Abstract
:1. Introduction
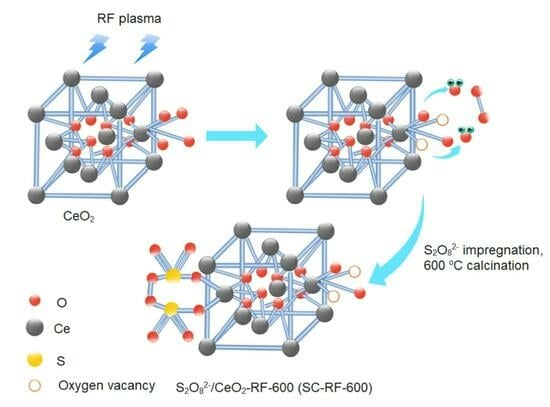
2. Results and Discussion
2.1. Hammett Titration
2.2. XRD
2.3. HR-TEM
2.4. FT-IR
2.5. Py-IR
2.6. N2 Adsorption–Desorption Experiments
2.7. XPS
2.8. Catalytic Performance
3. Experiments
3.1. Materials
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Catalytic Esterification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, H.Z.; Zhang, Y.; Xue, F.; Sun, Z.L. Research progress of /MxOy solid superacid catalysts. Chem. Ind. Eng. Prog. 2018, 37, 1759–1809. [Google Scholar]
- Hino, M.; Kobayashi, S.; Arata, K. Solid catalyst treated with anion. 2. Reactions of butane and isobutane catalyzed by zirconium oxide treated with sulfate ion. Solid superacid catalyst. J. Am. Chem. Soc. 1979, 101, 6439–6441. [Google Scholar] [CrossRef]
- Wang, H.G.; Shi, G.L.; Yu, F.; Li, R.F. Mild synthesis of biofuel over a microcrystalline /ZrO2 catalyst. Fuel Process. Technol. 2016, 145, 9–13. [Google Scholar] [CrossRef]
- Xia, Y.D.; Hua, W.M.; Gao, Z. n-Butane isomerization on solid superacids of ZrO2 modified by persulfate. J. Chem. 1999, 57, 1325–1331. [Google Scholar]
- Li, P.; Gu, Y.; Yu, Z.; Gao, P.; An, Y.; Li, J. TiO2-SnO2/ mesoporous solid superacid decorated nickel-based material as efficient electrocatalysts for methanol oxidation reaction. Electrochim. Acta 2019, 297, 864–871. [Google Scholar] [CrossRef]
- Dzhikiya, O.V.; Smolikov, M.D.; Zatolokina, E.V.; Kazantsev, K.V.; Belyi, A.S. Isomerization of n-hexane on Pd//ZrO2/Al2O3 and mechanical mixtures Pd/Al2O3 (Pd/SiO2) + /ZrO2/Al2O3. Procedia Eng. 2016, 152, 116–121. [Google Scholar] [CrossRef]
- Lin, F.; Shao, B.; Li, Z.; Zhang, J.Y.; Wang, H.; Zhang, S.H.; Haruta, M.; Huang, J.H. Visible light photocatalysis over solid acid: Enhanced by gold plasmonic effect. Appl. Catal. B Environ. 2017, 218, 480–487. [Google Scholar] [CrossRef]
- Zhang, S.; Su, L.; Liu, L.; Fang, G.Z. Degradation on hydrogenolysis of soda lignin using CuO//ZrO2 as catalyst. Ind. Crops Prod. 2015, 77, 451–457. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, Q.; Liu, X.; Fan, J.; Huang, Z. Introduction manner of sulfate acid for improving the performance of /CeO2 on selective catalytic reduction of NO by NH3. J. Rare Earths 2016, 34, 667–674. [Google Scholar] [CrossRef]
- Li, S.X.; Xu, Y.R.; Wang, S. Preparation of solid superacid /TiO2-HZSM-5 catalyst and its catalytic performance in esterification. Chem. Ind. Eng. Prog. 2015, 34, 745–750. [Google Scholar]
- Li, J.; Ma, C.; Zhu, S.; Yu, F.; Dai, B.; Yang, D. A Review of recent advances of dielectric barrier discharge plasma in catalysis. Nanomaterials 2019, 9, 1428. [Google Scholar] [CrossRef]
- Duan, S.; Liu, X.; Wang, Y.; Meng, Y.; Alsaedi, A.; Hayat, T.; Li, J. Plasma surface modification of materials and their entrapment of water contaminant: A review. Plasma Process. Polym. 2017, 14, 1600218. [Google Scholar] [CrossRef]
- Li, H.P.; Yu, D.R.; Sun, W.T.; Liu, D.X.; Li, J.; Han, X.W.; Li, Z.Y.; Sun, B.; Wu, Y. State-of-the-art of atmospheric discharge plasmas. High Volt. Eng. 2016, 42, 3697–3727. [Google Scholar]
- Xia, S.Y.; Mi, J.F.; Du, S.N. Research progress of non-thermal plasma treatment of volatile organic compounds. Appl. Chem. 2021, 50, 1130–1135. [Google Scholar]
- Su, F.-M.; Zhang, D.; Liang, F. Progress in preparation and modification of nano-catalytic materials by low-temperature plasma. Chin. J. Appl. Chem. 2019, 36, 882–891. [Google Scholar]
- Wang, B.; Xiong, Y.; Han, Y.; Hong, J.; Zhang, Y.; Li, J.; Jing, F.; Chu, W. Preparation of stable and highly active Ni/CeO2 catalysts by glow discharge plasma technique for glycerol steam reforming. Appl. Catal. B Environ. 2019, 249, 257–265. [Google Scholar] [CrossRef]
- Li, Y.; Jang, B.W.L. Selective hydrogenation of acetylene over Pd/Al2O3 catalysts: Effect of non-thermal RF plasma preparation methodologies. Top. Catal. 2017, 60, 997–1008. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, Q.; Xiao, Z.; Li, X.; Huo, J.; Wang, S.; Dai, L. Plasma-engraved Co3O4 nanosheets with oxygen vacancies and high surface area for the oxygen evolution reaction. Angew. Chem. Int. Ed. Engl. 2016, 55, 5277–5281. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Dai, Q.; Wang, X. Morphology effect of Ru/CeO2 catalysts for the catalytic combustion of chlorobenzene. Appl. Catal. B Environ. 2014, 158–159, 96–105. [Google Scholar] [CrossRef]
- Griehl, C.; Weigt, J.; Jeschkeit, H. Use of tryptophan peptides for an HPLC racemization test with fluorescence detection. J. High Resolut. Chromatogr. 1994, 17, 700–704. [Google Scholar] [CrossRef]
- Xiong, J.; Zhu, H.F.; Zhao, Y.J.; Lan, Y.J.; Jiang, J.W.; Yang, J.J.; Zhang, S.F. Synthesis and antitumor activity of amino acid ester derivatives containing 5-fluorouracil. Molecules 2009, 14, 3142–3152. [Google Scholar] [CrossRef]
- Vinod, V.T.P.; Sashidhar, R.B.; Sarma, V.U.M.; Raju, S.S. Comparative amino acid and fatty acid compositions of edible gums kondagogu (Cochlospermum gossypium) and karaya (Sterculia urens). Food Chem. 2010, 123, 57–62. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, K.; Luo, J.; Tian, B.; Sun, J.; Liu, X.; Zhu, W.; Zou, Z. Solid superacid -/SnO2-Nd2O3 catalyzed esterification of α-aromatic amino acids. Mol. Catal. 2023, 535, 112833. [Google Scholar] [CrossRef]
- Paul, M.A.; Long, F.A. H0 and related indicator acidity function. Chem. Rev. 1957, 57, 1–45. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Xu, Y.; Chen, C.G.; Ou, Z.W. Studies on the formation mechanism of /ZrO2 solid superacid. J. Chongqing Univ. (Nat. Sci. Ed.) 1999, 20, 77–81. [Google Scholar]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R. Modified Scherrer equation to estimate more accurately nano-crystallite size using XRD. World J. Nano Sci. Eng. 2012, 2, 154–160. [Google Scholar] [CrossRef]
- Bingfu, L.; Ken-ichi, M.; Takashi, H.; Hanzawa, H. Synthesis and photoluminescence properties of CaAlSiN3: Eu2+ nanocrystals. Chem. Lett. 2010, 39, 104–105. [Google Scholar]
- Huang, Z.; Deng, J.; Wang, H.; Zhang, Y.; Duan, J.; Tang, Z.; Cao, Z.; Qi, J.; He, D.; Lu, T. Fast low-temperature densification of translucent bulk nanograin Gd2Zr2O7 ceramics with average grain size below 10 nm. J. Alloys Compd. 2020, 830, 154617. [Google Scholar] [CrossRef]
- Wang, W.W.; Yu, W.Z.; Du, P.P.; Xu, H.; Jin, Z.; Si, R.; Ma, C.; Shi, S.; Jia, C.J.; Yan, C.H. Crystal plane effect of ceria on supported copper oxide cluster catalyst for CO oxidation: Importance of metal–support interaction. ACS Catal. 2017, 7, 1313–1329. [Google Scholar] [CrossRef]
- Wu, Z.; Li, M.; Overbury, S.H. On the structure dependence of CO oxidation over CeO2 nanocrystals with well-defined surface planes. J. Catal. 2012, 285, 61–73. [Google Scholar] [CrossRef]
- Song, H.; Cui, H.; Song, H.; Li, F. The effect of Zn–Fe modified /ZrO2-Al2O3 catalyst for n-pentane hydroisomerization. Res. Chem. Intermed. 2015, 42, 3029–3038. [Google Scholar] [CrossRef]
- Zhao, Q.; Meng, S.; Wang, J.; Li, Z.; Liu, J.; Guo, Y. Preparation of solid superacid /TiO2–exfoliated graphite (EG) and its catalytic performance. Ceram. Int. 2014, 40, 16183–16187. [Google Scholar] [CrossRef]
- Gu, Y. Honeycomb-like mesoporous NiO-SnO2/ solid superacid for the efficient reaction of methanol oxidation. Int. J. Electrochem. Sci. 2020, 15, 2481–2498. [Google Scholar] [CrossRef]
- Song, H.; Zhao, L.; Wang, N.; Li, F. Isomerization of n-pentane over La-Ni-/ZrO2-Al2O3 solid superacid catalysts: Deactivation and regeneration. Appl. Catal. A Gen. 2016, 526, 37–44. [Google Scholar] [CrossRef]
- Ma, H.-Q. Preparation and catalytic properties study of solid superacid catalyst /ZrO2-Al2O3: Modified by Lanthanum. Mater. Guide B 2014, 28, 48–64. [Google Scholar]
- Yin, H.L.; Tan, Z.Y.; Liao, Y.-T.; Feng, Y.J. Application of /TiO2 solid superacid in decontaminating radioactive pollutants. J. Environ. Radioact. 2006, 87, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.Y.; Zhang, P.W.; Yang, G. Study on Pt--ZrO2-Al2O3 solid superacid catalyst. Pet. Process. Petrochem. 2020, 51, 33–40. [Google Scholar]
- Mai, H.X.; Sun, L.D.; Zhang, Y.W.; Si, R.; Feng, W.; Zhang, H.P.; Liu, H.C.; Yan, C.H. Shape-selective synthesis and oxygen storage behavior of Ceria nanopolyhedra, nanorods. Am. Chem. Soc. 2005, 109, 24380–24385. [Google Scholar] [CrossRef]
- Velmurugan, R.; Krishnakumar, B.; Swaminathan, M. Synthesis of Pd co-doped nano-TiO2- and its synergetic effect on the solar photodegradation of Reactive Red 120 dye. Mater. Sci. Semicond. Process. 2014, 25, 163–172. [Google Scholar] [CrossRef]
- Liu, B.; Li, C.; Zhang, G.; Yao, X.; Chuang, S.S.C.; Li, Z. Oxygen vacancy promoting dimethyl carbonate synthesis from CO2 and methanol over Zr-doped CeO2 nanorods. ACS Catal. 2018, 8, 10446–10456. [Google Scholar] [CrossRef]
- Degueil-Castaing, M.; Rahm, A. Reduction of ketones by tributyltin hydride: The effect of high pressure on steric hindrance and rearrangement processes. J. Org. Chem. 1986, 51, 1672–1676. [Google Scholar] [CrossRef]
- Eleeza, J.; Boahene, P.; Vedachalam, S.; Adjaye, J. Influence of catalyst acidity on fine particle deposition during hydrotreating of bitumen-derived heavy gas oil. Energy Fuels 2021, 35, 16735–16749. [Google Scholar] [CrossRef]
- Perosa, A. Greeneer Syntheses & Solvents for Fine and Pharmaceutical Chemicals. Ph.D. Thesis, Università Ca’ Foscari Venezia, Venezia, Italy, 2012. [Google Scholar]
- Justino, A.; Carvalho AC, S.; de Vasconcelos, L.G.; Gai, B.M.; Stein, A.L.; Siqueira, A.B. Tryptophan methyl ester: A proposal of the thermal decomposition mechanism. J. Therm. Anal. Calorim. 2022, 147, 7741–7748. [Google Scholar] [CrossRef]
- Hu, X.C.; Fu, X.P.; Wang, W.W.; Wang, X.; Wu, K.; Si, R.; Ma, C.; Jia, C.J.; Yan, C.H. Ceria-supported ruthenium clusters transforming from isolated single atoms for hydrogen production via decomposition of ammonia. Appl. Catal. B Environ. 2020, 268, 118424. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, C.Y.; Jang, B.W.L.; Zhu, A.M. Radio-frequency H2 plasma treatment of AuPd/TiO2 catalyst for selective hydrogenation of acetylene in excess ethylene. Catal. Today 2015, 256, 161–169. [Google Scholar] [CrossRef]










| Indicator | 2,4-DNFB | 3-NT |
|---|---|---|
| H0 | ≤−14.5 | ≤−12.0 |
| Result | + | + |
| Entry | Cat. | BA Density (μmol·g−1) | LA Density (μmol·g−1) | Total Acid Density (μmol·g−1) | BA Density/LA Density |
|---|---|---|---|---|---|
| 1 | SC-400 | 7.33 | 36.56 | 43.89 | 0.20 |
| 2 | SC-500 | 9.81 | 38.84 | 48.65 | 0.25 |
| 3 | SC-600 | 14.52 | 30.80 | 45.32 | 0.47 |
| 4 | SC-RF-600 | 13.83 | 65.66 | 79.49 | 0.21 |
| Entry | Sample | SBET Average Value (m2/g) | SBET Standard Error (%) | Pore Volume Average Value (cm3/g) | Pore Volume Standard Error (%) | Pore Size Average Value (nm) | Pore Size Standard Error (%) |
|---|---|---|---|---|---|---|---|
| 1 | SC-400 | 25.61 | 2.81 | 0.16 | 0.01 | 25.82 | 2.99 |
| 2 | SC-500 | 28.87 | 1.41 | 0.19 | 0 | 26.77 | 1.40 |
| 3 | SC-600 | 23.46 | 2.23 | 0.21 | 0 | 36.08 | 3.81 |
| 4 | SC-RF-600 | 37.60 | 1.31 | 0.33 | 0.01 | 36.01 | 2.00 |
| Entry | Sample | Atomic Content (at%) | |||||
|---|---|---|---|---|---|---|---|
| C 1s | Ce 3d | O 1S | S 2p | OV | Ce3+/(Ce3+ + Ce4+) | ||
| 1 | SC-400 | 35.47 | 9.78 | 49.16 | 5.58 | 21.61 | 26.44 |
| 3 | SC-500 | 34.69 | 10.85 | 49.32 | 5.13 | 24.55 | 26.84 |
| 3 | SC-600 | 31.35 | 11.83 | 51.95 | 4.88 | 28.52 | 28.73 |
| 4 | SC-RF-600 | 11.95 | 17.42 | 58.37 | 4.64 | 31.11 | 31.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Zhu, C.; Zhang, X.; Tian, B.; Zhu, W.; Huang, B.
Wang K, Zhu C, Zhang X, Tian B, Zhu W, Huang B.
Wang, Kaiqiang, Changhui Zhu, Xudong Zhang, Baohe Tian, Wenchao Zhu, and Bangdou Huang.
2023. "