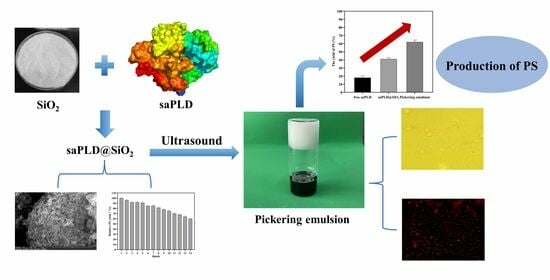
Immobilization of Phospholipase D for Production of Phosphatidylserine by a Pickering Emulsion Strategy
Abstract
:1. Introduction
2. Results and Discussion
2.1. Immobilization Conditions of saPLD
2.2. Characterization of saPLD@SiO2
2.2.1. Scanning Electron Microscopy
2.2.2. Confocal Laser Scanning Microscopy
2.2.3. Fourier Transform Infrared Spectroscopy
2.3. Properties of saPLD@SiO2
2.3.1. Optimum Temperatures and Thermal Stability
2.3.2. Optimum pH and pH Stability
2.4. Operational Stability of saPLD@SiO2
2.5. Storage Stability of saPLD@SiO2
2.6. Preparation and Characterization of Pickering Emulsion
2.7. Synthesis of Phosphatidylserine
3. Materials and Methods
3.1. Materials and Reagents
3.2. Activity Assay of PLD
3.3. Preparation of saPLD@SiO2
3.4. Characterization of saPLD@SiO2
3.4.1. Scanning Electron Microscopy
3.4.2. Confocal Laser Scanning Microscopy
3.4.3. Fourier Transform Infrared Spectroscopy
3.5. Enzymatic Properties of saPLD@SiO2
3.5.1. Optimum Temperatures and Thermal Stability
3.5.2. Optimum pH and pH Stability
3.6. Operational Stability of saPLD@SiO2
3.7. Storage Stability of saPLD@SiO2
3.8. Preparation and Characterization of Pickering Emulsion
3.9. Synthesis of Phosphatidylserine
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, W.B.; Gong, J.S.; Hou, H.J.; Li, H.; Lu, Z.M.; Xu, H.Y.; Xu, Z.H.; Shi, J.S. Mining of a phospholipase D and its application in enzymatic preparation of phosphatidylserine. Bioengineered 2018, 9, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Starks, M.A.; Starks, S.L.; Kingsley, M.; Purpura, M.; Jager, R. The effects of phosphatidylserine on endocrine response to moderate intensity exercise. J. Int. Soc. Sports Nutr. 2008, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Liu, A.; Dahmen, U.; Dirsch, O. Dual role of chloroquine in liver ischemia reperfusion injury: Reduction of liver damage in early phase, but aggravation in late phase. Cell Death Dis. 2013, 4, e694–e703. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.L.; Resende, R.; Ferreiro, E.; Rego, A.C.; Pereira, C.F. Multiple defects in energy metabolism in alzheimer’s disease. Curr. Drug Targets 2010, 11, 1193–1206. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Chen, M.; Xu, W.; Zhang, W.L.; Zhang, T.; Guang, C.E.; Mu, W.M. Microbial phospholipase D: Identification, modification and application. Trends Food Sci. Technol. 2020, 96, 145–156. [Google Scholar] [CrossRef]
- Vance, J.E.; Tasseva, G. Formation and function of phosphatidylserine and phosphatidylethanolamine in mammalian cells. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2013, 1831, 543–554. [Google Scholar] [CrossRef]
- Saeed, M.; van Brakel, M.; Zalba, S.; Schooten, E.; Rens, J.A.P.; Koning, G.A.; Debets, R.; ten Hagen, T.L.M. Targeting melanoma with immunoliposomes coupled to anti-MAGE A1 TCR-like single-chain antibody. Int. J. Nanomed. 2016, 11, 955–975. [Google Scholar] [CrossRef]
- Delwaide, P.J.; Gyselynck-Mambourg, A.M.; Hurlet, A.; Ylieff, M.J.A.N.S. Double-blind randomized controlled study of phosphatidylserine in senile demented patients. Acta Neurol. Scand. 2009, 73, 136–140. [Google Scholar] [CrossRef]
- Liu, Y.H.; Huang, L.; Fu, Y.; Zheng, D.; Ma, J.Y.; Li, Y.Z.; Xu, Z.H.; Lu, F.P. A novel process for phosphatidylserine production using a Pichia pastoris whole-cell biocatalyst with overexpression of phospholipase D from Streptomyces halstedii in a purely aqueous system. Food Chem. 2019, 274, 535–542. [Google Scholar] [CrossRef]
- Zhang, P.; Gong, J.S.; Qin, J.F.; Li, H.; Hou, H.J.; Zhang, X.M.; Xu, Z.H.; Shi, J.S. Phospholipids (PLs) know-how: Exploring and exploiting phospholipase D for its industrial dissemination. Crit. Rev. Biotechnol. 2021, 41, 1257–1278. [Google Scholar] [CrossRef]
- Feng, X.X.; Yang, S.X.; Zhang, Y.H.; Zhiyuan, C.; Tang, K.Q.; Li, G.; Yu, H.; Leng, J.T.; Wang, Q.Y. GmPGL2, Encoding a pentatricopeptide repeat protein, is essential for chloroplast rna editing and biogenesis in soybean. Front. Plant Sci. 2021, 12, 690973. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.Q.; Hu, F. Efficient synthesis of phosphatidylserine in 2-methyltetrahydrofuran. J. Biotechnol. 2013, 163, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Li, B.L.; Wang, J.; Li, H.Y.; Zhao, B.X. High-yield and sustainable production of phosphatidylserine in purely aqueous solutions via adsorption of phosphatidylcholine on Triton-X-100-modified silica. J. Agric. Food Chem. 2017, 65, 10767–10774. [Google Scholar] [CrossRef] [PubMed]
- Selvy, P.E.; Lavieri, R.R.; Lindsley, C.W.; Brown, H.A. Phospholipase D: Enzymology, functionality, and chemical modulation. Chem. Rev. 2011, 111, 6064–6119. [Google Scholar] [CrossRef] [PubMed]
- Shimbo, K.; Iwasaki, Y.; Yamane, T.; Ina, K.J.B. Purification and properties of phospholipase D from Streptomyces antibioticus. Biosci. Biotechnol. Biochem. 1993, 11, 1946–1948. [Google Scholar] [CrossRef]
- Leiros, I.; McSweeney, S.; Hough, E. The reaction mechanism of phospholipase D from Streptomyces sp strain PMF. snapshots along the reaction pathway reveal a pentacoordinate reaction intermediate and an unexpected final product. J. Mol. Biol. 2004, 339, 805–820. [Google Scholar] [CrossRef]
- Stieglitz, K.A.; Seaton, B.A.; Roberts, M.F. Proteolytically-clipped form of S. chromofuscus phospholipase D induces vesicle leakiness & enhances vesicle aggregation and fusion. Biophys. J. 2001, 80, 184A. [Google Scholar]
- Hagishita, T.; Nishikawa, M.; Hatanaka, T. Isolation of phospholipase D producing microorganisms with high transphosphatidylation activity. Biotechnol. Lett. 2000, 22, 1587–1590. [Google Scholar] [CrossRef]
- Diauudin, F.N.; Noor, S.A.M.; Rashid, J.I.A.; Knight, V.F.; Yunus, W.; Ong, K.K.; Kasim, N.A.M.; Taufik, S.; Samsuri, A.; Shamsudin, I.J.; et al. Preparation and characterisation of polypyrrole-iron oxyhydroxide nanocomposite as sensing material. Adv. Mater. Sci. Eng. 2020, 2020, 8762969. [Google Scholar] [CrossRef]
- Chen, L.; Zou, M.; Hong, F.F. Evaluation of fungal laccase immobilized on natural nanostructured bacterial cellulose. Front. Microbiol. 2015, 6, 1245–1255. [Google Scholar] [CrossRef]
- Bohari, N.A.; Shafiquzzaman, S.; Kamaruzaman, A.J.B. Development of formaldehyde biosensor for determination of formalin in fish samples; malabar red snapper (lutjanus malabaricus) and longtail tuna (thunnus tonggol). Biosensors. 2016, 6, 32. [Google Scholar] [CrossRef]
- Chatzikonstantinou, A.V.; Gkantzou, E.; Thomou, E.; Chalmpes, N.; Lyra, K.M.; Kontogianni, V.G.; Spyrou, K.; Patila, M.; Gournis, D.; Stamatis, H. Enzymatic Conversion of Oleuropein to Hydroxytyrosol Using Immobilized beta-Glucosidase on Porous Carbon Cuboids. Nanomaterials 2019, 9, 1166. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Liu, S.; Zhang, Q. Degradation and Detection of Endocrine Disruptors by Laccase-Mimetic Polyoxometalates. Front. Chem. 2022, 10, 854045. [Google Scholar] [CrossRef] [PubMed]
- Bosio, V.E.; Islan, G.A.; Martinez, Y.N.; Duran, N.; Castro, G.R. Nanodevices for the immobilization of therapeutic enzymes. Crit. Rev. Biotechnol. 2016, 36, 447–464. [Google Scholar] [CrossRef]
- Carvalho, N.B.; Vidal, B.T.; Barbosa, A.S.; Pereira, M.M.; Mattedi, S.; Freitas, L.D.; Lima, A.S.; Soares, C.M.F. Lipase immobilization on silica xerogel treated with protic ionic liquid and its application in biodiesel production from different oils. Int. J. Mol. Sci. 2018, 19, 1829. [Google Scholar] [CrossRef]
- Miguel, S.; Legrand, G.; Duriot, L.; Delporte, M.; Menin, B.; Michel, C.; Olry, A.; Chataigne, G.; Salwinski, A.; Bygdell, J.; et al. A GDSL lipase-like from Ipomoea batatas catalyzes efficient production of 3,5-diCQA when expressed in Pichia pastoris. Commun. Biol. 2020, 3, 673–686. [Google Scholar] [CrossRef]
- Fathi, Z.; Doustkhah, E.; Ebrahimipour, G.; Darvishi, F. Noncovalent immobilization of yarrowia lipolytica lipase on dendritic-like amino acid-functionalized silica nanoparticles. Biomolecules 2019, 9, 502. [Google Scholar] [CrossRef]
- Degorska, O.; Szada, D.; Zdarta, A.; Smulek, W.; Jesionowski, T.; Zdarta, J. Immobilized lipase in resolution of ketoprofen enantiomers: Examination of biocatalysts properties and process characterization. Pharmaceutics 2022, 14, 1443. [Google Scholar] [CrossRef]
- Meir, I.; Alfassi, G.; Arazi, Y.; Rein, D.M.; Fishman, A.; Cohen, Y. Lipase Catalyzed Transesterification of model long-chain molecules in double-shell cellulose-coated oil-in-water emulsion particles as microbioreactors. Int. J. Mol. Sci. 2022, 23, 12122. [Google Scholar] [CrossRef]
- Xi, Y.K.; Liu, B.; Wang, S.X.; Wei, S.H.; Yin, S.W.; Ngai, O.; Yang, X.Q. CO2-responsive Pickering emulsions stabilized by soft protein particles for interfacial biocatalysis. Chem. Sci. 2022, 13, 2884–2890. [Google Scholar] [CrossRef]
- Ren, G.H.; Li, Z.Z.; Lu, D.X.; Li, B.; Ren, L.L.; Di, W.W.; Yu, H.Q.; He, J.X.; Sun, D.J. pH and Magnetism dual-responsive pickering emulsion stabilized by dynamic covalent Fe3O4 nanoparticles. Nanomaterials 2022, 12, 2587. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Liu, Z.S.; Shi, J.; Tang, H.; Xiang, X.; Huang, F.H.; Zheng, M.M. Carbon nanoparticle-stabilized pickering emulsion as a sustainable and high-performance interfacial catalysis platform for enzymatic esterification/transesterification. Acs Sustain. Chem. Eng. 2019, 7, 7619–7629. [Google Scholar] [CrossRef]
- Pota, G.; Bifulco, A.; Parida, D.; Zhao, S.; Rentsch, D.; Amendola, E.; Califano, V.; Costantini, A. Tailoring the hydrophobicity of wrinkled silica nanoparticles and of the adsorption medium as a strategy for immobilizing lipase: An efficient catalyst for biofuel production. Microporous Mesoporous Mater. 2021, 328, 111504. [Google Scholar] [CrossRef]
- Yang, T.; Thomas, A.M.; Dangi, B.B.; Kaiser, R.I.; Mebel, A.M.; Millar, T.J. Directed gas phase formation of silicon dioxide and implications for the formation of interstellar silicates. Nat. Commun. 2018, 9, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.H.; Zhang, Z.H.; Ma, X.Y.; Tian, H.; Lu, F.P.; Liu, Y.H. Efficient secretion expression of phospholipase D in Bacillus subtilis and its application in synthesis of phosphatidylserine by enzyme immobilization. Int. J. Biol. Macromol. 2021, 169, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Younus, H.; Rajcani, J.; Ulbrich-Hofmann, R.; Saleemuddin, M. Behaviour of a recombinant cabbage (Brassica oleracea) phospholipase D immobilized on CNBr-activated and antibody supports. Biotechnol. Appl. Biochem. 2004, 40, 95–99. [Google Scholar] [CrossRef]
- Islam, M.M.; Chakraborty, M.; Pandya, P.; Al Masum, A.; Gupta, N.; Mukhopadhyay, S. Binding of DNA with Rhodamine B: Spectroscopic and molecular modeling studies. Dye. Pigment. 2013, 99, 412–422. [Google Scholar] [CrossRef]
- Potter, M.N.; Green, J.R.; Mutus, B. Fluorescein isothiocyanate, a platform for the selective and sensitive detection of S-Nitrosothiols and hydrogen sulfide. Talanta 2022, 237, 122981–122988. [Google Scholar] [CrossRef]
- Shabanian, M.; Hajibeygi, M.; Raeisi, A. FTIR Characterization of Layered Double Hydroxides and Modified Layered Double Hydroxides. In Layered Double Hydroxide Polymer Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2020; pp. 77–101. [Google Scholar]
- Califano, V.; Costantini, A.; Silvestri, B.; Venezia, V.; Cimino, S.; Sannino, F. The effect of pore morphology on the catalytic performance of β-glucosidase immobilized into mesoporous silica. Pure Appl. Chem. 2019, 91, 1583–1592. [Google Scholar] [CrossRef]
- Ha Li, S.S.; Li, Y.; Long, N.B.; Jiang, F.; Zhang, R.F. Highly active and stable nanobiocatalyst based on in-situ cross-linking of phospholipase D for the synthesis of phosphatidylserine. Int. J. Biol. Macromol. 2018, 117, 1188–1194. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, M.; Li, X.; Liu, B.; Li, B.; Wang, J. Immobilization of Bio-imprinted Phospholipase D and Its Catalytic Behavior for Transphosphatidylation in the Biphasic System. Appl. Biochem. Biotechnol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.Q.; Zhang, H.Y.; Sun, J.A.; Liu, Z.; Huang, W.C.; Xue, C.H.; Mao, X.Z. Immobilization of phospholipase D on silica-coated magnetic nanoparticles for the synthesis of functional phosphatidylserine. Catalysts 2019, 9, 361. [Google Scholar] [CrossRef]
- Li, Y.; Wu, J.Q.; Long, N.B.; Zhang, R.F. Efficient immobilization of phospholipase D on novel polymer supports with hierarchical pore structures. Int. J. Biol. Macromol. 2019, 141, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Salvador, J.L.; Balea, A.; Monte, M.C.; Blanco, A.; Negro, C. Pickering emulsions containing cellulose microfibers produced by mechanical treatments as stabilizer in the food industry. Appl. Sci. 2019, 9, 359. [Google Scholar] [CrossRef]
- de Carvalho-Guimaraes, F.B.; Correa, K.L.; de Souza, T.P.; Amado, J.R.R.; Ribeiro-Costa, R.M.; Silva, J.O.C. A Review of Pickering Emulsions: Perspectives and Applications. Pharmaceuticals 2022, 15, 1413. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.J.; Ao, F.; Ge, X.M.; Shen, W. Food-Grade Pickering Emulsions: Preparation, Stabilization and Applications. Molecules 2020, 25, 3202. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Cai, W.B.; Lv, W.; Zhao, P.; Shen, Y.M.; Zhang, L.F.; Ma, B.; Yuan, L.J.; Duan, Y.Y.; Yao, K.C. A new strategy for accurate targeted diagnosis and treatment of cutaneous malignant melanoma: Dual-mode phase-change lipid nanodroplets as ultrasound contrast agents. Int. J. Nanomed. 2019, 14, 7079–7093. [Google Scholar] [CrossRef]
- Wang, S.R.; Wang, T.Y.; Li, X.Y.; Cui, Y.J.; Sun, Y.; Yu, G.P.; Cheng, J.J. Fabrication of emulsions prepared by rice bran protein hydrolysate and ferulic acid covalent conjugate: Focus on ultrasonic emulsification. Ultrason. Sonochem. 2022, 88, 106064–106074. [Google Scholar] [CrossRef]
- Zhang, T.T.; Jiang, H.; Hong, L.Z.; Ngai, T. Multiple Pickering emulsions stabilized by surface-segregated micelles with adaptive wettability. Chem. Sci. 2022, 13, 10752–10758. [Google Scholar] [CrossRef]
- Tng, D.J.H.; Song, P.Y.; Lin, G.M.; Soehartono, A.M.; Yang, G.; Yang, C.B.; Yin, F.; Tan, C.H.; Yong, K.T. Synthesis and characterization of multifunctional hybrid-polymeric nanoparticles for drug delivery and multimodal imaging of cancer. Int. J. Nanomed. 2015, 10, 5771–5786. [Google Scholar] [CrossRef]
- Foroushani, P.H.; Rahmani, E.; Alemzadeh, I.; Vossoughi, M.; Pourmadadi, M.; Rahdar, A.; Diez-Pascual, A.M. Curcumin Sustained Release with a Hybrid Chitosan-Silk Fibroin Nanofiber Containing Silver Nanoparticles as a Novel Highly Efficient Antibacterial Wound Dressing. Nanomaterials 2022, 12, 3426. [Google Scholar] [CrossRef] [PubMed]
- Arora, H.; Culler, M.D.; Decker, E.A. Production of a High-phosphatidylserine lecithin that synergistically inhibits lipid oxidation with alpha-tocopherol in oil-in-water emulsions. Foods 2022, 11, 1014. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.K.; Cui, R.G.; Lan, D.M.; Wang, F.H.; Wang, Y.H. Acyl chain specificity of marine streptomyces klenkii phospholipase D and its application in enzymatic preparation of phosphatidylserine. Int. J. Mol. Sci. 2021, 22, 10580. [Google Scholar] [CrossRef]
- Mable, C.J.; Warren, N.J.; Thompson, K.L.; Mykhaylyk, O.O.; Armes, S.P. Framboidal ABC triblock copolymer vesicles: A new class of efficient Pickering emulsifier. Chem. Sci. 2015, 6, 6179–6188. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Lu, F.P.; Chen, G.Q.; Snyder, C.; Sun, J.; Li, Y.; Wang, J.L.; Xiao, J. High-level expression, purification and characterization of a recombinant medium-temperature alpha-amylase from Bacillus subtilis. Biotechnol. Lett. 2010, 32, 119–124. [Google Scholar] [CrossRef]












Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Zhang, S.; Liu, D.; Huang, Z.; Ge, Y.; Hou, J.; Lu, F.; Liu, Y. Immobilization of Phospholipase D for Production of Phosphatidylserine by a Pickering Emulsion Strategy. Catalysts 2023, 13, 1318. https://doi.org/10.3390/catal13101318
Sun H, Zhang S, Liu D, Huang Z, Ge Y, Hou J, Lu F, Liu Y. Immobilization of Phospholipase D for Production of Phosphatidylserine by a Pickering Emulsion Strategy. Catalysts. 2023; 13(10):1318. https://doi.org/10.3390/catal13101318
Chicago/Turabian StyleSun, Hui, Shujing Zhang, Dianqing Liu, Zhiqi Huang, Yuxin Ge, Jiayi Hou, Fuping Lu, and Yihan Liu. 2023. "Immobilization of Phospholipase D for Production of Phosphatidylserine by a Pickering Emulsion Strategy" Catalysts 13, no. 10: 1318. https://doi.org/10.3390/catal13101318