Optimization and Determination of Kinetic Parameters of the Synthesis of 5-Lauryl-hydroxymethylfurfural Catalyzed by Lipases
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Esterification to Produce 5-Lauryl-hydroxymethylfurfural Catalyzed by Novozym 435®
2.2. Optimization of Esterification to Produce 5-lauryl-hydroxymethylfurfural Catalyzed by Immobilized TL Lipase
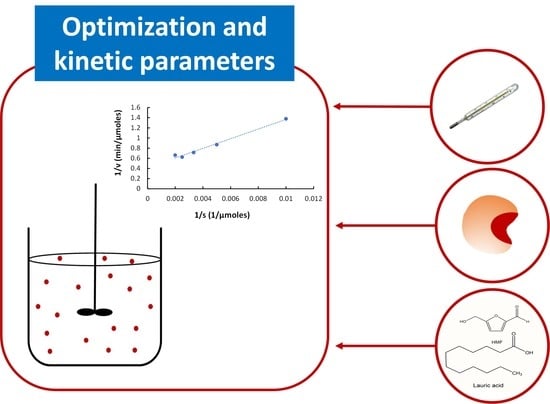
2.3. Determination of the Kinetic Parameters of the Esterification Reaction
3. Materials and Methods
3.1. Chemicals
3.2. Biocatalyst Synthesis Activity Assays
3.3. High-Performance Liquid Chromatography (HPLC) Analysis
3.4. Enzymatic Esterification of HMF with Lauric Acid in a Batch Reactor
3.5. Conversion Optimization
3.6. Determination of Kinetic Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.; Wang, J.; Nielsen, J. Yeast synthetic biology advances biofuel production. Curr. Opin. Microbiol. 2022, 65, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Raud, M.; Kikas, T.; Sippula, O.; Shurpali, N.J. Potentials, and challenges in lignocellulosic biofuel production technology. Renew. Sustain. Energy Rev. 2019, 111, 44–56. [Google Scholar] [CrossRef]
- Velvizhi, G.; Balakumar, K.; Shetti, N.P.; Ahmad, E.; Pant, K.K.; Aminabhavi, T.M. Integrated biorefinery processes for conversion of lignocellulosic biomass to value-added materials: Paving a path towards a circular economy. Bioresour. Technol. 2022, 343, 126151. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, I.; D’Angelo, S.C.; Galán-Martín, Á.; Pozo, C.; Pérez-Ramírez, J.; Guillén-Gosálbez, G. Process modelling and life cycle assessment coupled with experimental work to shape the future sustainable production of chemicals and fuels. React. Chem. Eng. 2021, 6, 1179–1194. [Google Scholar] [CrossRef] [PubMed]
- Sayed, E.T.; Wilberforce, T.; Elsaid, K.; Hussein-Rabaia, M.K.; Abdelkareem, M.A.; Chae, K.-J.; Olabi, A.G.A. Critical review on environmental impacts of renewable energy systems and mitigation strategies: Wind, hydro, biomass and geothermal. Sci. Total Environ. 2021, 766, 144505. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues da Silva, M.; Sánchez Bragagnolo, F.; Carneiro, R.L.; de Oliveira Carvalho Pereira, I.; Aquino Ribeiro, J.A.; Martins Rodrigues, C.; Jelley, R.E.; Fedrizzi, B.; Soleo Funari, C. Metabolite characterization of fifteen by-products of the coffee production chain: From farm to factory. Food Chem. 2022, 369, 130753. [Google Scholar] [CrossRef]
- Domínguez de María, P.; Guajardo, N. Biocatalytic valorization of furans: Opportunities for inherently unstable substrates. ChemSusChem 2017, 10, 4123–4134. [Google Scholar] [CrossRef]
- Domínguez de María, P.; Grande, P.M.; Leitner, W. Current trends in pretreatment and fractionation of lignocellulose as reflected in industrial patents activities. Chem. Ing. Tech. 2015, 87, 1686–1695. [Google Scholar] [CrossRef]
- Huang, K.; Luo, J.; Wu, Y.; Xu, Y. β-Factor-based separation characteristics of bio-derived chemicals present in lignocellulosic hydrolyzates using vacuum distillation. ACS Sustain. Chem. Eng. 2019, 7, 2406–2424. [Google Scholar] [CrossRef]
- Rout, P.K.; Nannaware, A.D.; Prakash, O.; Kalra, A.; Rajasekharam, R. Synthesis of hydroxymethylfurfural from cellulose using green processes: A promising biochemical and biofuel feedstock. Chem. Eng. Sci. 2016, 142, 318–346. [Google Scholar] [CrossRef]
- Wiermans, L.; Hofzumahaus, S.; Schotten, C.; Weigan, L.; Schallmey, M.; Schallmey, A.; Domínguez de María, P. Transesterifications and Peracid-Assisted Oxidations in Aqueous Media Catalyzed by Mycobacterium smegmatis Acyl Transferase. ChemCatChem 2013, 5, 3719–3724. [Google Scholar] [CrossRef]
- Guo, W.; Kortenbach, T.; Qi, W.; Hensen, E.; Heeres, H.J.; Yue, J. Selective tandem catalysis for the synthesis of 5-hydroxymethylfurfural from glucose over in-situ phosphate titania catalysts: Insights into structure, bi-functionality, and performance in flow microreactors. Appl. Catal. B 2022, 301, 120800. [Google Scholar] [CrossRef]
- Razayan, A.; Wang, K.; Nie, R.; Lu, T.; Wang, J.; Zhang, Y.; Xu, C.C. Synthesis of bifunctional tin-based silica–carbon catalysts, Sn/KIT-1/C, with tunable acid sites for the catalytic transformation of glucose into 5-hydroxymethylfurfural. Chem. Eng. J. 2022, 429, 132261. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Nezafat, Z.; Momenbeik, F.Y.; Orooji, Y. Polystyrene immobilized Brønsted acid ionic liquid as an efficient and recyclable catalyst for the synthesis of 5-hydroxymethylfurfural from fructose. J. Mol. Liq. 2022, 345, 117811. [Google Scholar] [CrossRef]
- Dedes, G.; Karnaouri, A.; Marianou, A.A.; Kalogiannis, K.G.; Michailof, C.M.; Lappas, A.A.; Topakas, E. Conversion of organosolv pretreated hardwood biomass into 5-hydroxymethylfurfural (HMF) by combining enzymatic hydrolysis and isomerization with homogeneous catalysis. Biotechnol. Biofuels 2021, 14, 172. [Google Scholar] [CrossRef] [PubMed]
- Barman, S.; Chakraborty, R. Sustainable HMF synthesis from waste cooked rice water using fly-ash based Al2SiO5 supported nano-photocatalyst under halogen-ultrasound synergistic-energy: LCA and DFT based simulation. J. Environ. Chem. Eng. 2021, 9, 10673. [Google Scholar] [CrossRef]
- Krystof, M.; Pérez-Sánchez, M.; Domínguez de María, P. Lipase-Catalyzed (Trans)esterification of 5-Hydroxy-methyl furfural and Separation from HMF Esters using Deep-Eutectic Solvents. ChemSusChem 2013, 6, 630–634. [Google Scholar] [CrossRef]
- Qin, Y.-Z.; Zong, M.-H.; Lou, W.-Y.; Li, N. Biocatalytic Upgrading of 5-Hydroxymethylfurfural (HMF) with Levulinic Acid to HMF Levulinate in Biomass-Derived Solvents. ACS Sustain. Chem. Eng. 2016, 4, 4050–4054. [Google Scholar] [CrossRef]
- Heo, J.B.; Lee, Y.-S.; Chung, C.-H. Conversion of inulin-rich raw plant biomass to 2,5-furan dicarboxylic acid (FDCA): Progress and challenges towards biorenewable plastics. Biotechnol. Adv. 2021, 53, 107838. [Google Scholar] [CrossRef]
- Hu, D.; Zhang, M.; Xu, H.; Wang, Y.; Yan, K. Recent advance on the catalytic system for efficient production of biomass-derived 5-hydroxymethylfurfural. Renew. Sustain. Energy Rev. 2021, 147, 111253. [Google Scholar] [CrossRef]
- Eid, N.; Ameduri, B.; Boutevin, B. Synthesis, and Properties of Furan Derivatives for Epoxy Resins. ACS Sustain. Chem. Eng. 2021, 9, 8018–8031. [Google Scholar] [CrossRef]
- Gruter, G.J.M. Furanix Technologies BV, Assignee. 5-(substituted methyl) 2-methylfuran. United States Patents US 20100212218A1, 26 August 2010. [Google Scholar]
- Gamayurova, V.S.; Zinoveva, M.E.; Shnaider, K.L.; Davletshina, G.A. Lipases in esterifications: A review. Catal. Ind. 2021, 13, 58–72. [Google Scholar] [CrossRef]
- Patti, A.; Sanfilippo, C. Stereoselective reactions catalyzed by lipases. Int. J. Mol. Sci. 2022, 23, 2675. [Google Scholar] [CrossRef] [PubMed]
- Salgado, C.A.; dos Santos, C.I.A.; Dantas Vanetti, M.C. Microbial lipases: Propitious biocatalysts for the food industry. Food Biosci. 2022, 45, 101509. [Google Scholar] [CrossRef]
- Remonatto, D.; Miotti, R.H.; Monti, R.; Bassan, J.C.; Veloso de Paula, A. Applications of immobilized lipases in enzymatic reactors: A review. Process Biochem. 2022, 114, 1–20. [Google Scholar] [CrossRef]
- Fenton, H.J.H.; Gostling, M. LXXXV.-Derivatives of methylfurfural. J. Chem. Soc. 1901, 79, 807–816. [Google Scholar] [CrossRef] [Green Version]
- Mäki-Arvela, P.; Salminen, E.; Riittonen, T.; Virtanen, P.; Kumar, N.; Mikkola, J. The Challenge of efficient Synthesis of biofuels from lignocellulose for future renewable transportation fuels. Int. J. Chem. Eng. 2012, 2012, 674761. [Google Scholar] [CrossRef] [Green Version]
- Weerathunga, H.; Sarina, S.; Zhu, H.; Waclawik, E. Oxidative Esterification of 5-Hydroxymethylfurfural into Dimethyl 2,5-Furandicarboxylate Using Gamma Alumina-Supported Gold Nanoparticles. ACS Omega 2021, 6, 4740–4748. [Google Scholar] [CrossRef]
- Hu, L.; He, A.; Liu, X.; Xia, J.; Xu, J.; Zhou, S.; Xu, J. Biocatalytic transformations of 5-hydroxymethylfurfural into high-value derivatives: Recent advances and future aspects. ACS Sustain. Chem. Eng. 2018, 6, 15915–15935. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, Z.; Xu, X. Enzymatic lipophilisation of phenolic acids through esterification with fatty alcohols in organic solvents. Food Chem. 2012, 132, 1311–1315. [Google Scholar] [CrossRef]
- Changa, S.-W.; Yanga, C.-J.; Chena, F.-Y.; Akohb, C.C.; Shieh, C.-J. Optimized synthesis of lipase-catalyzed l-ascorbyl laurate by Novozym® 435. J. Mol. Catal. B Enzym. 2009, 56, 7–12. [Google Scholar] [CrossRef]
- Wafti, N.S.A.; Yunus, R.; Lau, H.L.N.; Yaw, T.C.S.; Aziz, S.A. Immobilized lipase-catalyzed transesterifcation for synthesis of biolubricant from palm oil methyl ester and trimethylolpropane. Bioprocess Biosyst. Eng. 2021, 44, 2429. [Google Scholar] [CrossRef]
- Bernal, C.; Guzman, F.; Illanes, A.; Wilson, L. Selective and eco-friendly synthesis of lipoaminoacid-based surfactants for food, using immobilized lipase and protease biocatalysts. Food Chem. 2018, 239, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Razak, N.; Annuar, M. Enzymatic Synthesis of Flavonoid Ester: Elucidation of Its Kinetic Mechanism and Equilibrium Thermodynamic Behavior. Ind. Eng. Chem. Res. 2015, 54, 5604–5612. [Google Scholar] [CrossRef]
- Lue, B.; Guo, Z.; Glasius, M. Scalable Preparation of High Purity Rutin Fatty Acid Esters. J. Am. Oil Chem. Soc. 2010, 87, 55–61. [Google Scholar] [CrossRef]
- Vuillemin, M.E.; Husson, E.; Laclef, S.; Jamali, A.; Lambertyn, V.; Pilard, S.; Cailleu, D.; Sarazin, C. Improving the environmental compatibility of enzymatic synthesis of sugar-based surfactants using green reaction media. Process Biochem. 2022, 117, 30–41. [Google Scholar] [CrossRef]
- da Silva Corrêa, L.; Henriques, R.; Rios, J.; Lerin, L.; Oliveira, D.; Furigo, A. Lipase-Catalyzed Esterification of Geraniol and Citronellol for the Synthesis of Terpenic Esters. Appl. Biochem. Biotechnol. 2020, 190, 574–583. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandão, G.C.; Da Silva, E.G.P.; Portugal, L.A.; dos Reis, P.S.; Souza, A.S.; et al. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef]






| Biocatalysts | Activity (U/g) |
|---|---|
| Novozym 435® | 74 |
| Immobilized TL lipase | 39 |
| Conditions | Values |
|---|---|
| Highest conversion (75%) | 40 °C, 65 mM HMF, and 16 U of Novozym 435® |
| Most significant variables | Concentration of HMF, temperature, activity of biocatalyst, temperature squared, squared concentration, and squared activity |
| Optimal value predicted by Statgraphics 19® (experimentally validated in this work) | 76% conversion, 40 °C, 65 mM HMF, and 16 U of Novozym 435® |
| Conditions | Values |
|---|---|
| Highest conversion (78%) | 50 °C, 30 mM HMF, and 16 U of biocatalytic activity |
| Most significant variables | Temperature, concentration of HMF, squared concentration, squared temperature, and activity and temperature interaction. |
| Optimal value predicted by Statgraphics 19® (experimentally validated in this work) | 80.6% of conversion, 30 mM of HMF, 50 °C, and 22 U of biocatalytic activity. |
| Novozym 435® | Immobilized TL Lipase | |||
|---|---|---|---|---|
| Temperature (°C) | Km (mM) | VMax (mM/min) | Km (mM) | VMax (mM/min) |
| 50 | 69.2 | 0.76 | 461.5 | 0.5 |
| 40 | 34 | 0.43 | 123.5 | 0.18 |
| 30 | 45 | 0.46 | 103.1 | 0.18 |
| Factors | Units | Levels | ||
|---|---|---|---|---|
| − | 0 | + | ||
| : Temperature | °C | 30 | 40 | 50 |
| : Concentration of HMF | mM | 30 | 65 | 100 |
| : Activity of biocatalyst | U | 10 | 16 | 22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uribe, J.; Lienqueo, M.E.; Guajardo, N. Optimization and Determination of Kinetic Parameters of the Synthesis of 5-Lauryl-hydroxymethylfurfural Catalyzed by Lipases. Catalysts 2023, 13, 19. https://doi.org/10.3390/catal13010019
Uribe J, Lienqueo ME, Guajardo N. Optimization and Determination of Kinetic Parameters of the Synthesis of 5-Lauryl-hydroxymethylfurfural Catalyzed by Lipases. Catalysts. 2023; 13(1):19. https://doi.org/10.3390/catal13010019
Chicago/Turabian StyleUribe, Jorge, María Elena Lienqueo, and Nadia Guajardo. 2023. "Optimization and Determination of Kinetic Parameters of the Synthesis of 5-Lauryl-hydroxymethylfurfural Catalyzed by Lipases" Catalysts 13, no. 1: 19. https://doi.org/10.3390/catal13010019