9684. [Google Scholar] [CrossRef] [PubMed]
- Clausen, B. In Situ Cell for Combined XRD and On-Line Catalysis Tests: Studies of Cu-Based Water Gas Shift and Methanol Catalysts. J. Catal. 1991, 132, 524–535. [Google Scholar] [CrossRef]
- Si, R.; Tao, J.; Evans, J.; Park, J.B.; Barrio, L.; Hanson, J.C.; Zhu, Y.; Hrbek, J.; Rodriguez, J.A. Effect of Ceria on Gold–Titania Catalysts for the Water–Gas Shift Reaction: Fundamental Studies for Au/CeOx/TiO2 (110) and Au/CeOx/TiO2 Powders. J. Phys. Chem. C 2012, 116, 23547–23555. [Google Scholar] [CrossRef]
- Patlolla, A.; Carino, E.V.; Ehrlich, S.N.; Stavitski, E.; Frenkel, A.I. Application of Operando XAS, XRD, and Raman Spectroscopy for Phase Speciation in Water Gas Shift Reaction Catalysts. ACS Catal. 2012, 2, 2216–2223. [Google Scholar] [CrossRef]
- Vovchok, D.; Guild, C.J.; Dissanayake, S.; Llorca, J.; Stavitski, E.; Liu, Z.; Palomino, R.M.; Waluyo, I.; Li, Y.; Frenkel, A.I.; et al. In Situ Characterization of Mesoporous Co/CeO2 Catalysts for the High-Temperature Water-Gas Shift. J. Phys. Chem. C 2018, 122, 8998–9008. [Google Scholar] [CrossRef]
- López Cámara, A.; Cortés Corberán, V.; Martínez-Arias, A.; Barrio, L.; Si, R.; Hanson, J.C.; Rodriguez, J.A. Novel Manganese-Promoted Inverse CeO2/CuO Catalyst: In Situ Characterization and Activity for the Water-Gas Shift Reaction. Catal. Today 2020, 339, 24–31. [Google Scholar] [CrossRef]
- Barrio, L.; Kubacka, A.; Zhou, G.; Estrella, M.; Martínez-Arias, A.; Hanson, J.C.; Fernández-García, M.; Rodriguez, J.A. Unusual Physical and Chemical Properties of Ni in Ce1−xNixO2−y Oxides: Structural Characterization and Catalytic Activity for the Water Gas Shift Reaction. J. Phys. Chem. C 2010, 114, 12689–12697. [Google Scholar] [CrossRef]
- Zhu, M.; Chen, J.; Shen, L.; Ford, M.E.; Gao, J.; Xu, J.; Wachs, I.E.; Han, Y.-F. Probing the Surface of Promoted CuO-Cr2O3-Fe2O3 Catalysts during CO2 Activation. Appl. Catal. B Environ. 2020, 271, 118943. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.-Y.; Senftle, T.P.; Zhu, J.; Zhang, G.; Guo, X.; Song, C. Variation in the In2O3 Crystal Phase Alters Catalytic Performance toward the Reverse Water Gas Shift Reaction. ACS Catal. 2020, 10, 3264–3273. [Google Scholar] [CrossRef]
- Lin, L.; Yao, S.; Rui, N.; Han, L.; Zhang, F.; Gerlak, C.A.; Liu, Z.; Cen, J.; Song, L.; Senanayake, S.D.; et al. Conversion of CO2 on a Highly Active and Stable Cu/FeOx/CeO2 Catalyst: Tuning Catalytic Performance by Oxide-Oxide Interactions. Catal. Sci. Technol. 2019, 9, 3735–3742. [Google Scholar] [CrossRef]
- Muhler, M. The Nature of the Iron Oxide-Based Catalyst for Dehydrogenation of Ethylbenzene to Styrene I. Solid-State Chemistry and Bulk Characterization. J. Catal. 1990, 126, 339–360. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X.; Liu, J.; Huang, C.; Zhao, K.; Zhao, Z.; Wang, X.; Li, F. A Molten Carbonate Shell Modified Perovskite Redox Catalyst for Anaerobic Oxidative Dehydrogenation of Ethane. Sci. Adv. 2020, 6, eaaz9339. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Gao, Y.; Wang, X.; Haribal, V.; Liu, J.; Neal, L.M.; Bao, Z.; Wu, Z.; Wang, H.; Li, F. A Tailored Multi-Functional Catalyst for Ultra-Efficient Styrene Production under a Cyclic Redox Scheme. Nat. Commun. 2021, 12, 1329. [Google Scholar] [CrossRef]
- Wei, Z.; Shao, F.; Wang, J. Recent Advances in Heterogeneous Catalytic Hydrogenation and Dehydrogenation of N-Heterocycles. Chin. J. Catal. 2019, 40, 980–1002. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Z. A Review of In Situ/Operando Studies of Heterogeneous Catalytic Hydrogenation of CO2 to Methanol. Catal. Today 2023, 420, 114029. [Google Scholar] [CrossRef]
- Mishchenko, D.D.; Vinokurov, Z.S.; Afonasenko, T.N.; Saraev, A.A.; Simonov, M.N.; Gerasimov, E.Y.; Bulavchenko, O.A. Insights into the Contribution of Oxidation-Reduction Pretreatment for Mn0.2Zr0.8O2−δ Catalyst of CO Oxidation Reaction. Materials 2023, 16, 3508. [Google Scholar] [CrossRef]
- Bulavchenko, O.A.; Vinokurov, Z.S.; Afonasenko, T.N.; Gerasimov, E.Y.; Konovalova, V.P. The Activation of MnOx-ZrO2 Catalyst in CO Oxidation: Operando XRD Study. Mater. Lett. 2022, 315, 131961. [Google Scholar] [CrossRef]
- Bulavchenko, O.A.; Vinokurov, Z.S.; Konovalova, V.P.; Afonasenko, T.N. OPERANDO X-RAY DIFFRACTION ANALYSIS OF THE MnOx–ZrO2 CATALYST DURING OXIDATION OF PROPANE. J. Struct. Chem. 2022, 63, 885–894. [Google Scholar] [CrossRef]
- Lashina, E.A.; Vinokurov, Z.S.; Saraev, A.A.; Kaichev, V.V. Self-Sustained Oscillations in Oxidation of Methane over Palladium: Experimental Study and Mathematical Modeling. J. Chem. Phys. 2022, 157, 044703. [Google Scholar] [CrossRef]
- Kaichev, V.V.; Vinokurov, Z.S.; Saraev, A.A. Self-Sustained Oscillations in Oxidation of Methane over Palladium: The Nature of “Low-Active” and “Highly Active” States. Catal. Sci. Technol. 2021, 11, 4392–4397. [Google Scholar] [CrossRef]
- Strong, P.J.; Xie, S.; Clarke, W.P. Methane as a Resource: Can the Methanotrophs Add Value? Environ. Sci. Technol. 2015, 49, 4001–4018. [Google Scholar] [CrossRef] [PubMed]
- Schwach, P.; Pan, X.; Bao, X. Direct Conversion of Methane to Value-Added Chemicals over Heterogeneous Catalysts: Challenges and Prospects. Chem. Rev. 2017, 117, 8497–8520. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, J.-M. Review on Dry Reforming of Methane, a Potentially More Environmentally-Friendly Approach to the Increasing Natural Gas Exploitation. Front. Chem. 2014, 2, 81. [Google Scholar] [CrossRef] [PubMed]
- Mondal, K.; Sasmal, S.; Badgandi, S.; Chowdhury, D.R.; Nair, V. Dry Reforming of Methane to Syngas: A Potential Alternative Process for Value Added Chemicals—A Techno-Economic Perspective. Environ. Sci. Pollut. Res. 2016, 23, 22267–22273. [Google Scholar] [CrossRef]
- Gili, A.; Schlicker, L.; Bekheet, M.F.; Görke, O.; Penner, S.; Grünbacher, M.; Götsch, T.; Littlewood, P.; Marks, T.J.; Stair, P.C.; et al. Surface Carbon as a Reactive Intermediate in Dry Reforming of Methane to Syngas on a 5% Ni/MnO Catalyst. ACS Catal. 2018, 8, 8739–8750. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Mechanisms of Catalyst Deactivation. Appl. Catal. Gen. 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Argyle, M.; Bartholomew, C. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef]
- Svintsitskiy, D.A.; Kardash, T.Y.; Lazareva, E.V.; Saraev, A.A.; Derevyannikova, E.A.; Vorokhta, M.; Šmíd, B.; Bondareva, V.M. NAP-XPS and In Situ XRD Study of the Stability of Bi-Modified MoVNbTeO Catalysts for Oxidative Dehydrogenation of Ethane. Appl. Catal. Gen. 2019, 579, 141–150. [Google Scholar] [CrossRef]
- Muhler, M. The Nature of the Iron Oxide-Based Catalyst for Dehydrogenation of Ethylbenzene to Styrene 2. Surface Chemistry of the Active Phase. J. Catal. 1992, 138, 413–444. [Google Scholar] [CrossRef]
- Muhler, M.; Schlögl, R.; Reller, A.; Ertl, G. The Nature of the Active Phase of the Fe/K-Catalyst for Dehydrogenation of Ethylbenzene. Catal. Lett. 1989, 2, 201–210. [Google Scholar] [CrossRef]
- Guan, C.; Liu, Z.; Wang, D.; Zhou, X.; Pang, Y.; Yu, N.; Van Bavel, A.P.; Vovk, E.; Yang, Y. Exploring the Formation of Carbonates on La2O3 Catalysts with OCM Activity. Catal. Sci. Technol. 2021, 11, 6516–6528. [Google Scholar] [CrossRef]
- Moura, L.G.; Borges, R.P.; Noronha, F.B.; Hori, C.E. Steam Reforming of Liquefied Petroleum Gas Using Catalysts Supported on Ceria-Silica. Int. J. Hydrogen Energy 2021, 46, 1801–1814. [Google Scholar] [CrossRef]
- Vamvakeros, A.; Matras, D.; Jacques, S.D.M.; Di Michiel, M.; Middelkoop, V.; Cong, P.; Price, S.W.T.; Bull, C.L.; Senecal, P.; Beale, A.M. Real-Time Tomographic Diffraction Imaging of Catalytic Membrane Reactors for the Oxidative Coupling of Methane. Catal. Today 2021, 364, 242–255. [Google Scholar] [CrossRef]
- Kim, I.S.; Li, Z.; Zheng, J.; Platero-Prats, A.E.; Mavrandonakis, A.; Pellizzeri, S.; Ferrandon, M.; Vjunov, A.; Gallington, L.C.; Webber, T.E.; et al. Sinter-Resistant Platinum Catalyst Supported by Metal–Organic Framework. Angew. Chem. Int. Ed. 2018, 57, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Fichtl, M.B.; Schlereth, D.; Jacobsen, N.; Kasatkin, I.; Schumann, J.; Behrens, M.; Schlögl, R.; Hinrichsen, O. Kinetics of Deactivation on Cu/ZnO/Al2O3 Methanol Synthesis Catalysts. Appl. Catal. Gen. 2015, 502, 262–270. [Google Scholar] [CrossRef]
- Sadeghi, S.; Vafajoo, L.; Kazemeini, M.; Fattahi, M. Modeling of the Methanol Synthesis Catalyst Deactivation in a Spherical Bed Reactor: An Environmental Challenge. APCBEE Procedia 2014, 10, 84–90. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, X.; Qiu, Z.; Feng, B.; Liu, Y.; Xing, A.; Fan, M. Improved Methanol Synthesis Performance of Cu/ZnO/Al2O3 Catalyst by Controlling Its Precursor Structure. Green Energy Environ. 2022, 7, 772–781. [Google Scholar] [CrossRef]
- Zarabi Golkhatmi, S.; Asghar, M.I.; Lund, P.D. A Review on Solid Oxide Fuel Cell Durability: Latest Progress, Mechanisms, and Study Tools. Renew. Sustain. Energy Rev. 2022, 161, 112339. [Google Scholar] [CrossRef]
- Mishchenko, D.D.; Arapova, M.V.; Bespalko, Y.N.; Vinokurov, Z.S.; Shmakov, A.N. In Situ XRD and TGA/DTA Study of Multiphase La- and Nd-Substituted Pr2NiO4 under IT-SOFC Cathode Operating Conditions. J. Alloys Compd. 2023, 967, 171693. [Google Scholar] [CrossRef]
- Popov, M.P.; Bychkov, S.F.; Bulina, N.V.; Nemudry, A.P. In Situ High-Temperature X-ray Diffraction of Hollow Fiber Membranes under Operating Conditions. J. Eur. Ceram. Soc. 2019, 39, 1717–1720. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, X.; Yang, W. Degradation Mechanism Analysis of Ba0.5Sr0.5Co0.8Fe0.2O3−δ Membranes at Intermediate-Low Temperatures. AIChE J. 2015, 61, 3879–3888. [Google Scholar] [CrossRef]
- Nikolaeva, O.; Kapishnikov, A.; Gerasimov, E. Structural Insight into La0.5Ca0.5Mn0.5Co0.5O3 Decomposition in the Methane Combustion Process. Nanomaterials 2021, 11, 2283. [Google Scholar] [CrossRef] [PubMed]
- Heenan, T.M.M.; Robinson, J.B.; Lu, X.; Tjaden, B.; Cervellino, A.; Bailey, J.J.; Brett, D.J.L.; Shearing, P.R. Understanding the Thermo-Mechanical Behaviour of Solid Oxide Fuel Cell Anodes Using Synchrotron X-ray Diffraction. Solid State Ion. 2018, 314, 156–164. [Google Scholar] [CrossRef]
- Li, T.; Heenan, T.M.M.; Rabuni, M.F.; Wang, B.; Farandos, N.M.; Kelsall, G.H.; Matras, D.; Tan, C.; Lu, X.; Jacques, S.D.M.; et al. Design of Next-Generation Ceramic Fuel Cells and Real-Time Characterization with Synchrotron X-ray Diffraction Computed Tomography. Nat. Commun. 2019, 10, 1497. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulavchenko, O.A.; Vinokurov, Z.S. In Situ X-ray Diffraction as a Basic Tool to Study Oxide and Metal Oxide Catalysts. Catalysts 2023, 13, 1421. https://doi.org/10.3390/catal13111421
Bulavchenko OA, Vinokurov ZS. In Situ X-ray Diffraction as a Basic Tool to Study Oxide and Metal Oxide Catalysts. Catalysts. 2023; 13(11):1421. https://doi.org/10.3390/catal13111421
Chicago/Turabian StyleBulavchenko, Olga A., and Zakhar S. Vinokurov. 2023. "In Situ X-ray Diffraction as a Basic Tool to Study Oxide and Metal Oxide Catalysts" Catalysts 13, no. 11: 1421. https://doi.org/10.3390/catal13111421