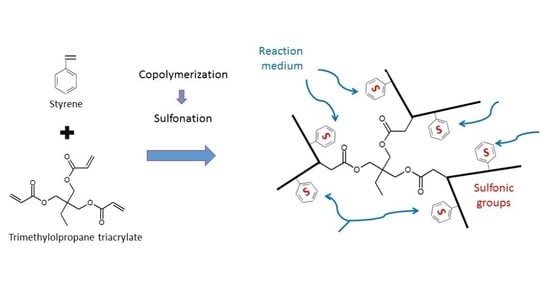
Synthesis of Vinyl–Trivinyl Acidic Resins for Application in Catalysis: Statistical Study and Site Accessibility Assessment
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Synthesis and Characterization
2.2. Catalytic Tests
2.3. Kinetic Model and Accessibility Assessment
3. Methods
3.1. Factorial Design
3.2. Suspension Copolymerization
3.3. Sulfonation Reaction
3.4. Ion-Exchange Capacity
3.5. Glycerol Acetylation
3.6. Swelling Index
3.7. Catalytic Efficiency
3.8. Kinetic Modeling
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Razzaq, R.; Shah, K.H.; Fahad, M.; Naeem, A.; Sherazi, T.A. Adsorption potential of macroporous Amberlyst-15 for Cd(II) removal from aqueous solutions Adsorption potential of macroporous Amberlyst-15 for Cd(II) removal from aqueous solutions. Mater. Res. Express 2020, 7, 025509. [Google Scholar] [CrossRef]
- Ramírez, E.; Bringu, R.; Fit, C.; Tejero, J.; Cunill, F. Assessment of ion exchange resins as catalysts for the direct transformation of fructose into butyl levulinate. Appl. Catal. A Gen. 2021, 612, 117988. [Google Scholar] [CrossRef]
- Silva, M.V.C.; Souza, A.B.; De Castro, H.F.; Aguiar, L.G.; De Oliveira, P.C.; De Freitas, L. Synthesis of 2-ethylhexyl oleate catalyzed by Candida antarctica lipase immobilized on a magnetic polymer support in continuous flow. Bioprocess Biosyst. Eng. 2020, 43, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, L.G.; Godoy, W.M.; Nápolis, L.; Faria, R.P.V.; Rodrigues, A.E. Modeling the Effect of Cross-Link Density on Resins Catalytic Activities. Ind. Eng. Chem. Res. 2021, 60, 6101–6110. [Google Scholar] [CrossRef]
- Pariente, S.; Tanchoux, N.; Fajula, F. Etherification of glycerol with ethanol over solid acid catalysts. Green Chem. 2009, 11, 1256–1261. [Google Scholar] [CrossRef]
- Jagadeeshbabu, P.E.; Sandesh, K.; Saidutta, M.B. Kinetics of esterification of acetic acid with methanol in the presence of ion exchange resin catalysts. Ind. Eng. Chem. Res. 2011, 50, 7155–7160. [Google Scholar] [CrossRef]
- Carpegiani, J.A.; Godoy, W.M.; Guimarães, D.H.P.; Aguiar, L.G. Glycerol acetylation catalyzed by an acidic styrene-co-dimethacrylate resin: Experiments and kinetic modeling. React. Kinet. Mech. Catal. 2020, 130, 447–461. [Google Scholar] [CrossRef]
- Güemez, M.B.; Requies, J.; Agirre, I.; Arias, P.L.; Barrio, V.L.; Cambra, J.F. Acetalization reaction between glycerol and n-butyraldehyde using an acidic ion exchange resin. Kinetic modelling. Chem. Eng. J. 2013, 228, 300–307. [Google Scholar] [CrossRef]
- Orjuela, A.; Yanez, A.J.; Santhanakrishnan, A.; Lira, C.T.; Miller, D.J. Kinetics of mixed succinic acid/acetic acid esterification with Amberlyst 70 ion exchange resin as catalyst. Chem. Eng. J. 2012, 188, 98–107. [Google Scholar] [CrossRef]
- Edebali, S.; Pehlivan, E. Evaluation of Amberlite IRA96 and Dowex 1×8 ion-exchange resins for the removal of Cr(VI) from aqueous solution. Chem. Eng. J. 2010, 161, 161–166. [Google Scholar] [CrossRef]
- Badia, J.H.; Fité, C.; Bringué, R.; Iborra, M.; Cunill, F. Catalytic Activity and Accessibility of Acidic Ion-Exchange Resins in Liquid Phase Etherification Reactions. Top. Catal. 2015, 58, 919–932. [Google Scholar] [CrossRef] [Green Version]
- Van de Steene, E.; De Clercq, J.; Thybaut, J.W. Ion-exchange resin catalyzed transesterification of ethyl acetate with methanol: Gel versus macroporous resins. Chem. Eng. J. 2014, 242, 170–179. [Google Scholar] [CrossRef]
- Chandane, V.S.; Rathod, A.P.; Wasewar, K.L.; Sonawane, S.S. Esterification of propionic acid with isopropyl alcohol over ion exchange resins: Optimization and kinetics. Korean J. Chem. Eng. 2017, 34, 249–258. [Google Scholar] [CrossRef]
- Serrano, D.P.; Melero, J.A.; Morales, G.; Iglesias, J.; Pizarro, P. Progress in the design of zeolite catalysts for biomass conversion into biofuels and bio-based chemicals. Catal. Rev.—Sci. Eng. 2018, 60, 1–70. [Google Scholar] [CrossRef]
- Mane, S.; Ponrathnam, S.; Chavan, N. Effect of Chemical Crosslinking on Properties of Polymer Microbeads: A Review. Can. Chem. Trans. 2016, 3, 473–485. [Google Scholar] [CrossRef]
- Tejero, M.A.; Ramírez, E.; Fité, C.; Tejero, J.; Cunill, F. Esterification of levulinic acid with butanol over ion exchange resins. Appl. Catal. A Gen. 2016, 517, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Theodoro, T.R.; Dias, J.R.; Penariol, J.L.; Moura, J.O.V.; Aguiar, L.G. Sulfonated poly (styrene-co-ethylene glycol dimethacrylate) with attractive ion exchange capacity. Polym. Adv. Technol. 2018, 29, 2759–2765. [Google Scholar] [CrossRef]
- Carpegiani, J.A. Study of Mathematical Modeling of Glycerol Acetylation with Different Catalysts. Master Thesis, Escola de Engenharia de Lorena, Universidade de São Paulo, Lorena, SP, Brazil, 2020. [Google Scholar]
- Silva, V.F.L.; Penariol, J.L.; Dias, J.R.; Theodoro, T.R.; Carpegiani, J.A.; Aguiar, L.G. Sulfonated Styrene—Dimethacrylate Resins with Improved Catalytic Activity. Kinet. Catal. 2019, 60, 654–660. [Google Scholar] [CrossRef]
- Kong, X.Z.; Gu, X.L.; Zhu, X.; Zhang, L. Precipitation polymerization in ethanol and ethanol/water to prepare uniform microspheres of poly(TMPTA-styrene). Macromol. Rapid Commun. 2009, 30, 909–914. [Google Scholar] [CrossRef]
- Karam, H.J.; Tien, L.; Dour, T.; Company, C. Analysis of Swelling of Crosslinked Rubber Gel with Occlusions. J. Appl. Polym. Sci. 1988, 30, 1969–1988. [Google Scholar] [CrossRef]
- Ho, L.-H.; Feng, S.-Y.; Yen, T.-M. A New Methodology for Customer Satisfaction Analysis: Taguchi’s Signal-to-Noise Ratio Approach. J. Serv. Sci. Manag. 2014, 07, 235–244. [Google Scholar] [CrossRef] [Green Version]
- Wolde-rufael, Y.; Idowu, S. Income distribution and CO2 emission: A comparative analysis for China and India. Renew. Sustain. Energy Rev. 2017, 74, 1336–1345. [Google Scholar] [CrossRef]
- Phadke, M.S.; Kackar, R.N.; Speeney, D.V.; Grieco, M.J. Off-line quality control in integrated circuit fabrication using experimental design. Bell Syst. Tech. J. 1983, 62, 1273–1309. [Google Scholar] [CrossRef]
- Okay, O. Macroporous copolymer networks. Prog. Polym. Sci. 2000, 25, 711–779. [Google Scholar] [CrossRef]
- Jerábek, K. Characterization of Swollen Polymer Gels Using Size Exclusion Chromatography. Anal. Chem. 1985, 57, 1598–1602. [Google Scholar] [CrossRef]
- NanoComposix Molecular Weight to Size Calculator. Available online: https://nanocomposix.com/pages/molecular-weight-to-size-calculator (accessed on 18 October 2022).
- Bringué, R.; Ramírez, E.; Tejero, J.; Cunill, F. Esteri fi cation of furfuryl alcohol to butyl levulinate over ion-exchange resins. Fuel 2019, 257, 116010. [Google Scholar] [CrossRef]
- Faria Neto, A.; Costa, A.F.B.; de Lima, M.F. Use of Factorial Designs and the Response Surface Methodology to Optimize a Heat Staking Process. Exp. Tech. 2018, 42, 319–331. [Google Scholar] [CrossRef] [Green Version]
- Godoy, W.M. Synthesis and Characterization of Polymerics Catalysts from Styrene Base, and Application in the Glycerol Acetylation. Master Thesis, University of São Paulo, Lorena, SP, Brazil, 2021. [Google Scholar]
- Godoy, W.M.; Carpegiani, J.A.; Pereira, F.M.; Guimarães, D.H.P.; Aguiar, L.G. Kinetic modeling of glycerol acetylation catalyzed by styrene–divinylbenzene and styrene-trimethylolpropane triacrylate sulfonated resins. React. Kinet. Mech. Catal. 2022, 135, 233–245. [Google Scholar] [CrossRef]
- Godoy, W.; Castro, G.; Nápolis, L.; Carpegiani, J.; Guimarães, D. Synthesis of Sulfonated Poly [Styrene-co-(Trimethylolpropane Triacrylate)] and Application in the Catalysis of Glycerol Acetylation. Macromol. Symp. 2020, 394, 1900169. [Google Scholar] [CrossRef]
- Banu, I.; Bumbac, G.; Bombos, D.; Velea, S.; Gălan, A.-M.; Bozga, G. Glycerol acetylation with acetic acid over Purolite CT-275. Product yields and process kinetics. Renew. Energy 2020, 148, 548–557. [Google Scholar] [CrossRef]
- Gomes, J.T.S.; Santos, J.H.S.; Abreu, C.A.M.; Medeiros, E.B.M.; Coelho, L.C.D.; Faria, R.P.V.; Rodrigues, A.E.; Lima Filho, N.M. Development and validation of analytical method for mono, di and triacetin analysis by HPLC/UV–Vis/DAD detection with 13C NMR identification. Results Chem. 2020, 2, 100063. [Google Scholar] [CrossRef]
- Perez, F.M.; Gatti, M.N.; Nichio, N.N.; Pompeo, F. Results in Engineering Bio-additives from glycerol acetylation with acetic acid: Chemical equilibrium model. Results Eng. 2022, 15, 100502. [Google Scholar] [CrossRef]







| Run | Factor | Response | ||
|---|---|---|---|---|
| YTMPTA | TTMPTA | PY (%) | SY (%) | |
| 1 | 0.10 | 30 | 77 | 89 |
| 2 | 0.10 | 150 | 85 | 82 |
| 3 | 0.10 | 270 | 88 | 82 |
| 4 | 0.06 | 30 | 73 | 85 |
| 5 | 0.06 | 150 | 90 | 93 |
| 6 | 0.06 | 270 | 92 | 80 |
| 7 | 0.02 | 30 | 73 | 83 |
| 8 | 0.02 | 150 | 74 | 82 |
| 9 | 0.02 | 270 | 57 | 73 |
| 10 | 0.06 | 150 | 93 | 91 |
| 11 | 0.06 | 150 | 100 | 93 |
| Resin | YTMPTA | TTMPTA (min) | IEC (mmol g−1) | Sw |
|---|---|---|---|---|
| S1 | 0.10 | 30 | 0.585 | 1.177 |
| S2 | 0.10 | 150 | 0.540 | 1.344 |
| S3 | 0.10 | 270 | 0.540 | 1.463 |
| S4 | 0.06 | 30 | 0.418 | 1.214 |
| S5 | 0.06 | 150 | 0.641 | 1.440 |
| S6 | 0.06 | 270 | 0.537 | 1.355 |
| S7 | 0.02 | 30 | 0.357 | 1.244 |
| S8 | 0.02 | 150 | 0.413 | 1.104 |
| S9 | 0.02 | 270 | 0.411 | 1.578 |
| Resin | YTMPTA | TTMPTA (min) | IEC (mmol g−1) | XG (%) | Ef |
|---|---|---|---|---|---|
| S1 | 0.10 | 30 | 0.585 | 86 | 98 |
| S2 | 0.10 | 150 | 0.540 | 66 | 90 |
| S3 | 0.10 | 270 | 0.540 | 63 | 87 |
| S4 | 0.06 | 30 | 0.418 | 78 | 173 |
| S5 | 0.06 | 150 | 0.641 | 72 | 83 |
| S6 | 0.06 | 270 | 0.537 | 88 | 181 |
| S7 | 0.02 | 30 | 0.357 | 64 | 147 |
| S8 | 0.02 | 150 | 0.413 | 34 | 84 |
| S9 | 0.02 | 270 | 0.411 | 90 | 206 |
| Run | Resin Formulation | (L2 mol−2 min−1) | |||
|---|---|---|---|---|---|
| TMPTA Content (%) | TMPTA Feed Time (min) | ||||
| S1 | 10 | 30 | 0.1100 | 0.0550 | 0.0041 |
| S2 | 10 | 150 | 0.0800 | 0.0210 | 0.0030 |
| S3 | 10 | 270 | 0.0650 | 0.0150 | 0.0022 |
| S4 | 6 | 30 | 0.1500 | 0.0390 | 0.0045 |
| S5 | 6 | 150 | 0.1240 | 0.0380 | 0.0034 |
| S6 | 6 | 270 | 0.1000 | 0.0570 | 0.0048 |
| S7 | 2 | 30 | 0.1200 | 0.0270 | 0.0031 |
| S8 | 2 | 150 | 0.0760 | 0.0180 | 0.0039 |
| S9 | 2 | 270 | 0.2100 | 0.0890 | 0.0061 |
| Level | Factors | |
|---|---|---|
| YTMPTA | TTMPTA (min) | |
| 1 | 0.10 | 30 |
| 2 | 0.06 | 150 |
| 3 | 0.02 | 270 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godoy, W.M.; Aguiar, L.G.; Graça, N.A.B.S.; Rodrigues, A.E. Synthesis of Vinyl–Trivinyl Acidic Resins for Application in Catalysis: Statistical Study and Site Accessibility Assessment. Catalysts 2023, 13, 181. https://doi.org/10.3390/catal13010181
Godoy WM, Aguiar LG, Graça NABS, Rodrigues AE. Synthesis of Vinyl–Trivinyl Acidic Resins for Application in Catalysis: Statistical Study and Site Accessibility Assessment. Catalysts. 2023; 13(1):181. https://doi.org/10.3390/catal13010181
Chicago/Turabian StyleGodoy, William M., Leandro G. Aguiar, Nuno A. B. S. Graça, and Alírio E. Rodrigues. 2023. "Synthesis of Vinyl–Trivinyl Acidic Resins for Application in Catalysis: Statistical Study and Site Accessibility Assessment" Catalysts 13, no. 1: 181. https://doi.org/10.3390/catal13010181