Low-Temperature Decomposition of Nitrous Oxide on Cs/MexCo3−xO4 (Me: Ni or Mg, x = 0–0.9) Oxides
Abstract
:1. Introduction
2. Results and Discussion
2.1. XRD Study of Samples
2.2. H2-TPR and O2-TPD Study of Samples
2.3. Catalytic Activity
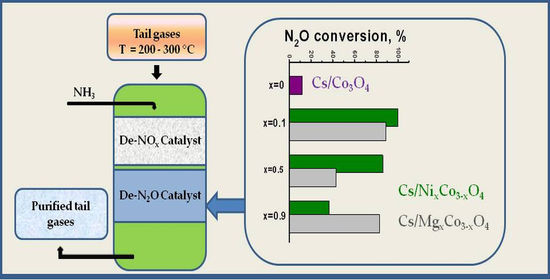
2.3.1. Dependence of Activity on the Content of Cs
2.3.2. The Decomposition of N2O
2.3.3. The Decomposition of N2O in the Presence of Inhibitors
3. Experimental
3.1. Samples Preparation
3.2. Catalysts Characterization
3.3. Activity Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chumachenko, V.A.; Isupova, L.A.; Ivanova, Y.A.; Ovchinnikova, E.V.; Reshetnikov, S.I.; Noskov, A.S. Technologies for Simultaneous Low-Temperature Catalytic Removal of NOx and N2O from the Tail Gases of Nitric Acid Plants. Chem. Sustain. Dev. 2020, 28, 203–212. [Google Scholar] [CrossRef]
- Isupova, L.A.; Ivanova, Y.A. Removal of nitrous oxide in nitric acid production. Kinet. Catal. 2019, 60, 744–760. [Google Scholar] [CrossRef]
- Konsolakis, M. Recent advances on nitrous oxide (N2O) decomposition over non–noble metal oxide catalysts: Catalytic performance, mechanistic considerations and surface chemistry aspects. ACS Catal. 2015, 5, 6397–6421. [Google Scholar] [CrossRef]
- Yu, H.; Wang, X. Apparent activation energies and reaction rates of N2O decomposition via different routes over Co3O4. Catal. Commun. 2018, 106, 40–43. [Google Scholar] [CrossRef]
- Kapteijn, F.; Marban, G.; Rodriguez-Mirasol, J.; Moulijn, J.A. Kinetic Analysis of the Decomposition of Nitrous Oxide over ZSM-5 Catalysts. J. Catal. 1997, 167, 256–265. [Google Scholar] [CrossRef]
- Dandl, H.; Emig, G. Mechanistic approach for the kinetics of the decomposition of nitrous oxide over calcined hydrotalcites. Appl. Catal. A Gen. 1998, 168, 261–268. [Google Scholar] [CrossRef]
- Ma, Z.; Ren, Y.; Lu, Y.B.; Peter, P.G. Catalytic decomposition of N2O on ordered crystalline metal oxides. J. Nanosci. Nanotechnol. 2013, 13, 5093–5103. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Wu, R.; Zhao, Y. Graphitic carbon nitride-supported cobalt oxides as a potential catalyst for decomposition of N2O. Appl. Surf. Sci. 2021, 538, 14815. [Google Scholar] [CrossRef]
- Yan, L.; Ren, T.; Wang, X.; Ji, D.; Suo, J. Catalytic decomposition of N2O over MxCo1−xCo2O4 (M = Ni, Mg) spinel oxides. Appl. Catal. B 2003, 45, 85–90. [Google Scholar] [CrossRef]
- Liu, Z.; He, F.; Ma, L.; Peng, S. Recent Advances in Catalytic Decomposition of N2O on Noble Metal and Metal Oxide. Catal. Catal. Surv. Asia 2016, 20, 121–132. [Google Scholar] [CrossRef]
- Zheng, L.; Li, H.J.; Xu, X.F. Catalytic decomposition of N2O over Mg-Co composite oxides hydrothermally prepared by using carbon sphere as template. J. Fuel Chem. Technol. 2018, 46, 569–577. [Google Scholar] [CrossRef]
- Zhang, H.J.; Jian, W.; Xu, X.F. Catalytic decomposition of N2O over NixCo 1−xCoAlO4 spinel oxides prepared by sol-gel method. J. Fuel Chem. Technol. 2015, 43, 81–87. [Google Scholar] [CrossRef]
- Russo, N.; Fino, D.; Saracco, G.; Specchiaet, V. N2O catalytic decomposition over various spinel-type oxides. Catal. Today 2007, 119, 228–232. [Google Scholar] [CrossRef]
- Abu-Zied, B.M.; Soliman, S.A.; Abdellah, S.E. Pure and Ni-substituted Co3O4 spinel catalysts for direct N2O decomposition. Chin. J. Catal. 2014, 35, 1105–1112. [Google Scholar] [CrossRef]
- Krezhov, K.; Konstantinov, P. Cationic distributions in the binary oxide spinels MxCo3−xO4 (M = Mg, Cu, Zn, Ni). Physica B 1997, 234–236, 157–158. [Google Scholar] [CrossRef]
- Krezhov, K.; Konstantinov, P. On the cationic distribution in MgxCo3−xO4 spinels. J. Phys. Condens. Matter 1992, 4, 543–548. [Google Scholar] [CrossRef]
- Wójcik, S.; Grzybek, G.; Stelmachowski, P.; Sojka, Z.; Kotarba, A. Bulk, Surface and Interface Promotion of Co3O4 for the Low-Temperature N2O Decomposition Catalysis. Catalysts 2020, 10, 41. [Google Scholar] [CrossRef] [Green Version]
- Stelmachowski, P.; Maniak, G.; Kotarba, A.; Sojka, Z. Strong electronic promotion of Co3O4 towards N2O decomposition by surface alkali dopants. Catal. Commun. 2009, 10, 1062–1065. [Google Scholar] [CrossRef]
- Maniak, G.; Stelmachowski, P.; Kotarba, A.; Sojka, Z.; Rico-Pérez, V.; Bueno-López, A. Rationales for the selection of the best precursor for potassium doping of cobalt spinel based deN2O catalyst. Appl. Catal. B Environ. 2013, 136–137, 302–307. [Google Scholar] [CrossRef]
- Rashmirekha, S.; Barsha, D.; Chinmaya, K.S.; Kali, S.; Tondepu, S.; Gamini, S.; Manickam, M. Influence of Synthesis Temperature on the Growth and Surface Morphology of Co3O4 Nanocubes for Supercapacitor. Appl. Nanomater. 2017, 7, 356. [Google Scholar] [CrossRef]
- Wang, H.Y.; Ruckenstein, E. CO2 reforming of CH4 over Co/MgO solid solution catalysts—Effect of calcination temperature and Co loading. Appl. Catal. A Gen. 2001, 209, 207–215. [Google Scholar] [CrossRef]
- Markov, L.; Petrov, K. Nickel-cobalt oxide spinels prepared by thermal decomposition of nickel(II)-cobalt(II) hydroxide nitrates. React. Solids 1986, 1, 319–327. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A 32, 751–767. [Google Scholar] [CrossRef]
- Lenglet, M.; Guillamet, R.; Dürr, J.; Gryffroy, D.; Vandenberghe, R.E. Electronic structure of NiCo2O4 by XANES, EXAFS and 61Ni Mössbauer studies. J. Solid State Commun. 1990, 74, 1035–1039. [Google Scholar] [CrossRef]
- Iliev, M.N.; Silwal, P.; Loukya, B.; Datta, R.; Kim, D.H.; Todorov, N.D.; Pachauri, N.; Gupta, A. Raman studies of cation distribution and thermal stability of epitaxial spinel NiCo2O4 films. J. Appl. Phys. 2013, 114, 033514. [Google Scholar] [CrossRef]
- Haenen, J.G.D.; Visscher, W.; Barendrecht, E.J. Oxygen Evolution on NiCo2O4 Electrodes. Ph.D. Thesis, Eindhoven University of Technology, Eindhoven, The Netherlands, 1985. [Google Scholar] [CrossRef]
- Chang, T.-C.; Lu, Y.-T.; Lee, C.-H.; Gupta, J.K.; Hardwick, L.J.; Hu, C.-C.; Chen, H.-Y.T. The Effect of Degrees of Inversion on the Electronic Structure of Spinel NiCo2O4: A Density Functional Theory Study. ACS Omega 2021, 6, 9692–9699. [Google Scholar] [CrossRef]
- Asano, K.; Ohnishi, C.; Iwamoto, S.; Shioya, Y.; Inoue, M. Potassium-doped Co3O4 catalyst for direct decomposition of N2O. Appl. Catal. B Environ. 2008, 78, 242–249. [Google Scholar] [CrossRef]
- Chromcakova, Z.; Obalova, L.; Kovanda, F.; Legut, D.; Titov, A.; Ritz, M.; Fridrichova, D.; Michalik, S.; Kustrowski, P.; Jirátová, K. Effect of precursor synthesis on catalytic activity of Co3O4 in N2O decomposition. Catal. Today 2015, 257, 18–25. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wen, W.; Mi, J.X.; Li, X.X.; Wang, R.H. The ordered mesoporous transition metal oxides for selective catalytic reduction of NOx at low temperature. Appl. Catal. B-Environ. 2015, 176–177, 454–463. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, P.; Cao, S.; Chen, H.; Zhou, S.; Liu, H.; Wang, H.; Zhang, J.; Liu, S.; Wei, S.; et al. Contemporaneous inverse manipulation of the valence configuration to preferred Co2+ and Ni3+ for enhanced overall water electrocatalysis. Appl. Catal. B 2021, 284, 119725. [Google Scholar] [CrossRef]
- Grzybek, G.; Wójcik, S.; Legutko, P.; Grybo’s, J.; Indyka, P.; Leszczynski, B.; Kotarba, A.; Sojka, Z. Thermal stability and repartition of potassium promoter between the support and active phase in the K-Co2.6Zn0.4O4/α-Al2O3 catalyst for N2O decomposition: Crucial role of activation temperature on catalytic performance. Appl. Catal. B Environ. 2017, 205, 597–604. [Google Scholar] [CrossRef]
- Grzybek, G.; Stelmachowski, P.; Gudyka, S.; Duch, J.; Cmil, K.; Kotarba, A.; Sojka, Z. Insights into the twofold role of Cs doping on deN2O activity of cobalt spinel catalyst—Towards rational optimization of the precursor and loading. Appl. Catal. B Environ. 2015, 168–169, 509–514. [Google Scholar] [CrossRef]
- Zabilsky, M.; Erzhavets, B.; Dzhinovich, P.; Pintar, A. Ordered mesoporous CuO–CeO2 mixed oxides as an effective catalyst for N2O decomposition. J. Chem. Eng. 2014, 254, 153–162. [Google Scholar] [CrossRef]
- Piskorz, W.; Zasada, F.; Stelmachowski, P.; Kotarba, A.; Sojka, Z. Decomposition of N2O over the surface of cobalt spinel: A DFT account of reactivity experiments. Catal. Today 2008, 137, 418–422. [Google Scholar] [CrossRef]
- Franken, T.; Palkovits, R. Investigation of potassium doped mixed spinels CuxCo3−xO4 as catalysts for an efficient N2O decomposition in real reaction conditions. Appl. Catal. B Environ. 2015, 176–177, 298–305. [Google Scholar] [CrossRef]
- Amrousse, R.; Tsutsumi, A.; Bachar, A.; Lahcene, D. N2O catalytic decomposition over nano-sized particles of Co–substituted Fe3O4 substrates. Appl. Catal. A Gen. 2013, 450, 253–260. [Google Scholar] [CrossRef]





| x | Phase Composition | Lattice Parameter, Ǻ | CSR, Ǻ | SBET, m2/g | T50, °C |
|---|---|---|---|---|---|
| 0 | Spinel | 8.083 | 380 | 12 | 275 |
| Cs/Ni-Co | |||||
| 0.1 | Spinel | 8.088 | 260 | 32 | 196 |
| 0.5 | Spinel, NiO | 8.094 | 310 | 23 | 224 |
| 0.9 | Spinel, NiO | 8.101 | 280 | 26 | 250 |
| Cs/Mg-Co | |||||
| 0.1 | Spinel | 8.084 | 210 | 36 | 210 |
| 0.5 | Spinel | 8.091 | 120 | 57 | 257 |
| 0.9 | Spinel | 8. 099 | 45 | 144 | 220 |
| x | , °C | ΣH2 × 10−3, mol/g | H2 × 10−3, mol/g Co3+ → Co2+ | H2 × 10−3, mol/g Co2+ → Co0 | , °C | ΣO × 1017, at/m2 |
|---|---|---|---|---|---|---|
| 0 | 212 | 16.6 | 4.0 | 12.6 | 150 | 3.3 |
| Cs/Ni-Co | ||||||
| 0.1 | 188 | 17.1 | 4.2 | 12.9 | 119 | 4.2 |
| 0.5 | 200 | 15.7 | 3.3 | 12.4 | 133 | 3.9 |
| 0.9 | 207 | 15.5 | 3.5 | 12.0 | 140 | 1.2 |
| Cs/Mg-Co | ||||||
| 0.1 | 200 | 16.1 | 4.2 | 11.9 | 100 | 3.4 |
| 0.5 | 207 | 15.6 | 4.0 | 11.6 | 121 | 1.2 |
| 0.9 | 188 | 13.2 | 3.8 | 9.4 | 136 | 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, Y.; Isupova, L. Low-Temperature Decomposition of Nitrous Oxide on Cs/MexCo3−xO4 (Me: Ni or Mg, x = 0–0.9) Oxides. Catalysts 2023, 13, 137. https://doi.org/10.3390/catal13010137
Ivanova Y, Isupova L. Low-Temperature Decomposition of Nitrous Oxide on Cs/MexCo3−xO4 (Me: Ni or Mg, x = 0–0.9) Oxides. Catalysts. 2023; 13(1):137. https://doi.org/10.3390/catal13010137
Chicago/Turabian StyleIvanova, Yulia, and Lyubov Isupova. 2023. "Low-Temperature Decomposition of Nitrous Oxide on Cs/MexCo3−xO4 (Me: Ni or Mg, x = 0–0.9) Oxides" Catalysts 13, no. 1: 137. https://doi.org/10.3390/catal13010137