Effect of Nitrogen Doping in GO as Support in ZnO/GO-N Compounds and Their Photocatalytic Assessment to Degrade the Lignin Molecule
Abstract
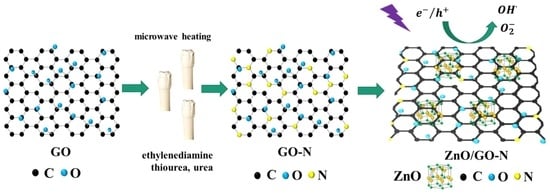
:1. Introduction
2. Results and Discussion
2.1. X-ray Diffraction
2.2. Field Emission Scanning Electron Microscopy
2.3. Transmission Electron Microscopy Studies
2.4. X-ray Photoelectron Spectroscopy
2.5. Specific Surface Area Determination
3. Photocatalytic Activity Tests
4. Materials and Methods
4.1. Chemicals
4.2. ZnO Synthesis
4.3. Synthesis of Graphene Oxide and Nitrogen-Doping
4.4. Synthesis of ZnO/GO-N Systems
4.5. Characterization
4.6. Degradation Studies
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kashinath, L.; Namratha, K.; Byrappa, K. Microwave Assisted Facile Hydrothermal Synthesis and Characterization of Zinc Oxide Flower Grown on Graphene Oxide Sheets for Enhanced Photodegradation of Dyes. Appl. Surf. Sci. 2015, 357, 1849–1856. [Google Scholar] [CrossRef]
- Pei, L.Z.; Wei, T.; Lin, N.; Yu, H.Y. Synthesis of Zinc Oxide and Titanium Dioxide Composite Nanorods and Their Photocatalytic Properties. Adv. Compos. Lett. 2016, 25, 9–15. [Google Scholar] [CrossRef]
- Hsieh, S.H.; Chen, W.J.; Yeh, T.H. Degradation of Methylene Blue Using ZnSe-Graphene Nanocomposites under Visible-Light Irradiation. Ceram. Int. 2015, 41, 13759–13766. [Google Scholar] [CrossRef]
- Li, H.; Lei, Z.; Liu, C.; Zhang, Z.; Lu, B. Photocatalytic Degradation of Lignin on Synthesized Ag-AgCl/ZnO Nanorods under Solar Light and Preliminary Trials for Methane Fermentation. Bioresour. Technol. 2015, 175, 494–501. [Google Scholar] [CrossRef]
- Oros, G.; Forgacs, E.; Cserha, T. Removal of Synthetic Dyes from Wastewaters: A Review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef]
- Feng, H.; Zhou, W.; Zhang, X.; Zhang, S.; Liu, B.; Zhen, D. Synthesis of Z-Scheme Mn-CdS/MoS2/TiO2 Ternary Photocatalysts for High-Efficiency Sunlight-Driven Photocatalysis. Adv. Compos. Lett. 2019, 28, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, V.R.A.; Azenha, M.A.; Pereira, C.M.; Silva, A.F. Preparation of Molecularly Imprinted Hollow TiO2 Microspheres for Selective Photocatalysis. Chem. Eng. J. Adv. 2021, 5, 100071. [Google Scholar] [CrossRef]
- Mehdi Sabzehmeidani, M.; Karimi, H.; Ghaedi, M. CeO2 Nanofibers-CdS Nanostructures n–n Junction with Enhanced Visible-Light Photocatalytic Activity. Arab. J. Chem. 2020, 13, 7583–7597. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, F.; Wu, T.; Wu, Q.; Huang, Z.; Su, H.; Zhang, Z. Photochemical Preparation of CdS Hollow Microspheres at Room Temperature and Their Use in Visible-Light Photocatalysis. J. Solid State Chem. 2011, 184, 644–648. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, G.; Zhang, C.; Xue, J.; Zhao, Q.; Li, R.; Wang, Y.; Fan, C. Assisting Bi2MoO6 Microspheres with Phenolic Resin-Based ACSs as Attractive Tailor-Made Supporter for Highly-Efficient Photocatalytic CO2 Reduction. Green Energy Environ. 2021, 6, 693–702. [Google Scholar] [CrossRef]
- Di Mauro, A.; Farrugia, C.; Abela, S.; Refalo, P.; Grech, M.; Falqui, L.; Privitera, V.; Impellizzeri, G. Synthesis of ZnO/PMMA Nanocomposite by Low-Temperature Atomic Layer Deposition for Possible Photocatalysis Applications. Mater. Sci. Semicond. Process. 2020, 118, 105214. [Google Scholar] [CrossRef]
- Kubiak, A.; Żółtowska, S.; Gabała, E.; Szybowicz, M.; Siwińska-Ciesielczyk, K.; Jesionowski, T. Controlled Microwave-Assisted and PH-Affected Growth of ZnO Structures and Their Photocatalytic Performance. Powder Technol. 2021, 386, 221–235. [Google Scholar] [CrossRef]
- Ohnishi, H.; Matsumura, M.; Tsubomura, H.; Iwasaki, M. Bleaching of Lignin Solution by a Photocatalyzed Reaction on Semiconductor Photocatalysts. Ind. Eng. Chem. Res. 1989, 28, 719–724. [Google Scholar] [CrossRef]
- Yogamalar, R.; Bose, A. Synthesis, Dopant Study and Device Fabrication of Zinc Oxide Nanostructures: Mini Review. Prog. Nanotechnol. 2013, 2, 1–20. [Google Scholar]
- Adegoke, K.A.; Iqbal, M.; Louis, H.; Bello, O.S. Synthesis, Characterization and Application of CdS/ZnO Nanorod Heterostructure for the Photodegradation of Rhodamine B Dye. Mater. Sci. Energy Technol. 2019, 2, 329–336. [Google Scholar] [CrossRef]
- Ramos-Corona, A.; Rangel, R.; Alvarado-Gil, J.J.; Bartolo-Pérez, P.; Quintana, P.; Rodríguez-Gattorno, G. Photocatalytic Performance of Nitrogen Doped ZnO Structures Supported on Graphene Oxide for MB Degradation. Chemosphere 2019, 236, 124368. [Google Scholar] [CrossRef]
- Kavil, J.; Alshahrie, A.; Periyat, P. CdS Sensitized TiO2 Nano Heterostructures as Sunlight Driven Photocatalyst. Nano-Struct. Nano-Objects 2018, 16, 24–30. [Google Scholar] [CrossRef]
- Meng, X.; Zong, P.; Wang, L.; Yang, F.; Hou, W.; Zhang, S.; Li, B.; Guo, Z.; Liu, S.; Zuo, G.; et al. Au-Nanoparticle-Supported ZnO as Highly Efficient Photocatalyst for H2O2 Production. Catal. Commun. 2020, 134, 105860. [Google Scholar] [CrossRef]
- Liu, S.H.; Wei, Y.S.; Lu, J.S. Visible-Light-Driven Photodegradation of Sulfamethoxazole and Methylene Blue by Cu2O/RGO Photocatalysts. Chemosphere 2016, 154, 118–123. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Jiang, D.; Schedin, F.; Booth, T.J.; Khotkevich, V.V.; Morozov, S.V.; Geim, A.K. Two-Dimensional Atomic Crystals. Proc. Natl. Acad. Sci. USA. 2005, 102, 10451–10453. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.D.; Li, Y.R.; Lee, Y.C. Synthesis of R-GO/TiO2 composites via the UV-Assisted Photocatalytic Reduction of Graphene Oxide. Appl. Surf. Sci. 2016, 380, 249–256. [Google Scholar] [CrossRef]
- Avouris, P.; Dimitrakopoulos, C. Graphene: Synthesis and Applications. Mater. Today 2012, 15, 86–97. [Google Scholar] [CrossRef]
- Praxedes, F.M.; Moreno, H.; Simões, A.Z.; Teixeira, V.C.; Nunes, R.S.; Amoresi, R.A.C.; Ramirez, M.A. Interface Matters: Design of an Efficient CaCu3Ti4O12-RGO Photocatalyst. Powder Technol. 2022, 404, 117478. [Google Scholar] [CrossRef]
- Kwan, Y.C.G.; Ng, G.M.; Huan, C.H.A. Identification of Functional Groups and Determination of Carboxyl Formation Temperature in Graphene Oxide Using the XPS O 1s Spectrum. Thin Solid Films 2015, 590, 40–48. [Google Scholar] [CrossRef]
- Ikram, R.; Jan, B.M.; Ahmad, W. An Overview of Industrial Scalable Production of Graphene Oxide and Analytical Approaches for Synthesis and Characterization. J. Mater. Res. Technol. 2020, 9, 11587–11610. [Google Scholar] [CrossRef]
- Gocyla, M.; Pisarek, M.; Holdynski, M.; Opallo, M. Electrochemical Detection of Graphene Oxide. Electrochem. commun. 2018, 96, 77–82. [Google Scholar] [CrossRef]
- Liu, L.; Dong, C.; Wu, K.; Ye, Y.; Wei, X. Synthesis of Nitrogen-Doped Graphene–ZnO Nanocomposites with Improved Photocatalytic Activity. Mater. Lett. 2014, 129, 170–173. [Google Scholar] [CrossRef]
- Rajbongshi, B.M.; Ramchiary, A.; Samdarshi, S. Influence of N-Doping on Photocatalytic Activity of ZnO Nanoparticles under Visible Light Irradiation. Mater. Lett. 2014, 134, 111–114. [Google Scholar] [CrossRef]
- Li, B.; Liu, T.; Wang, Y.; Wang, Z. ZnO/Graphene-Oxide Nanocomposite with Remarkably Enhanced Visible-Light-Driven Photocatalytic Performance. J. Colloid Interface Sci. 2012, 377, 114–121. [Google Scholar] [CrossRef]
- Samadi, M.; Zirak, M.; Naseri, A.; Khorashadizade, E.; Moshfegh, A.Z. Recent Progress on Doped ZnO Nanostructures for Visible-Light Photocatalysis. Thin Solid Films 2016, 605, 2–19. [Google Scholar] [CrossRef] [Green Version]
- Chamoli, P.; Das, K.M.; Kar, K. Temperature Dependence Green Reduction of Graphene Oxide by Urea. Adv. Mater. Lett. 2017, 8, 217–222. [Google Scholar] [CrossRef]
- Liu, S.; Sun, H.; Suvorova, A.; Wang, S. One-Pot Hydrothermal Synthesis of ZnO-Reduced Graphene Oxide Composites Using Zn Powders for Enhanced Photocatalysis. Chem. Eng. J. 2013, 229, 533–539. [Google Scholar] [CrossRef] [Green Version]
- Meng, F.; Ge, F.; Chen, Y.; Xu, G.; Huang, F. Local Structural Changes Induced by Ion Bombardment in Magnetron Sputtered ZnO: Al Films: Raman, XPS, and XAS Study. Surf. Coatings Technol. 2019, 365, 2–9. [Google Scholar] [CrossRef]
- Al, B.S.M.; Al, F.; Al, B.; Kuvarega, A.T.; Al, S.M.Z.; Kim, Y.; Selvaraj, R. Hydrothermal Synthesis of CdS Sub-Microspheres for Photocatalytic Degradation of Pharmaceuticals. Appl. Surf. Sci. 2018, 457, 559–565. [Google Scholar] [CrossRef]
- Guo, C.; Tian, K.; Wang, L.; Liang, F.; Wang, F.; Chen, D.; Ning, J.; Zhong, Y.; Hu, Y. Approach of Fermi Level and Electron-Trap Level in Cadmium Sulfide Nanorods via Molybdenum Doping with Enhanced Carrier Separation for Boosted Photocatalytic Hydrogen Production. J. Colloid Interface Sci. 2021, 583, 661–671. [Google Scholar] [CrossRef]
- Wang, H.; Maiyalagan, T.; Wang, X. Review on Recent Progress in Nitrogen-Doped Graphene: Synthesis, Characterization, and Its Potential Applications. ACS Catal. 2012, 2, 781–794. [Google Scholar] [CrossRef]
- Wang, Y.; Shao, Y.; Matson, D.W.; Li, J.; Lin, Y. Nitrogen-Doped Graphene and Its Biosensing. ACS Nano 2010, 4, 1790–1798. [Google Scholar] [CrossRef]
- Gao, H.; Song, L.; Guo, W.; Huang, L.; Yang, D.; Wang, F.; Zuo, Y.; Fan, X.; Liu, Z.; Gao, W.; et al. A Simple Method to Synthesize Continuous Large Area Nitrogen-Doped Graphene. Carbon 2012, 50, 4476–4482. [Google Scholar] [CrossRef]
- Khan, M.E.; Khan, M.M.; Cho, M.H. CdS-Graphene Nanocomposite for Efficient Visible-Light-Driven Photocatalytic and Photoelectrochemical Applications. J. Colloid Interface Sci. 2016, 482, 221–232. [Google Scholar] [CrossRef]
- Putri, L.K.; Tan, L.L.; Ong, W.J.; Chang, W.S.; Chai, S.P. Graphene Oxide: Exploiting Its Unique Properties toward Visible-Light-Driven Photocatalysis. Appl. Mater. Today 2016, 4, 9–16. [Google Scholar] [CrossRef]
- Ramos-Corona, A.; Rangel, R.; Espino, J.; Lara, J.; Nuñez, R.; Bartolo-Pérez, P.; Alvarado-Gil, J.J. High-Yield of Lignin Degradation under N-ZnO/Graphene Oxide Compounds. Catal. Today 2022, 392–393, 81–92. [Google Scholar] [CrossRef]
- Nguyen-Phan, T.-D.; Pham, V.H.; Shin, E.W.; Pham, H.-D.; Kim, S.; Chung, J.S.; Kim, E.J.; Hur, S.H. The Role of Graphene Oxide Content on the Adsorption-Enhanced Photocatalysis of Titanium Dioxide/Graphene Oxide Composites. Chem. Eng. J. 2011, 170, 226–232. [Google Scholar] [CrossRef]
- Qin, H.; Li, W.; Xia, Y.; He, T. Photocatalytic Activity of Heterostructures Based on ZnO and N-Doped ZnO. ACS Appl. Mater. Interfaces 2011, 3, 3152–3156. [Google Scholar] [CrossRef] [PubMed]
- Rangel, R.; Cedeño, V.; Ramos-Corona, A.; Gutiérrez, R.; Alvarado-Gil, J.J.; Ares, O.; Bartolo-Pérez, P.; Quintana, P. Tailoring Surface and Photocatalytic Properties of ZnO and Nitrogen-Doped ZnO Nanostructures Using Microwave-Assisted Facile Hydrothermal Synthesis. Appl. Phys. A 2017, 123, 552. [Google Scholar] [CrossRef]
- Chen, D.; Feng, H.; Li, J. Graphene Oxide: Preparation, Functionalization, and Electrochemical Applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef] [PubMed]
- Dahm, A.; Lucia, L.A. Titanium Dioxide Catalyzed Photodegradation of Lignin in Industrial Effluents. Ind. Eng. Chem. Res. 2004, 43, 7996–8000. [Google Scholar] [CrossRef]









| Compound | Atomic Content N (%) | Atomic Content C (%) | Atomic Content O (%) | Atomic Content Zn (%) |
|---|---|---|---|---|
| ZnO | - | - | 52.66 | 47.34 |
| GO | - | 84.52 | 15.48 | - |
| ZnO/GO-U | 14.08 | 70.84 | 9.15 | 5.93 |
| ZnO/GO-T | 12.14 | 72.77 | 9.52 | 5.57 |
| ZnO/GO-E | 5.32 | 83.71 | 8.44 | 2.53 |
| Compound | Atomic Content of N (%) | Pyridinic-N (%) | Pyrrolic-N (%) | Graphitic-N (%) |
|---|---|---|---|---|
| GO-U | 16.86 | 4.89 | 6.66 | 5.29 |
| GO-T | 12.45 | 3.28 | 4.38 | 4.77 |
| GO-E | 6.36 | 2.13 | 1.11 | 3.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rangel, R.; Ramos-Corona, A.; Espino, J.; Quintana, P.; Bartolo-Pérez, P.; García, R. Effect of Nitrogen Doping in GO as Support in ZnO/GO-N Compounds and Their Photocatalytic Assessment to Degrade the Lignin Molecule. Catalysts 2023, 13, 69. https://doi.org/10.3390/catal13010069
Rangel R, Ramos-Corona A, Espino J, Quintana P, Bartolo-Pérez P, García R. Effect of Nitrogen Doping in GO as Support in ZnO/GO-N Compounds and Their Photocatalytic Assessment to Degrade the Lignin Molecule. Catalysts. 2023; 13(1):69. https://doi.org/10.3390/catal13010069
Chicago/Turabian StyleRangel, R., A. Ramos-Corona, J. Espino, P. Quintana, P. Bartolo-Pérez, and R. García. 2023. "Effect of Nitrogen Doping in GO as Support in ZnO/GO-N Compounds and Their Photocatalytic Assessment to Degrade the Lignin Molecule" Catalysts 13, no. 1: 69. https://doi.org/10.3390/catal13010069