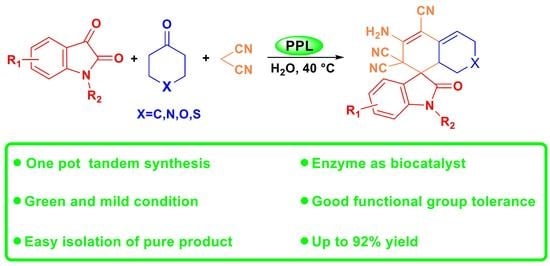
Green Synthesis of Spirooxindoles via Lipase-Catalyzed One-Pot Tandem Reaction in Aqueous Media
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Reaction Conditions for the One-Pot Tandem Process
2.2. Substrate Scopes for the One-Pot Tandem Process
2.3. Mechanism of the One-Pot Tandem Process
3. Materials and Methods
3.1. General Information
3.2. General Procedure for Lipase-Catalyzed Synthesis of 4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calmanti, R.; Selva, M.; Perosa, A. Tandem catalysis: One–pot synthesis of cyclic organic carbonates from olefins and carbon dioxide. Green Chem. 2021, 23, 1921–1941. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, H. Tandem Reactions Combining Biocatalysts and Chemical Catalysts for Asymmetric Synthesis. Catalysts 2016, 6, 194. [Google Scholar] [CrossRef] [Green Version]
- Diego, C.; Roberto, M.S.; Roberto, F.L. Design of Artificial Enzymes Bearing Several Active Centers: New Trends, Opportunities and Problems. Int. J. Mol. Sci. 2022, 23, 5304. [Google Scholar]
- Finnigan, W.; Cutlan, R.; Snajdrova, R.; Adams, J.P.; Littlechild, J.A.; Harmer, N.J. Engineering a Seven Enzyme Biotransformation using Mathematical Modelling and Characterized Enzyme Parts. ChemCatChem 2019, 11, 3474–3489. [Google Scholar] [CrossRef] [Green Version]
- Cutlan, R.; De Rose, S.; Isupov, M.N.; Littlechild, J.A.; Harmer, N.J. Using enzyme cascades in biocatalysis: Highlight on transaminases and carboxylic acid reductases. BBA—Proteins Proteom. 2020, 1868, 140322. [Google Scholar] [CrossRef]
- Rios, R. Enantioselective methodologies for the synthesis of spiro compounds. Chem. Soc. Rev. 2012, 41, 1060–1074. [Google Scholar] [CrossRef]
- Carreira, E.M.; Fessard, T.C. Four–Membered Ring–Containing Spirocycles: Synthetic Strategies and Opportunities. Chem. Rev. 2014, 114, 8257–8322. [Google Scholar] [CrossRef]
- Ding, A.; Meazza, M.; Guo, H.; Yang, J.W.; Rios, R. New development in the enantioselective synthesis of spiro compounds. Chem. Soc. Rev. 2018, 47, 5946–5996. [Google Scholar] [CrossRef]
- Cheng, D.; Ishihara, Y.; Tan, B.; Barbas, C.F. Organocatalytic Asymmetric Assembly Reactions: Synthesis of Spirooxindoles via Organocascade Strategies. ACS Catal. 2014, 4, 743–762. [Google Scholar] [CrossRef]
- Pavlovska, T.L.; Redkin, R.G.; Lipson, V.V.; Atamanuk, D.V. Molecular diversity of spirooxindoles. Synthesis and biological activity. Mol. Divers 2016, 20, 299–344. [Google Scholar] [CrossRef]
- Kathirvelan, D.; Haribabu, J.; Reddy, B.S.; Balachandran, C.; Duraipandiyan, V. Facile and diastereoselective synthesis of 3,2’–spiropyrrolidine–oxindoles derivatives, their molecular docking and antiproliferative activities. Bioorg. Med. Chem. Lett. 2015, 25, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Vintonyak, V.V.; Warburg, K.; Kruse, H.; Grimme, S.; Hubel, K.; Rauh, D.; Waldmann, H. Identification of Thiazolidinones Spiro–Fused to Indolin–2–ones as Potent and Selective Inhibitors of the Mycobacterium tuberculosis Protein Tyrosine Phosphatase B. Angew. Chem. Int. Ed. 2010, 49, 5902–5905. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Marvin, C.C.; Pettersson, M.; Martin, S.F. Enantioselective Total Syntheses of Citrinadins A and B. Stereochemical Revision of Their Assigned Structures. J. Am. Chem. Soc. 2014, 136, 14184–14192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hari Babu, T.; Abragam Joseph, A.; Muralidharan, D.; Perumal, P.T. A novel method for the synthesis of functionalized spirocyclic oxindoles by one–pot tandem reaction of vinyl malononitriles with isatylidene malononitriles. Tetrahedron Lett. 2010, 51, 994–996. [Google Scholar] [CrossRef]
- Hegade, P.G.; Chinchkar, S.D.; Pore, D.M. DABCO catalyzed pseudo multi–component synthesis of functionalized spirooxindoles. Monatsh. Chem. 2016, 147, 1243–1249. [Google Scholar] [CrossRef]
- Reddy Gajulapalli, V.P.; Vinayagam, P.; Kesavan, V. Enantioselective assembly of functionalized carbocyclic spirooxindoles using anl–proline derived thiourea organocatalyst. RSC Adv. 2015, 5, 7370–7379. [Google Scholar] [CrossRef]
- Dandia, A.; Mahawar, D.K.; Saini, P.; Saini, S.; Gupta, S.L.; Rathore, K.S.; Parewa, V. Site–specific role of bifunctional graphitic carbon nitride catalyst for the sustainable synthesis of 3,3–spirocyclic oxindoles in aqueous media. RSC Adv. 2021, 11, 28452–28465. [Google Scholar] [CrossRef]
- Li, F.; Li, Z.; Tang, X.; Cao, X.; Wang, C.; Li, J.; Wang, L. Hemoglobin: A New Biocatalyst for the Synthesis of 2–substituted BenzoxazolesviaOxidative Cyclization. ChemCatChem 2019, 11, 1192–1195. [Google Scholar] [CrossRef]
- Li, F.; Tang, X.; Xu, Y.; Wang, C.; Zhang, L.; Zhang, J.; Liu, J.; Li, Z.; Wang, L. Hemoglobin–Catalyzed Synthesis of Indolizines Under Mild Conditions. Eur. J. Org. Chem. 2019, 2019, 7720–7724. [Google Scholar] [CrossRef]
- Li, F.; Tang, X.; Xu, Y.; Wang, C.; Wang, Z.; Li, Z.; Wang, L. A Dual–Protein Cascade Reaction for the Regioselective Synthesis of Quinoxalines. Org. Lett. 2020, 22, 3900–3904. [Google Scholar] [CrossRef]
- Xu, Y.; Li, F.; Zhao, N.; Su, J.; Wang, C.; Wang, C.; Li, Z.; Wang, L. Environment–friendly and efficient synthesis of 2–aminobenzo–xazoles and 2–aminobenzothiazoles catalyzed by Vitreoscilla hemoglobin incorporating a cobalt porphyrin cofactor. Green Chem. 2021, 23, 8047–8052. [Google Scholar] [CrossRef]
- Li, F.; Xu, Y.; Wang, C.; Wang, C.; Zhao, R.; Wang, L. Efficient synthesis of cyano–containing multi–substituted indoles catalyzed by lipase. Bioorg. Chem. 2021, 107, 104583. [Google Scholar] [CrossRef] [PubMed]
- Dutt, S.; Goel, V.; Garg, N.; Choudhury, D.; Mallick, D.; Tyagi, V. Biocatalytic Aza-Michael Addition of Aromatic Amines to Enone Using α-Amylase in Water. Adv. Synth. Catal. 2020, 362, 858–866. [Google Scholar] [CrossRef]
- Xu, Y.; Smith, R.; Vivoli, M.; Ema, M.; Goos, N.; Gehrke, S.; Harmer, N.J.; Wagner, G.K. Covalent inhibitors of LgtC: A blueprint for the discovery of non–substrate–like inhibitors for bacterial glycosyltransferases. Bioorg. Med. Chem. 2017, 25, 3182–3194. [Google Scholar] [CrossRef] [Green Version]
- Finnigan, W.; Thomas, A.; Cromar, H.; Gough, B.; Snajdrova, R.; Adams, J.P.; Littlechild, J.A.; Harmer, N.J. Characterization of Carboxylic Acid Reductases as Enzymes in the Toolbox for Synthetic Chemistry. ChemCatChem 2017, 9, 1005–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vivoli, M.; Pang, J.; Harmer, N.J. A half–site multimeric enzyme achieves its cooperativity without conformational changes. Sci. Rep. 2017, 7, 16529. [Google Scholar] [CrossRef] [Green Version]
- Iwasaki, J.; Lorimer, D.D.; Vivoli-Vega, M.; Kibble, E.A.; Peacock, C.S.; Abendroth, J.; Mayclin, S.J.; Dranow, D.M.; Pierce, P.G.; Fox, D.; et al. Broad–spectrum in vitro activity of macrophage infectivity potentiator inhibitors against Gram–negative bacteria and Leishmania major. J. Antimicrob. Chemother. 2022, 77, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Chai, S.; Lai, Y.; Xu, J.; Zheng, H.; Zhu, Q.; Zhang, P. One-Pot Synthesis of Spirooxindole Derivatives Catalyzed by Lipase in the Presence of Water. Adv. Synth. Catal. 2011, 353, 371–375. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, X.; Wu, Q.; Lin, X. Diastereoselective synthesis of spirooxindole derivatives via biocatalytic domino reaction. Tetrahedron 2015, 71, 616–621. [Google Scholar] [CrossRef]
- Fu, Y.; Lu, Z.; Fang, K.; He, X.; Huang, H.; Hu, Y. Promiscuous enzyme–catalyzed cascade reaction in water: Synthesis of dicoumarol derivatives. Bioorg. Med. Chem. Lett. 2019, 29, 1236–1240. [Google Scholar] [CrossRef]
- Ding, X.; Dong, C.L.; Guan, Z.; He, Y.H. Concurrent Asymmetric Reactions Combining Photocatalysis and Enzyme Catalysis: Direct Enantioselective Synthesis of 2,2–Disubstituted Indol–3–ones from 2–Arylindoles. Angew. Chem. Int. Ed. 2019, 58, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Bavandi, H.; Habibi, Z.; Yousefi, M. Porcine pancreas lipase as a green catalyst for synthesis of bis–4–hydroxy coumarins. Bioorg. Chem. 2020, 103, 104139. [Google Scholar] [CrossRef] [PubMed]
- Chaitanya, P.K.; Prabhu, N.P. Stability and Activity of Porcine Lipase Against Temperature and Chemical Denaturants. Appl. Biochem. Biotechnol. 2014, 174, 2711–2724. [Google Scholar] [CrossRef] [PubMed]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou–Shehada, S.; Dunn, P.J. CHEM21 selection guide of classical– and less classical–solvents. Green Chem. 2016, 18, 288–296. [Google Scholar] [CrossRef] [Green Version]
- Arana-Peña, S.; Rios, N.S.; Mendez-Sanchez, C.; Lokha, Y.; Gonçalves, L.R.B.; Fernández-Lafuente, R. Use of polyethylenimine to produce immobilized lipase multilayers biocatalysts with very high volumetric activity using octyl-agarose beads: Avoiding enzyme release during multilayer production. Enzyme Microb. Technol. 2020, 137, 109535. [Google Scholar] [CrossRef]
- Arana-Pena, S.; Carballares, D.; Morellon-Sterling, R.; Rocha-Martin, J.; Fernandez-Lafuente, R. The combination of covalent and ionic exchange immobilizations enables the coimmobilization on vinyl sulfone activated supports and the reuse of the most stable immobilized enzyme. Int. J. Biol. Macromol. 2022, 199, 51–60. [Google Scholar] [CrossRef]
- Xiang, X.; Ding, S.; Suo, H.; Xu, C.; Gao, Z.; Hu, Y. Fabrication of chitosan-mesoporous silica SBA-15 nanocomposites via functional ionic liquid as the bridging agent for PPL immobilization. Carbohydr. Polym. 2018, 182, 245–253. [Google Scholar] [CrossRef]
- Remonatto, D.; Miotti Jr, R.H.; Monti, R.; Bassan, J.C.; de Paula, A.V. Applications of immobilized lipases in enzymatic reactors: A review. Process Biochem. 2022, 114, 1–20. [Google Scholar] [CrossRef]
- Hu, R.; Niu, Z.; Lu, Y.; Zhu, H.; Mao, Z.; Yan, K.; Hu, X.; Chen, H. Immobilization for Lipase: Enhanced Activity and Stability by Flexible Combination and Solid Support. Appl. Biochem. Biotechnol. 2022, 194, 5963–5976. [Google Scholar] [CrossRef]






 | ||
|---|---|---|
| Entry | Lipase 1 | Yield (%) 2 |
| 1 | PPL | 88 |
| 2 | PSL | 40 |
| 3 | BSA | 45 |
| 4 | CALB | 35 |
| 5 | Novozym 435 | 38 |
| 6 | PPL 3 | N.D 4 |
| 7 | Control | N.D 4 |
 | ||
|---|---|---|
| Entry | Solvent 1 | Yield (%) 2 |
| 1 | EtOH | 86 |
| 2 | DMF 3 | 90 |
| 3 | DMSO 3 | 90 |
| 4 | H2O | 88 |
| 5 | THF | 35 |
| 6 | Toluene | 23 |
| 7 | EA | N.D 4 |
| 8 | DCM | N.D 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Wang, C.; Xie, H.; Xu, Y.; Wang, C.; Du, C.; Wang, Z.; Wang, L. Green Synthesis of Spirooxindoles via Lipase-Catalyzed One-Pot Tandem Reaction in Aqueous Media. Catalysts 2023, 13, 143. https://doi.org/10.3390/catal13010143
Tang Y, Wang C, Xie H, Xu Y, Wang C, Du C, Wang Z, Wang L. Green Synthesis of Spirooxindoles via Lipase-Catalyzed One-Pot Tandem Reaction in Aqueous Media. Catalysts. 2023; 13(1):143. https://doi.org/10.3390/catal13010143
Chicago/Turabian StyleTang, Yong, Ciduo Wang, Hanqing Xie, Yuelin Xu, Chunyu Wang, Chuang Du, Zhi Wang, and Lei Wang. 2023. "Green Synthesis of Spirooxindoles via Lipase-Catalyzed One-Pot Tandem Reaction in Aqueous Media" Catalysts 13, no. 1: 143. https://doi.org/10.3390/catal13010143