Synergistic Effects of Ionizing Radiation Process in the Integrated Coagulation–Sedimentation, Fenton Oxidation, and Biological Process for Treatment of Leachate Wastewater
Abstract
:1. Introduction
2. Result and Discussions
2.1. Leachate Treatment Using Ionizing Radiation
2.2. Lechate Treatment Using Coagulation Process
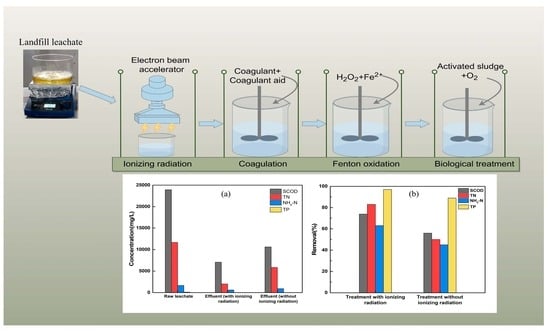
2.3. Comparative Study of Coagulation–Sedimentation after Ionizing Radiation Treatment and Coagulation Process Combined with Ionizing Radiation
2.4. Effects of Fenton Oxidation
2.5. Biological Treatment as Post-Treatment to Combined Ionizing Radiation, Coagulation–Sedimentation and Fenton Oxidation Process
3. Materials and Methods
3.1. Collection and Characterization of Landfill Leachate
3.2. Leachate Treatment Using Ionizing Radiation
3.3. Leachate Treatment Using Coagulation
3.4. Simultaneous Ionizing Radiation with Coagulation–Sedimentation
3.5. Leachate Treatment Using Fenton Oxidation
3.6. Biological Treatment of Pretreated Landfill Leachate
3.7. Analytical Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Chen, Y.; Deng, J.; Wang, J. N-doped aluminum-graphite (Al-Gr-N) composite for enhancing in-situ production and activation of hydrogen peroxide to treat landfill leachate. Appl. Catal. B Environ. 2021, 297, 120407. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J. Treatment of fresh leachate from a municipal solid waste incineration plant by combined radiation with coagulation process. Radiat. Phys. Chem. 2020, 166, 108501. [Google Scholar] [CrossRef]
- Wijekoon, P.; Koliyabandara, P.A.; Cooray, A.T.; Lam, S.S.; Athapattu, B.C.; Vithanage, M. Progress and prospects in mitigation of landfill leachate pollution: Risk, pollution potential, treatment and challenges. J. Hazard. Mater. 2022, 421, 126627. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mukhopadhyay, S.; Hashim, M.A.; Sen Gupta, B. Contemporary environmental issues of landfill leachate: Assessment and remedies. Crit. Rev. Environ. Sci. Technol. 2015, 45, 472–590. [Google Scholar] [CrossRef]
- Wdowczyk, A.; Szymańska-Pulikowska, A. Analysis of the possibility of conducting a comprehensive assessment of landfill leachate contamination using physicochemical indicators and toxicity test. Ecotoxicol. Environ. Saf. 2021, 221, 112434. [Google Scholar] [CrossRef]
- Remmas, N.; Manfe, N.; Zerva, I.; Melidis, P.; Raga, R.; Ntougias, S. A critical review on the microbial ecology of landfill leachate treatment systems. Sustainability 2023, 15, 949. [Google Scholar] [CrossRef]
- Tugtas, A.E.; Cavdar, P.; Calli, B. Bio-electrochemical post-treatment of anaerobically treated landfill leachate. Bioresour. Technol. 2013, 128, 266–272. [Google Scholar] [CrossRef]
- Borba, F.H.; Leichtweis, J.; Bueno, F.; Pellenz, L.; Inticher, J.J.; Seibert, D. Pollutant removal and acute toxicity assessment (Artemia salina) of landfill leachate treated by photo-Fenton process mediated by oxalic acid. J. Water Process Eng. 2019, 28, 159–168. [Google Scholar] [CrossRef]
- Show, P.L.; Pal, P.; Leong, H.Y.; Juan, J.C.; Ling, T.C. A review on the advanced leachate treatment technologies and their performance comparison: An opportunity to keep the environment safe. Environ. Monit. Assess. 2019, 191, 227. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; He, L.; Dai, Z.; Sun, R.; Jiang, S.; Lu, Z.; Liang, Y.; Ren, L.; Sun, S.; Zhang, Y.; et al. Review on recent progress of bioremediation strategies in Landfill leachate-A green approach. J. Water Process Eng. 2022, 50, 103229. [Google Scholar] [CrossRef]
- De Carluccio, M.; Sabatino, R.; Eckert, E.M.; Di Cesare, A.; Corno, G.; Rizzo, L. Co-treatment of landfill leachate with urban wastewater by chemical, physical and biological processes: Fenton oxidation preserves autochthonous bacterial community in the activated sludge process. Chemosphere 2023, 313, 137578. [Google Scholar] [CrossRef] [PubMed]
- Hasnine, M.T.; Anand, N.; Zoungrana, A.; Palani, S.G.; Yuan, Q. An overview of physicochemical and biological treatment of landfill leachate. In Circular Economy in Municipal Solid Waste Landfilling: Biomining & Leachate Treatment: Sustainable Solid Waste Management: Waste to Wealth; Springer: Berlin/Heidelberg, Germany, 2022; pp. 115–152. [Google Scholar]
- Fazzino, F.; Bilardi, S.; Moraci, N.; Calabrò, P.S. Integrated treatment at laboratory scale of a mature landfill leachate via active filtration and anaerobic digestion: Preliminary results. Water 2021, 13, 2845. [Google Scholar] [CrossRef]
- Pasalari, H.; Esrafili, A.; Rezaee, A.; Gholami, M.; Farzadkia, M. Electrochemical oxidation pretreatment for enhanced methane potential from landfill leachate in anaerobic co-digestion process: Performance, Gompertz model, and energy assessment. Chem. Eng. J. 2021, 422, 130046. [Google Scholar] [CrossRef]
- Anjum, M.; Anees, M.; Qadeer, S.; Khalid, A.; Kumar, R.; Barakat, M.A. A recent progress in the leachate pretreatment methods coupled with anaerobic digestion for enhanced biogas production: Feasibility, trends, and techno-economic evaluation. Int. J. Mol. Sci. 2023, 24, 763. [Google Scholar] [CrossRef]
- De Torres-Socías, E.; Prieto-Rodríguez, L.; Zapata, A.; Fernández-Calderero, I.; Oller, I.; Malato, S. Detailed treatment line for a specific landfill leachate remediation. Brief economic assessment. Chem. Eng. J. 2015, 261, 60–66. [Google Scholar] [CrossRef]
- Oloibiri, V.; Ufomba, I.; Chys, M.; Audenaert, W.T.; Demeestere, K.; Van Hulle, S.W. A comparative study on the efficiency of ozonation and coagulation–flocculation as pretreatment to activated carbon adsorption of biologically stabilized landfill leachate. Waste Manag. 2015, 43, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bai, Z. Fe-based catalysts for heterogeneous catalytic ozonation of emerging contaminants in water and wastewater. Chem. Eng. J. 2017, 312, 79–98. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, Q.; Wang, J. Zn-Fe-CNTs catalytic in situ generation of H2O2 for Fenton-like degradation of sulfamethoxazole. J. Hazard. Mater. 2018, 342, 166–176. [Google Scholar] [CrossRef]
- Tang, J.; Wang, J. Metal organic framework with coordinatively unsaturated sites as efficient Fenton-like catalyst for enhanced degradation of sulfamethazine. Environ. Sci. Technol. 2018, 52, 5367–5377. [Google Scholar] [CrossRef] [PubMed]
- Hosny, M.; Fawzy, M. Sustainable synthesis of a novel hydrothermally carbonized AuNPs-hydrochar nanocomposite for the photocatalytic degradation of cephalexin. Biomass Convers. Biorefin. 2023, 1–18. [Google Scholar] [CrossRef]
- Eltaweil, A.S.; Abdelfatah, A.M.; Hosny, M.; Fawzy, M. Novel biogenic synthesis of a Ag@ Biochar nanocomposite as an antimicrobial agent and photocatalyst for methylene blue degradation. ACS Omega 2022, 7, 8046–8059. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jiang, F.; Sun, D.; Qiu, B. Catalytic ozonation for advanced treatment of incineration leachate using (MnO2-Co3O4)/AC as a catalyst. Chem. Eng. J. 2017, 325, 624–631. [Google Scholar] [CrossRef]
- Suresh, D.P.; Kowald, C.; Lassalle, J.; Staack, D. Remediation of Poly-and Perfluorinated Chemical Substances (PFAS) in the Environment by Ionizing Technology. In Ionizing Radiation Technologies: Managing and Extracting Value from Wastes; John Wiley & Sons: Hoboken, NJ, USA, 2022; pp. 223–228. [Google Scholar]
- Trojanowicz, M. Removal of persistent organic pollutants (POPs) from waters and wastewaters by the use of ionizing radiation. Sci. Total Environ. 2020, 718, 134425. [Google Scholar] [CrossRef]
- Bao, Q.; Dong, J.; Dong, Z.; Yang, M. A review on ionizing radiation-based technologies for the remediation of contaminated groundwaters and soils. Chem. Eng. J. 2022, 446, 136964. [Google Scholar] [CrossRef]
- Wang, J.; Zhuan, R.; Chu, L. The occurrence, distribution and degradation of antibiotics by ionizing radiation: An overview. Sci. Total Environ. 2019, 646, 1385–1397. [Google Scholar] [CrossRef]
- Wu, C.; Chen, W.; Gu, Z.; Li, Q. A review of the characteristics of Fenton and ozonation systems in landfill leachate treatment. Sci. Total Environ. 2021, 762, 143131. [Google Scholar] [CrossRef]
- Qian, X.; Ren, M.; Fang, M.; Kan, M.; Yue, D.; Bian, Z.; Li, H.; Jia, J.; Zhao, Y. Hydrophilic mesoporous carbon as iron (III)/(II) electron shuttle for visible light enhanced Fenton-like degradation of organic pollutants. Appl. Catal. B Environ. 2018, 231, 108–114. [Google Scholar] [CrossRef]
- Yu, S.; Xiaofang, Y.; Zhaoyi, Y.; Dongsheng, W.; Chaocheng, Z. Organic pollutant removal from high-salinity wastewater by coagulation-Fenton integrated process. Chin. J. Environ. Eng. 2017, 11, 4958–4964. [Google Scholar]
- Chen, G.; Wu, G.; Li, N.; Lu, X.; Zhao, J.; He, M.; Yan, B.; Zhang, H.; Duan, X.; Wang, S. Landfill leachate treatment by persulphate related advanced oxidation technologies. J. Hazard. Mater. 2021, 418, 126355. [Google Scholar] [CrossRef]
- Mahtab, M.S.; Islam, D.T.; Farooqi, I.H. Optimization of the process variables for landfill leachate treatment using Fenton based advanced oxidation technique. Eng. Sci. Technol. Int. J. 2021, 24, 428–435. [Google Scholar] [CrossRef]
- Lei, Y.; Hou, J.; Fang, C.; Tian, Y.; Naidu, R.; Zhang, J.; Zhang, X.; Zeng, Z.; Cheng, Z.; He, J.; et al. Ultrasound-based advanced oxidation processes for landfill leachate treatment: Energy consumption, influences, mechanisms and perspectives. Ecotoxicol. Environ. Saf. 2023, 263, 115366. [Google Scholar] [CrossRef] [PubMed]
- Bae, B.U.; Jung, E.S.; Kim, Y.R.; Shin, H.S. Treatment of landfill leachate using activated sludge process and electron-beam radiation. Water Res. 1999, 33, 2669–2673. [Google Scholar] [CrossRef]
- He, S.; Sun, W.; Wang, J.; Chen, L.; Zhang, Y.; Yu, J. Enhancement of biodegradability of real textile and dyeing wastewater by electron beam irradiation. Radiat. Phys. Chem. 2016, 124, 203–207. [Google Scholar] [CrossRef]
- Shin, H.S.; Kim, Y.R.; Han, B.; Makarov, I.E.; Ponomarev, A.V.; Pikaev, A.K. Application of electron beam to treatment of wastewater from papermill. Radiat. Phys. Chem. 2002, 65, 539–547. [Google Scholar] [CrossRef]
- Lim, S.J.; Kim, T.H. Combined treatment of swine wastewater by electron beam irradiation and ion-exchange biological reactor system. Sep. Purif. Technol. 2015, 146, 42–49. [Google Scholar] [CrossRef]
- Chu, L.; Yu, S.; Wang, J. Gamma radiolytic degradation of naphthalene in aqueous solution. Radiat. Phys. Chem. 2016, 123, 97–102. [Google Scholar] [CrossRef]
- Wang, J.; Chu, L. Irradiation treatment of pharmaceutical and personal care products (PPCPs) in water and wastewater: An overview. Radiat. Phys. Chem. 2016, 125, 56–64. [Google Scholar] [CrossRef]
- Wojnárovits, L.; Takács, E. Rate constants of dichloride radical anion reactions with molecules of environmental interest in aqueous solution: A review. Environ. Sci. Pollut. Res. 2021, 28, 41552–41575. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Bai, J.; Li, L.; Chen, S.; Zhou, T.; Wang, J.; Xia, L.; Xu, Q.; Zhou, B. Extremely efficient decomposition of ammonia N to N2 using ClO− from reactions of HO− and HOCl generated in situ on a novel bifacial photoelectroanode. Environ. Sci. Technol. 2019, 53, 6945–6953. [Google Scholar] [CrossRef]
- Wang, S.; Hu, J.; He, S.; Wang, J. Removal of ammonia and phenol from saline chemical wastewater by ionizing radiation: Performance, mechanism and toxicity. J. Hazard. Mater. 2022, 433, 128727. [Google Scholar] [CrossRef]
- Zuo, S.; Zhang, Y.; Guo, R.; Chen, J. Efficient removal of ammonia nitrogen by an electrochemical process for spent caustic wastewater treatment. Catalysts 2022, 12, 1357. [Google Scholar] [CrossRef]
- Son, Y.S.; Kim, K.H.; Kim, K.J.; Kim, J.C. Ammonia decomposition using electron beam. Plasma Chem. Plasma Process. 2013, 33, 617–629. [Google Scholar] [CrossRef]
- Naceradska, J.; Pivokonska, L.; Pivokonsky, M. On the importance of pH value in coagulation. J. Water Supply Res. Technol.—AQUA 2019, 68, 222–230. [Google Scholar] [CrossRef]
- Vedrenne, M.; Vasquez-Medrano, R.; Prato-Garcia, D.; Frontana-Uribe, B.A.; Ibanez, J.G. Characterization and detoxification of a mature landfill leachate using a combined coagulation–flocculation/photo Fenton treatment. J. Hazard. Mater. 2012, 205, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Amor, C.; De Torres-Socías, E.; Peres, J.A.; Maldonado, M.I.; Oller, I.; Malato, S.; Lucas, M.S. Mature landfill leachate treatment by coagulation/flocculation combined with Fenton and solar photo-Fenton processes. J. Hazard. Mater. 2015, 286, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Maleki, A.; Zazouli, M.A.; Izanloo, H.; Rezaee, R. Composting plant leachate treatment by coagulation-flocculation process. Am.-Eurasian J. Agric. Environ. Sci. 2009, 5, 638–643. [Google Scholar]
- Rui, L.M.; Daud, Z.; Latif, A.A.A. Coagulation-flocculation in leachate treatment by using ferric chloride and alum as coagulant. Int. J. Eng. Res. Appl. 2012, 2, 1929–1934. [Google Scholar]
- Sudoh, R.; Islam, M.S.; Sazawa, K.; Okazaki, T.; Hata, N.; Taguchi, S.; Kuramitz, H. Removal of dissolved humic acid from water by coagulation method using polyaluminum chloride (PAC) with calcium carbonate as neutralizer and coagulant aid. J. Environ. Chem. Eng. 2015, 3, 770–774. [Google Scholar] [CrossRef]
- Oladoja, N.A. Advances in the quest for substitute for synthetic organic polyelectrolytes as coagulant aid in water and wastewater treatment operations. Sustain. Chem. Pharm. 2016, 3, 47–58. [Google Scholar] [CrossRef]
- Bratby, J. Coagulation and Flocculation in Water and Wastewater Treatment; IWA Publishing: London UK, 2016. [Google Scholar]
- Tran, N.; Drogui, P.; Blais, J.F.; Mercier, G. Phosphorus removal from spiked municipal wastewater using either electrochemical coagulation or chemical coagulation as tertiary treatment. Sep. Purif. Technol. 2012, 95, 16–25. [Google Scholar] [CrossRef]
- Shi, J.; Dang, Y.; Qu, D.; Sun, D. Effective treatment of reverse osmosis concentrate from incineration leachate using direct contact membrane distillation coupled with a NaOH/PAM pre-treatment process. Chemosphere 2019, 220, 195–203. [Google Scholar] [CrossRef]
- Bao, H.; Liu, Y.; Jia, H. A study of irradiation in the treatment of wastewater. Radiat. Phys. Chem. 2002, 63, 633–636. [Google Scholar] [CrossRef]
- Dosta, J.; Rovira, J.; Galí, A.; Macé, S.; Mata-Alvarez, J. Integration of a Coagulation/Flocculation step in a biological sequencing batch reactor for COD and nitrogen removal of supernatant of anaerobically digested piggery wastewater. Bioresour. Technol. 2008, 99, 5722–5730. [Google Scholar] [CrossRef]
- Zhang, H.; Choi, H.J.; Huang, C.P. Optimization of Fenton process for the treatment of landfill leachate. J. Hazard. Mater. 2005, 125, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y. Physical and oxidative removal of organics during Fenton treatment of mature municipal landfill leachate. J. Hazard. Mater. 2007, 146, 334–340. [Google Scholar] [CrossRef]
- Wang, K.; Li, L.; Tan, F.; Wu, D. Treatment of landfill leachate using activated sludge technology: A review. Archaea 2018, 2018, 1039453. [Google Scholar] [CrossRef]
- Ching, Y.C.; Redzwan, G. Biological treatment of fish processing saline wastewater for reuse as liquid fertilizer. Sustainability 2017, 9, 1062. [Google Scholar] [CrossRef]
- Ogata, Y.; Ishigaki, T.; Nakagawa, M.; Yamada, M. Effect of increasing salinity on biogas production in waste landfills with leachate recirculation: A lab-scale model study. Biotechnol. Rep. 2016, 10, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Alkaabi, S.; Van Geel, P.J.; Warith, M.A. Effect of saline water and sludge addition on biodegradation of municipal solid waste in bioreactor landfills. Waste Manag. Res. 2009, 27, 59–69. [Google Scholar] [CrossRef]
- He, H.; Chen, Y.; Li, X.; Cheng, Y.; Yang, C.; Zeng, G. Influence of salinity on microorganisms in activated sludge processes: A review. Int. Biodeterior. Biodegrad. 2017, 119, 520–527. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association/American Water Works Association/Water Environment Federation: Washington, DC, USA, 2005. [Google Scholar]
- Khalaf, N.N.; Alabdraba, W.S. Removal of acetaminophen from aqueous solutions by hybrid Fenton oxidation and adsorption. IOP Conf. Ser. Earth Environ. Sci. 2022, 1120, 012008. [Google Scholar] [CrossRef]






| Raw Leachate | Ionizing Radiation (50 kGy) | Coagulation–Sedimentation | Fenton Oxidation | Effluent | ||||
| Concentration (mg L−1) | Concentration (mg L−1) | Removal (%) | Concentration (mg L−1) | Removal (%) | Concentration (mg L−1) | Removal (%) | Removal (%) | |
| SCOD | 23,950 | 22,730 | 5.1 | 12,920 | 43 | 7070 | 69 | 74 |
| T-N | 11,650 | 4500 | 61 | 3990 | 11 | 2020 | 55 | 83 |
| NH4-N | 1662.5 | 982.3 | 41 | 959 | 2.4 | 622 | 37 | 63 |
| T-P | 109.8 | 54 | 51 | 13.6 | 75 | 3.1 | 94 | 97 |
| Raw Leachate | Coagulation–Sedimentation | Fenton Oxidation | Effluent | |||||
| Concentration (mg L−1) | Concentration (mg L−1) | Removal (%) | Concentration (mg L−1) | Removal (%) | Concentration (mg L−1) | Removal (%) | Removal (%) | |
| SCOD | 23,950 | Not applicable | 18,980 | 21 | 10,620 | 56 | 56 | |
| T-N | 11,650 | 10,240 | 12 | 5840 | 50 | 50 | ||
| NH4-N | 1662.5 | 1412.4 | 15 | 912 | 45 | 45 | ||
| T-P | 109.8 | 53 | 52 | 12 | 89 | 89 | ||
| (a) | Total organic carbon (TOC) concentration | |||||
| Cl− concentration | 0.5% | 4% | 8% | |||
| Irradiation intensity | 0 kGy | 50 kGy | 0 kGy | 50 kGy | 0 kGy | 50 kGy |
| Initial TOC (mg L−1) | 140 mg L−1 | 80 mg L−1 | 83 mg L−1 | 68 mg L−1 | 58 mg L−1 | 50 mg L−1 |
| Final TOC (mg L−1) | 54 mg L−1 | 30 mg L−1 | 64 mg L−1 | 51 mg L−1 | 91 mg L−1 | 72 mg L−1 |
| (b) | Total nitrogen (TN) concentration | |||||
| Cl− concentration | 0.5% | 4% | 8% | |||
| Irradiation intensity | 0 kGy | 50 kGy | 0 kGy | 50 kGy | 0 kGy | 50 kGy |
| Initial TN (mg L−1) | 40 mg L−1 | 23 mg L−1 | 66 mg L−1 | 69 mg L−1 | 65 mg L−1 | 55 mg L−1 |
| Final TN (mg L−1) | 26 mg L−1 | 6.2 mg L−1 | 84 mg L−1 | 77 mg L−1 | 58 mg L−1 | 57 mg L−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Sinharoy, A.; Lee, G.-Y.; Lee, M.-J.; Lee, B.-C.; Chung, C.-M. Synergistic Effects of Ionizing Radiation Process in the Integrated Coagulation–Sedimentation, Fenton Oxidation, and Biological Process for Treatment of Leachate Wastewater. Catalysts 2023, 13, 1376. https://doi.org/10.3390/catal13101376
Liu S, Sinharoy A, Lee G-Y, Lee M-J, Lee B-C, Chung C-M. Synergistic Effects of Ionizing Radiation Process in the Integrated Coagulation–Sedimentation, Fenton Oxidation, and Biological Process for Treatment of Leachate Wastewater. Catalysts. 2023; 13(10):1376. https://doi.org/10.3390/catal13101376
Chicago/Turabian StyleLiu, Sha, Arindam Sinharoy, Ga-Young Lee, Myun-Joo Lee, Byung-Cheol Lee, and Chong-Min Chung. 2023. "Synergistic Effects of Ionizing Radiation Process in the Integrated Coagulation–Sedimentation, Fenton Oxidation, and Biological Process for Treatment of Leachate Wastewater" Catalysts 13, no. 10: 1376. https://doi.org/10.3390/catal13101376