The Synthesis of a Pt/SAPO-11 Composite with Trace Pt Loading and Its Catalytic Application in n-Heptane Hydroisomerization
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phase Structure
2.2. Morphology
2.3. Textural Properties
2.4. Acid Properties
2.5. Valence Structure
2.6. Catalytic Properties
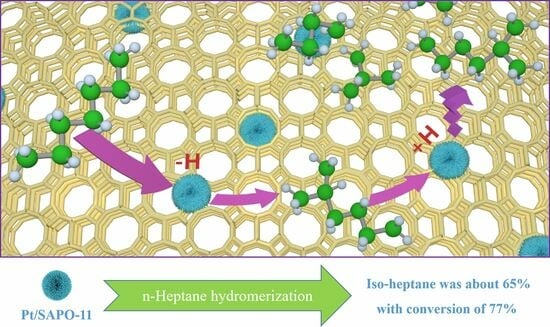
2.7. Mechanism of n-Heptane Hydroisomerization over xPt/SAPO-11
3. Experimental Section
3.1. Materials
3.2. Preparation
3.3. Characterization
3.4. Hydroisomerization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, Y.; Hu, W.; Du, Y.; Li, J. Species and impacts of metal sites over bifunctional catalyst on long chain n-alkane hydroisomerization: A review. Appl. Catal. A Gen. 2021, 611, 117916. [Google Scholar] [CrossRef]
- Wang, W.; Liu, C.; Wu, W. Bifunctional catalysts for the hydroisomerization of n-alkanes: The effects of metal–acid balance and textural structure. Catal. Sci. Technol. 2019, 9, 4162–4187. [Google Scholar] [CrossRef]
- Prachee, M.; Majmutov, A.; Chen, J. Isomerization catalysts and technologies for biorefining: Opportunities for producing sustainable aviation fuels. Fuel 2023, 351, 128994. [Google Scholar]
- Monteiro, R.R.C.; dos Santos, I.A.; Arcanjo, M.R.A.; Cavalcante, C.L., Jr.; de Luna, F.M.T.; Fernandez-Lafuente, R.; Vieira, R.S. Production of jet biofuels by catalytic hydroprocessing of esters and fatty acids: A review. Catalysts 2022, 12, 237. [Google Scholar] [CrossRef]
- Li, T.; Zhang, L.; Tao, Z.; Hu, C.; Zhao, C.; Yi, F.; Gao, X.; Wen, X.; Yang, Y.; Li, Y. Synthesis and characterization of amorphous silica-alumina with enhanced acidity and its application in hydro-isomerization/cracking. Fuel 2020, 279, 118487. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Wang, Q. N-dodecane hydroisomerization over Pt/ZSM-22: Controllable microporous Brönsted acidity distribution and shape-selectivity. Appl. Catal. A Gen. 2020, 590, 117335. [Google Scholar] [CrossRef]
- Tao, S.; Li, X.; Lv, G.; Wang, C.; Xu, R.; Ma, H.; Tian, Z. Highly mesoporous SAPO-11 molecular sieves with tunable acidity: Facile synthesis, formation mechanism and catalytic performance in hydroisomerization of n-dodecane. Catal. Sci. Technol. 2017, 7, 5775–5784. [Google Scholar] [CrossRef]
- Jaroszewska, K.; Fedyna, M.; Trawczyński, J. Hydroisomerization of long-chain n-alkanes over Pt/AlSBA-15 zeolite bimodal catalysts. Appl. Catal. B Environ. 2019, 255, 117756. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Kaka khel, T.A.; Azkaar, M.; Engblom, S.; Murzin, D.Y. Catalytic hydroisomerization of long-chain hydrocarbons for the production of fuels. Catalysts 2018, 8, 534. [Google Scholar] [CrossRef]
- Guo, C.; Wang, W.; Zhang, Y.; Lin, H.; Jia, G.; Li, T.; Xin, Q.; Bai, X.; Wu, W. Influences of the metal-acid proximity of Pd-SAPO-31 bifunctional catalysts for n-hexadecane hydroisomerization. Fuel Process. Technol. 2021, 214, 106717. [Google Scholar] [CrossRef]
- Jin, D.; Li, L.; Ye, G.; Ding, H.; Zhao, X.; Zhu, K.; Coppens, M.O.; Zhou, X. Manipulating the mesostructure of silicoaluminophosphate SAPO-11 via tumbling-assisted, oriented assembly crystallization: A pathway to enhance selectivity in hydroisomerization. Catal. Sci. Technol. 2018, 8, 5044–5061. [Google Scholar] [CrossRef]
- Yadav, R.; Sakthivel, A. Silicoaluminophosphate molecular sieves as potential catalysts for hydroisomerization of alkanes and alkenes. Appl. Catal. A Gen. 2014, 481, 143–160. [Google Scholar] [CrossRef]
- Lv, G.; Wang, C.; Chi, K.; Liu, H.; Wang, P.; Ma, H.; Qu, W.; Tian, Z. Effect of Pt site distribution on the catalytic performance of Pt/SAPO-11 for n-dodecane hydroisomerization. Catal. Today 2018, 316, 43–50. [Google Scholar] [CrossRef]
- Wu, Q.; Yuan, J.; Guo, C.; Liu, Z.; Li, X.; Liu, Z.; Wang, W.; Wu, W. The hydroisomerization of n-hexadecane over Pd/SAPOs bifunctional catalysts with different opening size: Features of the diffusion properties in pore channels and the metal-acid synergistic catalysis. Fuel Process. Technol. 2023, 244, 107692. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, B.; Wu, B.; Chen, H.; Xu, J.; Yang, Y.; Li, Y. Shape-controlled synthesis of Pt particles and their catalytic performances in the n-hexadecane hydroconversion. Catal. Today 2016, 259, 331–339. [Google Scholar] [CrossRef]
- Lucus, A.; Sánchez, P.; Dorado, F.; Ramos, M.; Valverde, J. Effect of the metal loading in the hydroisomerization of n-octane over beta agglomerated zeolite based catalysts. Appl. Catal. A Gen. 2005, 294, 215–225. [Google Scholar] [CrossRef]
- Batalha, N.; Pinard, L.; Bouchy, C.; Guillon, E.; Guisnet, M. n-Hexadecane hydroisomerization over Pt-HBEA catalysts. Quantification and effect of the intimacy between metal and protonic sites. J. Catal. 2013, 307, 122–131. [Google Scholar] [CrossRef]
- Sun, N.; Wang, H.; Luo, A.; Yang, Z.; Wang, Y.; Kang, L. Synthesis and hydrogen isomerization performance of ordered mesoporous nanosheet SAPO-11 molecular sieves. J. Solid State Chem. 2022, 309, 122972. [Google Scholar] [CrossRef]
- Dang, T.T.H.; Bartoszek, M.; Schneider, M.; Hoang, D.L.; Bentrup, U.; Martin, A. Chloride-free Cu-modified SAPO-37 catalyst for the oxidative carbonylation of methanol in the gas phase. Appl. Catal. B 2012, 122, 115–122. [Google Scholar] [CrossRef]
- Du, Y.; Feng, B.; Jiang, Y.; Yuan, L.; Huang, K.; Li, J. Non-solvent synthesis and n-hexadecane hydroisomerization performance of SAPO-11 catalyst. Eur. J. Inorg. Chem. 2018, 22, 2599–2606. [Google Scholar] [CrossRef]
- Wang, D.; Liu, J.; Cheng, X.; Kang, X.; Wu, A.; Tian, C.; Fu, H. Trace Pt clusters dispersed on SAPO-11 promoting the synergy of metal sites with acid sites for high-effective hydroisomerization of n-alkanes. Small Methods 2019, 3, 1800510. [Google Scholar] [CrossRef]
- Song, X.; Bai, X.; Wu, W.; Kikhtyanin, O.V.; Zhao, A.; Xiao, L.; Su, X.; Zhang, J.; Wei, X. The effect of palladium loading on the catalytic performance of Pd/SAPO-11 for n-decane hydroisomerization. Mol Catal. 2017, 433, 84–90. [Google Scholar] [CrossRef]
- Wang, X.; Guo, S.; Niu, Z. Synthesis of a ZSM-5 (core)/SAPO-11 (shell) composite zeolite and its catalytic performance in the methylation of naphthalene with methanol. RSC Adv. 2023, 13, 2081–2089. [Google Scholar] [CrossRef] [PubMed]
- Mériaudeau, P.; Tuan, V.A.; Nghiem, V.T.; Lai, S.Y.; Hung, L.N.; Naccache, C. SAPO-11, SAPO-34, and SAPO-41 molecular sieves: Synthesis, characterization, and catalytic properties in n-octane hydroisomerization. J. Catal. 1997, 169, 55–66. [Google Scholar] [CrossRef]
- Nie, R.; Lei, H.; Pan, S.; Wang, L.; Fei, J.; Hou, Z. Core-shell structured CuO–ZnO@H-ZSM-5 catalysts for CO hydrogenation to dimethyl ether. Fuel 2012, 96, 419–425. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Y.; Liu, D.; Meng, X.; Liu, C. Hydroisomerization of n-octane bimetallic Ni-Cu/SAPO-11 catalysts. Appl. Catal. A Gen. 2017, 543, 274–282. [Google Scholar] [CrossRef]
- Qin, H.; Feng, N.; Lv, Q.; Wan, H.; Guan, G. Pt Single Atom-Anchored CeOx/SAPO-11 for Highly Efficient Hydroisomerization of n-Heptane. Fuel Process. Technol. 2023, 241, 107604. [Google Scholar] [CrossRef]
- Niu, P.; Xi, H.; Ren, J.; Lin, M.; Wang, Q.; Jia, L.; Hou, B.; Li, D. High selectivity for n-dodecane hydroisomerization over highly siliceous ZSM-22 with low Pt loading. Catal. Sci. Technol. 2017, 7, 5055–5068. [Google Scholar] [CrossRef]
- Kim, M.Y.; Lee, K.; Choi, M. Cooperative effects of secondary mesoporosity and acid site location in Pt/SAPO-11 on n-dodecane hydroisomerization selectivity. J. Catal. 2014, 319, 232–238. [Google Scholar] [CrossRef]
- Ma, J.; Liu, X.; Yuan, H. In situ synthesis of mesoporous Pt/SAPO–11 for the preparation of biological aviation kerosene. J. Porous Mat. 2022, 29, 1387–1398. [Google Scholar] [CrossRef]
- Souverijins, W.; Martens, J.A.; Froment, G.F.; Jacobs, P.A. Hydrocracking of isoheptadecanes on Pt/H-ZSM-22: An example of pore mouth catalysis. J. Catal. 1998, 174, 177–184. [Google Scholar] [CrossRef]
- Geng, L.; Gong, J.; Qiao, G.; Ye, S.; Zheng, J.; Zhang, N.; Chen, B. Effect of metal precursors on the performance of Pt/SAPO-11 catalysts for n-doecane hydrosiomerization. ACS Omga 2019, 4, 12598–12605. [Google Scholar] [CrossRef] [PubMed]
- Shamanaeva, I.A.; Parkhomchuk, E.V.; Bukhtiyarova, G.A. Hydrodeoxygenation isomerization of methyl palmitate over SAPO-11-supported Ni-phosphide catalysts. Catalyst 2022, 12, 1486. [Google Scholar] [CrossRef]
- Yuan, Z.; Cheng, Y.; Ma, S.; Jiang, Z.; Zhou, Y.; Wang, Y.; Zhang, W.; Suo, Y. Instant exactness synthesis and n-heptane hydroisomerization of high performance Ni/SAPO-11 catalyst. J. Porous Mat. 2020, 5, 1455–1466. [Google Scholar] [CrossRef]
- Yang, H.; Du, X.; Lei, X.; Lei, X.; Zhou, K.; Tian, Y.; Li, D.; Hu, C. Unraveling enhanced activity and coke resistance of Pt-based catalyst in bio-aviation fuel refining. Appl. Energy 2021, 301, 117469. [Google Scholar] [CrossRef]
- Cui, X.; Liu, Y.X.; Liu, X.M. Controlling acidic sites to improve hydroisomerization performance of Pt/SAPO-11 catalysts. Catal. Lett. 2015, 145, 1464–1473. [Google Scholar] [CrossRef]
- Lyu, Y.C.; Yu, Z.M.; Yang, Y.; Liu, Y.X.; Zhao, X.X.; Liu, X.M.; Mintova, S.; Yan, Z.F.; Zhao, G.F. Metal and acid sites instantaneously prepared over Ni/SAPO-11 bifunctional catalyst. J. Catal. 2019, 374, 208–216. [Google Scholar] [CrossRef]
- Jin, D.; Ye, G.; Zheng, J.; Yang, W.; Zhu, K.; Coppens, M.O.; Zhou, X. Hierarchical silicoaluminophosphate catalysts with enhanced hydroisomerization selectivity by directing the orientated assembly of premanufactured building blocks. ACS Catal. 2017, 7, 5887. [Google Scholar] [CrossRef]
- Pastvova, J.; Kaucky, D.; Moravkova, J.; Rathousky, J.; Sklenak, S.; Vorokhta, M.; Brabec, L.; Pilar, R.; Jakubec, I.; Tabor, E.; et al. Effect of Enhanced Accessibility of Acid Sites in Micromesoporous Mordenite Zeolites on Hydroisomerization of n-Hexane. ACS Catal. 2017, 7, 5781. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Y.; Zhao, J.; Gou, J.; Sun, K.; Liu, C. Zinc-modifieed Pt/SAPO-11 for improving the isomerization selectivity to dibranched alkanes. Chin. J. Catal. 2017, 38, 509–517. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Y.; Sun, Q.; Dai, Z.; Gies, H.; Wu, Q.; Pan, S.; Bian, C.; Tian, Z.; Meng, X.; et al. Hybridization chain reactions on silica coated Qbeads for the colorimetric detection of multiplex microRNAs. Chem. Commun. 2017, 53, 4954. [Google Scholar]
- Wang, Y.; Tao, Z.; Wu, B.; Xu, J.; Huo, C.; Li, K.; Chen, H.; Yang, Y.; Li, Y. Effect of metal precursors on the performance of Pt/ZSM-22 catalysts for n-hexadecane hydroisomerization. J. Catal. 2015, 322, 1–13. [Google Scholar] [CrossRef]










| Parameters | SAPO-11 | 0.1Pt/SAPO-11 | 0.2Pt/SAPO-11 | 0.5Pt/SAPO-11 | 0.8Pt/SAPO-11 | 1.0Pt/SAPO-11 |
|---|---|---|---|---|---|---|
| S/(m2 g−1) | 121 | 119 | 118 | 117 | 115 | 115 |
| Vpore/(cm3 g−1) | 0.101 | 0.098 | 0.087 | 0.085 | 0.083 | 0.078 |
| dpore/(Å) | 22.6 | 21.9 | 21.3 | 22.5 | 18.7 | 18.9 |
| Sample | Acid Amount (mmol/g) | |||
|---|---|---|---|---|
| Total | Weak | Medium | Strong | |
| SAPO-11 | 0.27 | 0.06 | 0.11 | 0.10 |
| 0.1Pt/SAPO-11 | 0.29 | 0.03 | 0.15 | 0.11 |
| 0.3Pt/SAPO-11 | 0.30 | 0.05 | 0.16 | 0.09 |
| 0.5Pt/SAPO-11 | 0.31 | 0.09 | 0.18 | 0.04 |
| 0.8Pt/SAPO-11 | 0.28 | 0.05 | 0.12 | 0.11 |
| 1.0Pt/SAPO-11 | 0.28 | 0.06 | 0.12 | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.; Chen, L.; Cui, Y.; Gao, H.; Zhou, Y.; Zhang, W.; Suo, Y.; Wang, Y. The Synthesis of a Pt/SAPO-11 Composite with Trace Pt Loading and Its Catalytic Application in n-Heptane Hydroisomerization. Catalysts 2023, 13, 1383. https://doi.org/10.3390/catal13101383
Jiang Z, Chen L, Cui Y, Gao H, Zhou Y, Zhang W, Suo Y, Wang Y. The Synthesis of a Pt/SAPO-11 Composite with Trace Pt Loading and Its Catalytic Application in n-Heptane Hydroisomerization. Catalysts. 2023; 13(10):1383. https://doi.org/10.3390/catal13101383
Chicago/Turabian StyleJiang, Zhen, Liduo Chen, Yanhong Cui, Huijie Gao, Yisi Zhou, Wei Zhang, Yanhua Suo, and Yingjun Wang. 2023. "The Synthesis of a Pt/SAPO-11 Composite with Trace Pt Loading and Its Catalytic Application in n-Heptane Hydroisomerization" Catalysts 13, no. 10: 1383. https://doi.org/10.3390/catal13101383