Applications of Fluorescent Carbon Dots as Photocatalysts: A Review
Abstract
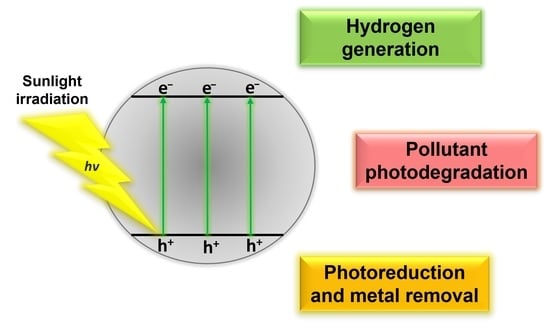
:1. Introduction
2. Application as Sole Photocatalysts
2.1. Hydrogen Generation via Water Splitting
2.2. Photocatalytic Degradation of Organic Pollutants
2.3. Photocatalytic Photoreduction Reaction and Metal Removal
3. Limitations and Future Challenges
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ajayan, P.M. Nanotubes from Carbon. Chem. Rev. 1999, 99, 1787–1800. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ratinac, K.R.; Ringer, S.P.; Thordarson, P.; Gooding, J.J.; Braet, F. Carbon nanomaterials in biosensors: Should you use nanotubes or graphene? Angew. Chem. Int. Ed. Engl. 2010, 49, 2114–2138. [Google Scholar] [CrossRef] [PubMed]
- De Jong, K.P.; Geus, J.W. Carbon Nanofibers: Catalytic Synthesis and Applications. Catal. Rev. 2007, 42, 481–510. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, X.; Qian, Y. Synthetic methodologies for carbon nanomaterials. Adv. Mater. 2010, 22, 1963–1966. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.N.; Sood, A.K.; Subrahmanyam, K.S.; Govindaraj, A. Graphene: The new two-dimensional nanomaterial. Angew. Chem. Int. Ed. Engl. 2009, 48, 7752–7777. [Google Scholar] [CrossRef]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Sun, Y.P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef]
- da Silva, J.C.G.E.; Gonçalves, H.M.R. Analytical and bioanalytical applications of carbon dots. TrAC Trends. Anal. Chem. 2011, 30, 1327–1336. [Google Scholar] [CrossRef]
- Hola, K.; Zhang, Y.; Wang, Y.; Giannelis, E.P.; Zboril, R.; Rogach, A.L. Carbon dots—Emerging light emitters for bioimaging, cancer therapy and optoelectronics. Nano Today 2014, 9, 590–603. [Google Scholar] [CrossRef]
- Baker, S.N.; Baker, G.A. Luminescent carbon nanodots: Emergent nanolights. Angew. Chem. Int. Ed. Engl. 2010, 49, 6726–6744. [Google Scholar] [CrossRef] [PubMed]
- Demchenko, A.P.; Dekaliuk, M.O. Novel fluorescent carbonic nanomaterials for sensing and imaging. Methods Appl. Fluoresc. 2013, 1, 042001. [Google Scholar] [CrossRef] [PubMed]
- Crista, D.M.A.; Esteves da Silva, J.C.G.; Pinto da Silva, L. Evaluation of Different Bottom-up Routes for the Fabrication of Carbon Dots. Nanomaterials 2020, 10, 1316. [Google Scholar] [CrossRef]
- Tan, M.; Zhang, L.; Tang, R.; Song, X.; Li, Y.; Wu, H.; Wang, Y.; Lv, G.; Liu, W.; Ma, X. Enhanced photoluminescence and characterization of multicolor carbon dots using plant soot as a carbon source. Talanta 2013, 115, 950–956. [Google Scholar] [CrossRef]
- Wang, J.; Sahu, S.; Sonkar, S.K.; Tackett Ii, K.N.; Sun, K.W.; Liu, Y.; Maimaiti, H.; Anilkumar, P.; Sun, Y.-P. Versatility with carbon dots—From overcooked BBQ to brightly fluorescent agents and photocatalysts. RSC Adv. 2013, 3, 15604–15607. [Google Scholar] [CrossRef]
- Lu, J.; Yang, J.X.; Wang, J.; Lim, A.; Wang, S.; Loh, K.P. One-pot synthesis of fluorescent carbon nanoribbons, nanoparticles, and graphene by the exfoliation of graphite in ionic liquids. ACS Nano 2009, 3, 2367–2375. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, H.U.; Park, E.S.; Lee, S.C.; Lee, J.W.; Jeong, S.W.; Kim, C.H.; Lee, Y.C.; Huh, Y.S.; Lee, J. Photoluminescent green carbon nanodots from food-waste-derived sources: Large-scale synthesis, properties, and biomedical applications. ACS Appl. Mater. Interfaces 2014, 6, 3365–3370. [Google Scholar] [CrossRef]
- Sendao, R.M.S.; Crista, D.M.A.; Afonso, A.C.P.; Martinez de Yuso, M.D.V.; Algarra, M.; Esteves da Silva, J.C.G.; Pinto da Silva, L. Insight into the hybrid luminescence showed by carbon dots and molecular fluorophores in solution. Phys. Chem. Chem. Phys. 2019, 21, 20919–20926. [Google Scholar] [CrossRef]
- Ma, C.B.; Zhu, Z.T.; Wang, H.X.; Huang, X.; Zhang, X.; Qi, X.; Zhang, H.L.; Zhu, Y.; Deng, X.; Peng, Y.; et al. A general solid-state synthesis of chemically-doped fluorescent graphene quantum dots for bioimaging and optoelectronic applications. Nanoscale 2015, 7, 10162–10169. [Google Scholar] [CrossRef] [Green Version]
- Sendão, R.; Yuso, M.d.V.M.d.; Algarra, M.; Esteves da Silva, J.C.G.; Pinto da Silva, L. Comparative life cycle assessment of bottom-up synthesis routes for carbon dots derived from citric acid and urea. J. Clean. Prod. 2020, 254, 120080. [Google Scholar] [CrossRef]
- Liu, R.; Wu, D.; Liu, S.; Koynov, K.; Knoll, W.; Li, Q. An aqueous route to multicolor photoluminescent carbon dots using silica spheres as carriers. Angew. Chem. Int. Ed. Engl. 2009, 48, 4598–4601. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Chen, F.; Lagally, M.G.; Denes, F.S. New strategy for synthesis and functionalization of carbon nanoparticles. Langmuir 2010, 26, 1991–1995. [Google Scholar] [CrossRef] [PubMed]
- Long, C.; Jiang, Z.; Shangguan, J.; Qing, T.; Zhang, P.; Feng, B. Applications of carbon dots in environmental pollution control: A review. Chem. Eng. J. 2021, 406, 126848. [Google Scholar] [CrossRef]
- Wang, R.; Lu, K.-Q.; Tang, Z.-R.; Xu, Y.-J. Recent progress in carbon quantum dots: Synthesis, properties and applications in photocatalysis. J. Mater. Chem. A 2017, 5, 3717–3734. [Google Scholar] [CrossRef]
- Peng, Z.; Han, X.; Li, S.; Al-Youbi, A.O.; Bashammakh, A.S.; El-Shahawi, M.S.; Leblanc, R.M. Carbon dots: Biomacromolecule interaction, bioimaging and nanomedicine. Coord. Chem. Rev. 2017, 343, 256–277. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef]
- Vale, N.; Silva, S.; Duarte, D.; Crista, D.M.A.; Pinto da Silva, L.; Esteves da Silva, J.C.G. Normal breast epithelial MCF-10A cells to evaluate the safety of carbon dots. RSC Med. Chem. 2021, 12, 245–253. [Google Scholar] [CrossRef]
- Wang, K.; Gao, Z.; Gao, G.; Wo, Y.; Wang, Y.; Shen, G.; Cui, D. Systematic safety evaluation on photoluminescent carbon dots. Nanoscale Res. Lett. 2013, 8, 122. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Chen, M.; Chen, G.; Zou, W.; Zhao, Y.; Zhang, H.; Zhao, Q. Visible-Ultraviolet Upconversion Carbon Quantum Dots for Enhancement of the Photocatalytic Activity of Titanium Dioxide. ACS Omega 2021, 6, 4247–4254. [Google Scholar] [CrossRef]
- Dager, A.; Uchida, T.; Maekawa, T.; Tachibana, M. Synthesis and characterization of Mono-disperse Carbon Quantum Dots from Fennel Seeds: Photoluminescence analysis using Machine Learning. Sci. Rep. 2019, 9, 14004. [Google Scholar] [CrossRef]
- Ladomenou, K.; Landrou, G.; Charalambidis, G.; Nikoloudakis, E.; Coutsolelos, A.G. Carbon dots for photocatalytic H2 production in aqueous media with molecular Co catalysts. Sustain. Energy Fuels 2021, 5, 449–458. [Google Scholar] [CrossRef]
- Yang, P.; Zhao, J.; Wang, J.; Cao, B.; Li, L.; Zhu, Z. Light-induced synthesis of photoluminescent carbon nanoparticles for Fe3+ sensing and photocatalytic hydrogen evolution. J. Mater. Chem. A 2015, 3, 136–138. [Google Scholar] [CrossRef]
- Bhunia, S.; Ghorai, N.; Burai, S.; Purkayastha, P.; Ghosh, H.N.; Mondal, S. Unraveling the Carrier Dynamics and Photocatalytic Pathway in Carbon Dots and Pollutants of Wastewater System. J. Phys. Chem. C 2021, 125, 27252–27259. [Google Scholar] [CrossRef]
- Zhou, Y.; Zahran, E.M.; Quiroga, B.A.; Perez, J.; Mintz, K.J.; Peng, Z.; Liyanage, P.Y.; Pandey, R.R.; Chusuei, C.C.; Leblanc, R.M. Size-Dependent Photocatalytic Activity of Carbon Dots with Surface-State Determined Photoluminescence. Appl. Catal. B 2019, 248, 157–166. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Liu, H.; Liu, C.; Wan, Y.; Long, Y.; Cai, Z. Recent Advances and Applications of Semiconductor Photocatalytic Technology. Appl. Sci. 2019, 9, 2489. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zheng, T.; Li, X.; Xu, J.; Zeng, H. Progress of Carbon Quantum Dots in Photocatalysis Applications. Part. Part. Syst. Charact. 2016, 33, 457–472. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 2002, 95, 735–758. [Google Scholar] [CrossRef]
- Yang, K.D.; Ha, Y.; Sim, U.; An, J.; Lee, C.W.; Jin, K.; Kim, Y.; Park, J.; Hong, J.S.; Lee, J.H.; et al. Graphene Quantum Sheet Catalyzed Silicon Photocathode for Selective CO2Conversion to CO. Adv. Funct. Mater. 2016, 26, 233–242. [Google Scholar] [CrossRef]
- Gao, G.; Jiao, Y.; Ma, F.; Jiao, Y.; Waclawik, E.; Du, A. Carbon nanodot decorated graphitic carbon nitride: New insights into the enhanced photocatalytic water splitting from ab initio studies. Phys. Chem. Chem. Phys. 2015, 17, 31140–31144. [Google Scholar] [CrossRef]
- Yeh, T.F.; Teng, C.Y.; Chen, S.J.; Teng, H. Nitrogen-doped graphene oxide quantum dots as photocatalysts for overall water-splitting under visible light illumination. Adv. Mater. 2014, 26, 3297–3303. [Google Scholar] [CrossRef]
- Qu, D.; Sun, Z.; Zheng, M.; Li, J.; Zhang, Y.; Zhang, G.; Zhao, H.; Liu, X.; Xie, Z. Three Colors Emission from S,N Co-doped Graphene Quantum Dots for Visible Light H2Production and Bioimaging. Adv. Opt. Mater. 2015, 3, 360–367. [Google Scholar] [CrossRef]
- Yu, H.; Shi, R.; Zhao, Y.; Waterhouse, G.I.; Wu, L.Z.; Tung, C.H.; Zhang, T. Smart Utilization of Carbon Dots in Semiconductor Photocatalysis. Adv. Mater. 2016, 28, 9454–9477. [Google Scholar] [CrossRef] [PubMed]
- Chai, N.-N.; Wang, H.-X.; Hu, C.-X.; Wang, Q.; Zhang, H.-L. Well-controlled layer-by-layer assembly of carbon dot/CdS heterojunctions for efficient visible-light-driven photocatalysis. J. Mater. Chem. A 2015, 3, 16613–16620. [Google Scholar] [CrossRef]
- Di, J.; Xia, J.; Ji, M.; Wang, B.; Yin, S.; Zhang, Q.; Chen, Z.; Li, H. Carbon Quantum Dots Modified BiOCl Ultrathin Nanosheets with Enhanced Molecular Oxygen Activation Ability for Broad Spectrum Photocatalytic Properties and Mechanism Insight. ACS Appl. Mater. Interfaces 2015, 7, 20111–20123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, H.; Ming, H.; Li, H.; Zhang, L.; Liu, Y.; Kang, Z. Carbon quantum dots/Ag3PO4 complex photocatalysts with enhanced photocatalytic activity and stability under visible light. J. Mater. Chem. 2012, 22, 10501–10506. [Google Scholar] [CrossRef]
- Martindale, B.C.; Hutton, G.A.; Caputo, C.A.; Reisner, E. Solar hydrogen production using carbon quantum dots and a molecular nickel catalyst. J. Am. Chem. Soc. 2015, 137, 6018–6025. [Google Scholar] [CrossRef]
- Qu, D.; Zheng, M.; Du, P.; Zhou, Y.; Zhang, L.; Li, D.; Tan, H.; Zhao, Z.; Xie, Z.; Sun, Z. Highly luminescent S, N co-doped graphene quantum dots with broad visible absorption bands for visible light photocatalysts. Nanoscale 2013, 5, 12272–12277. [Google Scholar] [CrossRef]
- Yu, H.; Zhao, Y.; Zhou, C.; Shang, L.; Peng, Y.; Cao, Y.; Wu, L.-Z.; Tung, C.-H.; Zhang, T. Carbon quantum dots/TiO2 composites for efficient photocatalytic hydrogen evolution. J. Mater. Chem. A 2014, 2, 3344–3351. [Google Scholar] [CrossRef]
- Yu, X.; Liu, J.; Yu, Y.; Zuo, S.; Li, B. Preparation and visible light photocatalytic activity of carbon quantum dots/TiO2 nanosheet composites. Carbon 2014, 68, 718–724. [Google Scholar] [CrossRef]
- Pan, J.; Sheng, Y.; Zhang, J.; Wei, J.; Huang, P.; Zhang, X.; Feng, B. Preparation of carbon quantum dots/TiO2nanotubes composites and their visible light catalytic applications. J. Mater. Chem. A 2014, 2, 18082–18086. [Google Scholar] [CrossRef]
- Guo, Y.; Yao, P.; Zhu, D.; Gu, C. A novel method for the development of a carbon quantum dot/carbon nitride hybrid photocatalyst that responds to infrared light irradiation. J. Mater. Chem. A 2015, 3, 13189–13192. [Google Scholar] [CrossRef]
- Campalani, C.; Cattaruzza, E.; Zorzi, S.; Vomiero, A.; You, S.; Matthews, L.; Capron, M.; Mondelli, C.; Selva, M.; Perosa, A. Biobased Carbon Dots: From Fish Scales to Photocatalysis. Nanomaterials 2021, 11, 524. [Google Scholar] [CrossRef] [PubMed]
- Bhati, A.; Anand, S.R.; Saini, D.; Gunture; Sonkar, S.K. Sunlight-induced photoreduction of Cr(VI) to Cr(III) in wastewater by nitrogen-phosphorus-doped carbon dots. npj Clean Water 2019, 2, 12. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Li, X.; Luo, M.; Chen, M.; Chen, W.; Yang, P.; Zhou, X. Synthesis of carbon dots with high photocatalytic reactivity by tailoring heteroatom doping. J. Colloid Interface Sci. 2022, 605, 330–341. [Google Scholar] [CrossRef]
- Saini, D.; Kaushik, J.; Garg, A.K.; Dalal, C.; Sonkar, S.K. N, S-codoped Carbon Dots for Nontoxic Cell Imaging and As a Sunlight-Active Photocatalytic Material for the Removal of Chromium. ACS Appl. Bio. Mater. 2020, 3, 3656–3663. [Google Scholar] [CrossRef]
- Kaushal, N.; Sharma, A.L.; Saha, A. Visible LED-based photo-redox properties of sulfur and nitrogen-doped carbon dots designed by solid-state synthesis. Mater. Adv. 2022, 3, 355–361. [Google Scholar] [CrossRef]
- Zhou, Y.; ElMetwally, A.E.; Chen, J.; Shi, W.; Cilingir, E.K.; Walters, B.; Mintz, K.J.; Martin, C.; Ferreira, B.; Zhang, W.; et al. Gel-like carbon dots: A high-performance future photocatalyst. J. Colloid Interface Sci. 2021, 599, 519–532. [Google Scholar] [CrossRef]
- Genc, M.T.; Yanalak, G.; Aksoy, I.; Aslan, E.; Patır, I.H. Green Carbon Dots (GCDs) for Photocatalytic Hydrogen Evolution and Antibacterial Applications. Chem. Sel. 2021, 6, 7317–7322. [Google Scholar] [CrossRef]
- Jana, B.; Reva, Y.; Scharl, T.; Strauss, V.; Cadranel, A.; Guldi, D.M. Carbon Nanodots for All-in-One Photocatalytic Hydrogen Generation. J. Am. Chem. Soc. 2021, 143, 20122–20132. [Google Scholar] [CrossRef]
- Xu, X.; Bao, Z.; Zhou, G.; Zeng, H.; Hu, J. Enriching Photoelectrons via Three Transition Channels in Amino-Conjugated Carbon Quantum Dots to Boost Photocatalytic Hydrogen Generation. ACS Appl. Mater. Interfaces 2016, 8, 14118–14124. [Google Scholar] [CrossRef]
- Saini, D.; Aggarwal, R.; Sonker, A.K.; Sonkar, S.K. Photodegradation of Azo Dyes in Sunlight Promoted by Nitrogen–Sulfur–Phosphorus Codoped Carbon Dots. ACS Appl. Nano Mater. 2021, 4, 9303–9312. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Low, J.; Fang, Y.; Xiao, J.; Chen, X. Engineering heterogeneous semiconductors for solar water splitting. J. Mater. Chem. A 2015, 3, 2485–2534. [Google Scholar] [CrossRef]
- Reddy, C.V.; Reddy, K.R.; Harish, V.V.N.; Shim, J.; Shankar, M.V.; Shetti, N.P.; Aminabhavi, T.M. Metal-organic frameworks (MOFs)-based efficient heterogeneous photocatalysts: Synthesis, properties and its applications in photocatalytic hydrogen generation, CO2 reduction and photodegradation of organic dyes. Int. J. Hydrogen Energy 2020, 45, 7656–7679. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Zhao, J.; Wang, J.; Cui, H.; Li, L.; Zhu, Z. Pure carbon nanodots for excellent photocatalytic hydrogen generation. RSC Adv. 2015, 5, 21332–21335. [Google Scholar] [CrossRef]
- Cao, L.; Sahu, S.; Anilkumar, P.; Bunker, C.E.; Xu, J.; Fernando, K.A.; Wang, P.; Guliants, E.A.; Tackett, K.N., 2nd; Sun, Y.P. Carbon nanoparticles as visible-light photocatalysts for efficient CO2 conversion and beyond. J. Am. Chem. Soc. 2011, 133, 4754–4757. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Ehrat, F.; Urban, P.; Teves, R.; Wyrwich, R.; Doblinger, M.; Feldmann, J.; Urban, A.S.; Stolarczyk, J.K. Effect of nitrogen atom positioning on the trade-off between emissive and photocatalytic properties of carbon dots. Nat. Commun. 2017, 8, 1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlsen, L.; Bruggemann, R.; Sailaukhanuly, Y. Application of selected partial order tools to analyze fate and toxicity indicators of environmentally hazardous chemicals. Ecol. Indic. 2013, 29, 191–202. [Google Scholar] [CrossRef]
- Richardson, S.D. Environmental mass spectrometry: Emerging contaminants and current issues. Anal. Chem. 2012, 84, 747–778. [Google Scholar] [CrossRef]
- Rigodanza, F.; Đorđević, L.; Arcudi, F.; Prato, M. Customizing the Electrochemical Properties of Carbon Nanodots by Using Quinones in Bottom-Up Synthesis. Angew. Chem. 2018, 130, 5156–5161. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Y.; Lu, J.; Zhou, Y. Novel cyclodextrin-based adsorbents for removing pollutants from wastewater: A critical review. Chemosphere 2020, 241, 125043. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, M.M.; Hoekstra, A.Y. Four billion people facing severe water scarcity. Sci. Adv. 2016, 2, e1500323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tollefson, J. How green is my future? Nature 2011, 473, 134–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sendao, R.M.S.; Esteves da Silva, J.C.G.; Pinto da Silva, L. Photocatalytic removal of pharmaceutical water pollutants by TiO2—Carbon dots nanocomposites: A review. Chemosphere 2022, 301, 134731. [Google Scholar] [CrossRef]
- Sharma, S.; Dutta, V.; Singh, P.; Raizada, P.; Rahmani-Sani, A.; Hosseini-Bandegharaei, A.; Thakur, V.K. Carbon quantum dot supported semiconductor photocatalysts for efficient degradation of organic pollutants in water: A review. J. Clean. Prod. 2019, 228, 755–769. [Google Scholar] [CrossRef]
- Umrao, S.; Sharma, P.; Bansal, A.; Sinha, R.; Singh, R.K.; Srivastava, A. Multi-layered graphene quantum dots derived photodegradation mechanism of methylene blue. RSC Adv. 2015, 5, 51790–51798. [Google Scholar] [CrossRef]
- Zhu, Z.; Yang, P.; Li, X.; Luo, M.; Zhang, W.; Chen, M.; Zhou, X. Green preparation of palm powder-derived carbon dots co-doped with sulfur/chlorine and their application in visible-light photocatalysis. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 227, 117659. [Google Scholar] [CrossRef]
- Hu, Y.; Guan, R.; Zhang, C.; Zhang, K.; Liu, W.; Shao, X.; Xue, Q.; Yue, Q. Fluorescence and photocatalytic activity of metal-free nitrogen-doped carbon quantum dots with varying nitrogen contents. Appl. Surf. Sci. 2020, 531, 147344. [Google Scholar] [CrossRef]
- Sharma, S.; Ibhadon, A.O.; Francesconi, M.G.; Mehta, S.K.; Elumalai, S.; Kansal, S.K.; Umar, A.; Baskoutas, S. Bi2WO6/C-Dots/TiO2: A Novel Z-Scheme Photocatalyst for the Degradation of Fluoroquinolone Levofloxacin from Aqueous Medium. Nanomaterials 2020, 10, 910. [Google Scholar] [CrossRef]
- Sharma, S.; Umar, A.; Mehta, S.K.; Ibhadon, A.O.; Kansal, S.K. Solar light driven photocatalytic degradation of levofloxacin using TiO2/carbon-dot nanocomposites. New J. Chem. 2018, 42, 7445–7456. [Google Scholar] [CrossRef]
- Wongso, V.; Chung, H.K.; Sambudi, N.S.; Sufian, S.; Abdullah, B.; Wirzal, M.D.H.; Ang, W.L. Silica–carbon quantum dots decorated titanium dioxide as sunlight-driven photocatalyst to diminish acetaminophen from aquatic environment. J. Photochem. Photobiol. A Chem. 2020, 394, 112436. [Google Scholar] [CrossRef]
- Koe, W.S.; Chong, W.C.; Pang, Y.L.; Koo, C.H.; Ebrahim, M.; Mohammad, A.W. Novel nitrogen and sulphur co-doped carbon quantum dots/titanium oxide photocatalytic membrane for in-situ degradation and removal of pharmaceutical compound. J. Water Process Eng. 2020, 33, 101068. [Google Scholar] [CrossRef]
- Aggarwal, R.; Saini, D.; Singh, B.; Kaushik, J.; Garg, A.K.; Sonkar, S.K. Bitter apple peel derived photoactive carbon dots for the sunlight induced photocatalytic degradation of crystal violet dye. Sol. Energy 2020, 197, 326–331. [Google Scholar] [CrossRef]
- Zhang, S.; Fan, X.; Guan, R.; Hu, Y.; Jiang, S.; Shao, X.; Wang, S.; Yue, Q. Carbon dots as metal-free photocatalyst for dye degradation with high efficiency within nine minutes in dark. Opt. Mater. 2022, 123, 111914. [Google Scholar] [CrossRef]
- Huang, B.; He, J.; Bian, S.; Zhou, C.; Li, Z.; Xi, F.; Liu, J.; Dong, X. S-doped graphene quantum dots as nanophotocatalyst for visible light degradation. Chin. Chem. Lett. 2018, 29, 1698–1701. [Google Scholar] [CrossRef]
- Umrao, S.; Abraham, S.; Theil, F.; Pandey, S.; Ciobota, V.; Shukla, P.K.; Rupp, C.J.; Chakraborty, S.; Ahuja, R.; Popp, J.; et al. A possible mechanism for the emergence of an additional band gap due to a Ti–O–C bond in the TiO2–graphene hybrid system for enhanced photodegradation of methylene blue under visible light. RSC Adv. 2014, 4, 59890–59901. [Google Scholar] [CrossRef]
- Yashwanth, H.J.; Rondiya, S.R.; Dzade, N.Y.; Dhole, S.D.; Phase, D.M.; Hareesh, K. Enhanced photocatalytic activity of N, P, co-doped carbon quantum dots: An insight from experimental and computational approach. Vacuum 2020, 180, 109589. [Google Scholar] [CrossRef]
- Peng, Z.; Zhou, Y.; Ji, C.; Pardo, J.; Mintz, K.J.; Pandey, R.R.; Chusuei, C.C.; Graham, R.M.; Yan, G.; Leblanc, R.M. Facile Synthesis of “Boron-Doped” Carbon Dots and Their Application in Visible-Light-Driven Photocatalytic Degradation of Organic Dyes. Nanomaterials 2020, 10, 1560. [Google Scholar] [CrossRef]
- Bramhaiah, K.; Bhuyan, R.; Mandal, S.; Kar, S.; Prabhu, R.; John, N.S.; Gramlich, M.; Urban, A.S.; Bhattacharyya, S. Molecular, Aromatic, and Amorphous Domains of N-Carbon Dots: Leading toward the Competitive Photoluminescence and Photocatalytic Properties. J. Phys. Chem. C 2021, 125, 4299–4309. [Google Scholar] [CrossRef]
- Das, G.S.; Shim, J.P.; Bhatnagar, A.; Tripathi, K.M.; Kim, T. Biomass-derived Carbon Quantum Dots for Visible-Light-Induced Photocatalysis and Label-Free Detection of Fe(III) and Ascorbic acid. Sci. Rep. 2019, 9, 15084. [Google Scholar] [CrossRef] [Green Version]
- Aghamali, A.; Khosravi, M.; Hamishehkar, H.; Modirshahla, N.; Behnajady, M.A. Synthesis and characterization of high efficient photoluminescent sunlight driven photocatalyst of N-Carbon Quantum Dots. J. Lumin. 2018, 201, 265–274. [Google Scholar] [CrossRef]
- Ibarbia, A.; Grande, H.J.; Ruiz, V. On the Factors behind the Photocatalytic Activity of Graphene Quantum Dots for Organic Dye Degradation. Part. Part. Syst. Charact. 2020, 37, 2000061. [Google Scholar] [CrossRef]
- Ma, Z.; Ming, H.; Huang, H.; Liu, Y.; Kang, Z. One-step ultrasonic synthesis of fluorescent N-doped carbon dots from glucose and their visible-light sensitive photocatalytic ability. New J. Chem. 2012, 36, 861–864. [Google Scholar] [CrossRef]
- Wang, C.; Xu, J.; Zhang, R.; Zhao, W. Facile and low-energy-consumption synthesis of dual-functional carbon dots from Cornus walteri leaves for detection of p-nitrophenol and photocatalytic degradation of dyes. Colloids Surf. A Physicochem. Eng. Asp. 2022, 640, 128351. [Google Scholar] [CrossRef]
- Smrithi, S.P.; Kottam, N.; Vergis, B.R. Heteroatom Modified Hybrid Carbon Quantum Dots Derived from Cucurbita pepo for the Visible Light Driven Photocatalytic Dye Degradation. Top. Catal. 2022. [Google Scholar] [CrossRef]
- Hu, S.; Tian, R.; Wu, L.; Zhao, Q.; Yang, J.; Liu, J.; Cao, S. Chemical regulation of carbon quantum dots from synthesis to photocatalytic activity. Chem. Asian J. 2013, 8, 1035–1041. [Google Scholar] [CrossRef]
- Sabet, M.; Mahdavi, K. Green synthesis of high photoluminescence nitrogen-doped carbon quantum dots from grass via a simple hydrothermal method for removing organic and inorganic water pollutions. Appl. Surf. Sci. 2019, 463, 283–291. [Google Scholar] [CrossRef]
- Bacon, M.; Bradley, S.J.; Nann, T. Graphene Quantum Dots. Part. Part. Syst. Charact. 2014, 31, 415–428. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, J.; Li, N.; Tian, J.; Li, K.; Jiang, J.; Liu, J.; Tian, Q.; Chen, P. Systematic Bandgap Engineering of Graphene Quantum Dots and Applications for Photocatalytic Water Splitting and CO2 Reduction. ACS Nano 2018, 12, 3523–3532. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Park, S.-J. TiO2 photocatalyst for water treatment applications. J. Ind. Eng. Chem. 2013, 19, 1761–1769. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, S.; Lu, X.; Wu, P.; Liu, J. Lanthanide-Boosted Singlet Oxygen from Diverse Photosensitizers along with Potent Photocatalytic Oxidation. ACS Nano 2019, 13, 14152–14161. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Lan, M.; Zhou, B.; Liu, W.; Guo, L.; Wang, H.; Jia, Q.; Niu, G.; Huang, X.; Zhou, H.; et al. A graphene quantum dot photodynamic therapy agent with high singlet oxygen generation. Nat. Commun. 2014, 5, 4596. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Li, D.; Wang, X. Effect of modified graphene quantum dots on photocatalytic degradation property. Diamond Relat. Mater. 2016, 69, 81–85. [Google Scholar] [CrossRef]
- Hutton, G.A.; Reuillard, B.; Martindale, B.C.; Caputo, C.A.; Lockwood, C.W.; Butt, J.N.; Reisner, E. Carbon Dots as Versatile Photosensitizers for Solar-Driven Catalysis with Redox Enzymes. J. Am. Chem. Soc. 2016, 138, 16722–16730. [Google Scholar] [CrossRef] [Green Version]
- Mondal, S.; Das, T.; Maity, A.; Seth, S.K.; Purkayastha, P. Synergic Influence of Reverse Micelle Confinement on the Enhancement in Photoinduced Electron Transfer to and from Carbon Nanoparticles. J. Phys. Chem. C 2015, 119, 13887–13892. [Google Scholar] [CrossRef]
- Amadio, E.; Cailotto, S.; Campalani, C.; Branzi, L.; Raviola, C.; Ravelli, D.; Cattaruzza, E.; Trave, E.; Benedetti, A.; Selva, M.; et al. Precursor-Dependent Photocatalytic Activity of Carbon Dots. Molecules 2019, 25, 101. [Google Scholar] [CrossRef] [Green Version]
- Martindale, B.C.M.; Hutton, G.A.M.; Caputo, C.A.; Prantl, S.; Godin, R.; Durrant, J.R.; Reisner, E. Enhancing Light Absorption and Charge Transfer Efficiency in Carbon Dots through Graphitization and Core Nitrogen Doping. Angew. Chem. Int. Ed. Engl. 2017, 56, 6459–6463. [Google Scholar] [CrossRef] [Green Version]
- Fernando, K.A.; Sahu, S.; Liu, Y.; Lewis, W.K.; Guliants, E.A.; Jafariyan, A.; Wang, P.; Bunker, C.E.; Sun, Y.P. Carbon quantum dots and applications in photocatalytic energy conversion. ACS Appl. Mater. Interfaces 2015, 7, 8363–8376. [Google Scholar] [CrossRef]
- Sahara, G.; Ishitani, O. Efficient Photocatalysts for CO2 Reduction. Inorg. Chem. 2015, 54, 5096–5104. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, H.; Song, X.; Shi, Y.; Si, D.; Li, H.; Hao, C. Insight into the CO2 photoreduction mechanism over 9-hydroxyphenal-1-one (HPHN) carbon quantum dots. J. Energy Chem. 2021, 52, 269–276. [Google Scholar] [CrossRef]
- Aggarwal, R.; Saini, D.; Sonkar, S.K.; Sonker, A.K.; Westman, G. Sunlight promoted removal of toxic hexavalent chromium by cellulose derived photoactive carbon dots. Chemosphere 2022, 287, 132287. [Google Scholar] [CrossRef] [PubMed]
- Omole, M.A.; K’Owino, I.O.; Sadik, O.A. Palladium nanoparticles for catalytic reduction of Cr(VI) using formic acid. Appl. Catal. B Environ. 2007, 76, 158–167. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, G.; Deng, Q.; Ng, D.H.; Zhao, H. Microwave-assisted fabrication of nanoparticulate TiO(2) microspheres for synergistic photocatalytic removal of Cr(VI) and methyl orange. ACS Appl. Mater. Interfaces 2014, 6, 3008–3015. [Google Scholar] [CrossRef]
- Abdullah, H.; Kuo, D.H. Facile Synthesis of n-type (AgIn)(x)Zn(2(1-x))S2/p-type Ag2S Nanocomposite for Visible Light Photocatalytic Reduction To Detoxify Hexavalent Chromium. ACS Appl. Mater. Interfaces 2015, 7, 26941–26951. [Google Scholar] [CrossRef]
- Norseth, T. The carcinogenicity of chromium. Environ. Health Perspect. 1981, 40, 121–130. [Google Scholar] [CrossRef]
- Zhitkovich, A. Importance of chromium-DNA adducts in mutagenicity and toxicity of chromium(VI). Chem. Res. Toxicol. 2005, 18, 3–11. [Google Scholar] [CrossRef]
- Li, L.; Zhang, J.; Li, Y.; Yang, C. Removal of Cr (VI) with a spiral wound chitosan nanofiber membrane module via dead-end filtration. J. Membr. Sci. 2017, 544, 333–341. [Google Scholar] [CrossRef]
- Charerntanyarak, L. Heavy metals removal by chemical coagulation and precipitation. Water Sci. Technol. 1999, 39, 135–138. [Google Scholar] [CrossRef]
- Edebali, S.; Pehlivan, E. Evaluation of Amberlite IRA96 and Dowex 1×8 ion-exchange resins for the removal of Cr(VI) from aqueous solution. Chem. Eng. J. 2010, 161, 161–166. [Google Scholar] [CrossRef]
- Ma, H.-L.; Zhang, Y.; Hu, Q.-H.; Yan, D.; Yu, Z.-Z.; Zhai, M. Chemical reduction and removal of Cr(vi) from acidic aqueous solution by ethylenediamine-reduced graphene oxide. J. Mater. Chem. 2012, 22, 5914–5916. [Google Scholar] [CrossRef]
- Crista, D.M.A.; Mello, G.P.C.; Shevchuk, O.; Sendao, R.M.S.; Simoes, E.F.C.; Leitao, J.M.M.; da Silva, L.P.; Esteves da Silva, J.C.G. 3-Hydroxyphenylboronic Acid-Based Carbon Dot Sensors for Fructose Sensing. J. Fluoresc 2019, 29, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Simoes, E.F.C.; da Silva, L.P.; da Silva, J.; Leitao, J.M.M. Hypochlorite fluorescence sensing by phenylboronic acid-alizarin adduct based carbon dots. Talanta 2020, 208, 120447. [Google Scholar] [CrossRef] [PubMed]
- Crista, D.M.A.; El Mragui, A.; Algarra, M.; Esteves da Silva, J.C.G.; Luque, R.; Pinto da Silva, L. Turning Spent Coffee Grounds into Sustainable Precursors for the Fabrication of Carbon Dots. Nanomaterials 2020, 10, 1209. [Google Scholar] [CrossRef]
- Li, J.; Tang, K.; Yu, J.; Wang, H.; Tu, M.; Wang, X. Nitrogen and chlorine co-doped carbon dots as probe for sensing and imaging in biological samples. R. Soc. Open Sci. 2019, 6, 181557. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Huang, Y.; Hu, Z.; Tong, C.; Zhang, Z.; Hu, S. Carbon dots codoped with nitrogen and sulfur are viable fluorescent probes for chromium(VI). Microchimica Acta 2017, 184, 1547–1553. [Google Scholar] [CrossRef]
- Zhong, Y.; Qiu, X.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Flexible Electrospun Carbon Nanofiber/Tin(IV) Sulfide Core/Sheath Membranes for Photocatalytically Treating Chromium(VI)-Containing Wastewater. ACS Appl. Mater. Interfaces 2016, 8, 28671–28677. [Google Scholar] [CrossRef]
- Essner, J.B.; Kist, J.A.; Polo-Parada, L.; Baker, G.A. Artifacts and Errors Associated with the Ubiquitous Presence of Fluorescent Impurities in Carbon Nanodots. Chem. Mater. 2018, 30, 1878–1887. [Google Scholar] [CrossRef]
- Tan, J.; Zou, R.; Zhang, J.; Li, W.; Zhang, L.; Yue, D. Large-scale synthesis of N-doped carbon quantum dots and their phosphorescence properties in a polyurethane matrix. Nanoscale 2016, 8, 4742–4747. [Google Scholar] [CrossRef]
- Christe, S.; Esteves da Silva, J.C.G.; Pinto da Silva, L. Evaluation of the Environmental Impact and Efficiency of N-Doping Strategies in the Synthesis of Carbon Dots. Materials 2020, 13, 504. [Google Scholar] [CrossRef]
- Fernandes, S.; Esteves da Silva, J.C.G.; Pinto da Silva, L. Comparative life cycle assessment of high-yield synthesis routes for carbon dots. NanoImpact 2021, 23, 100332. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lu, C.; Li, S.; Liu, S.; Wang, L.; Cai, W.; Xu, W.; Yang, X.; Liu, Y.; Zhang, R. A high-yield and versatile method for the synthesis of carbon dots for bioimaging applications. J. Mater. Chem. B 2017, 5, 1935–1942. [Google Scholar] [CrossRef] [PubMed]
- Jing, S.; Zhao, Y.; Sun, R.-C.; Zhong, L.; Peng, X. Facile and High-Yield Synthesis of Carbon Quantum Dots from Biomass-Derived Carbons at Mild Condition. ACS Sustain. Chem. Eng. 2019, 7, 7833–7843. [Google Scholar] [CrossRef]
- Algarra, M.; Orfãos, L.d.; Alves, C.S.; Moreno-Tost, R.; Pino-González, M.S.; Jiménez-Jiménez, J.; Rodríguez-Castellón, E.; Eliche-Quesada, D.; Castro, E.; Luque, R. Sustainable Production of Carbon Nanoparticles from Olive Pit Biomass: Understanding Proton Transfer in the Excited State on Carbon Dots. ACS Sustain. Chem. Eng. 2019, 7, 10493–10500. [Google Scholar] [CrossRef]








| Ref. | CDs’ Precursors | Synthesis/ Purification | CDs Loading | Pollutant/ Concentration | Light Source/Power | Dye Removal Efficiency and Time |
|---|---|---|---|---|---|---|
| [77] | Graphene | Hydrothermal/ Centrifugation | NA | MB-83 mg L−1 | LED (520 nm) 1 W/mm2 | 93.3%/60 mins |
| [54] | Xylose | Hydrothermal/ Filtering Dialysis | 30 mg | MB-1 g L−1 | Xe lamp 150 mW/cm2 | 92.06%/2.5 mins |
| [57] | Citric acid Urea | Solvothermal/ Acetone wash | 12 mg | MB-10 mg L−1 MO-10 mg L−1 RhB-10 mg L−1 | Hg/Xe lamp | 100%/20 mins 100%/40 mins 100%/60 mins |
| [93] | Pyrene | Hydrothermal/ Centrifugation Filtering Dialysis | 0.3 mg | RhB-10 mg L−1 MB-10 mg L−1 | LED and CFL Flux of 1000 lm | 55%/360 mins 39%/360 mins |
| [34] | Citric acid 1,2-phenylenediamien | Microwave/ Filtration Chromatography | 3 mg | RhB-0.5 mg L−1 MB-2.5 mg L−1 | Hg/Xe lamp 310 W | 100%/150 mins 100%/150 mins |
| [78] | Palm powder Thionyl chloride | Hydrothermal/ Filtering Dialysis | NA-added in solution | MB-15 mg L−1 RhB-15 mg L−1 | Xe lamp 400 mW/cm2 | 94.2%/50 mins 71.7%/50 mins |
| [88] | Glucose Ethylenediamine Phosphoric acid | Microwave/ Centrifugation Dialysis | 2 mg | MB-10 mg L−1 | Sunlight ~832 W/m2 | 100%/25 mins |
| [89] | Citric acid 1,2-diboranyethane Dimethylformamide | Hydrothermal/ Centrifugation Dialysis | 3 mg | MB-2.5 mg L−1 RhB-0.5 mg L−1 | Hg/Xe lamp 310 W | 100%/170 mins 100%/170 mins |
| [90] | Citric acid Hexamethylene-1,6-diamine | Hydrothermal/ Centrifugation Dialysis | 10 mg | MB-5 µM | Xe lamp 110 mW/cm−2 | 96.7%/40 mins |
| [91] | Pear juice | Hydrothermal/ Filtration | NA-added in solution | MB-45 µM | Tungsten lamp 60 W | 99.5%/130 mins |
| [92] | Citric acid Diethylenetriamine | Hydrothermal None | 0.2 mg | MB-18.75 mg L−1 | Sunlight and Halogen lamp (500 W) | 97%/160 mins |
| [61] | Polyethylene glycol Phosphoric acid Cysteine | Microwave/ Dialysis | 25 mg | MO-20 mg L−1 MY-20 mg L−1 | Sunlight | 100%/90 mins 100%/60 mins |
| [94] | Glucose Ammonia | Ultrasonic treatment/ Dialysis | NA-added in solution | MO-10 ppm | Xe lamp 150 W | 90%/120 mins |
| [95] | Cornus walteri leaves Malei anhydride Hydrogen peroxide | Hydrothermal/ Centrifugation Filtration Dialysis | 1 mg | MO-30 mg L−1 MG-30 mg L−1 MV- 30 mg L−1 | Sunlight | 97.1%/50 mins 98.0%/40 mins 63.6%/60 mins |
| [84] | Bitter apple peel | Calcination/ None | 10 mg | CV-200 ppm | Sunlight | 100%/90 mins |
| [79] | 2-aminonaphthalene Potassium persulfate | Hydrothermal/ Centrifugation Dialysis | NA-added in solution | IC-10 mg L−1 | Natural light | 97%/120 mins |
| [85] | Glyoxal Ethanol | Solvothermal None | 0.265 mg | IC-38.8 mg L−1 | Sunlight | 91%/4.5 mins |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sendão, R.M.S.; Esteves da Silva, J.C.G.; Pinto da Silva, L. Applications of Fluorescent Carbon Dots as Photocatalysts: A Review. Catalysts 2023, 13, 179. https://doi.org/10.3390/catal13010179
Sendão RMS, Esteves da Silva JCG, Pinto da Silva L. Applications of Fluorescent Carbon Dots as Photocatalysts: A Review. Catalysts. 2023; 13(1):179. https://doi.org/10.3390/catal13010179
Chicago/Turabian StyleSendão, Ricardo M. S., Joaquim C. G. Esteves da Silva, and Luís Pinto da Silva. 2023. "Applications of Fluorescent Carbon Dots as Photocatalysts: A Review" Catalysts 13, no. 1: 179. https://doi.org/10.3390/catal13010179