Preparation of a Novel NiAlO Composite Oxide Catalyst for the Dehydrogenation of Methylcyclohexane
Abstract
:1. Introduction
2. Results and Discussion
2.1. N2 Adsorption-Desorption Measurement
2.2. Determination of Nickel Content in Catalyst
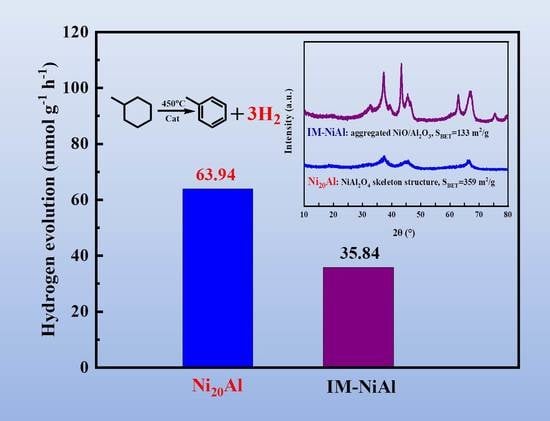
2.3. XRD
2.4. TEM Analysis
2.5. XPS Analysis
2.6. Catalytic Performance in the Dehydrogenation of MCH
3. Experimental
3.1. Materials
3.2. Catalyst Preparation
3.2.1. Preparation of NixAl Catalyst
3.2.2. Preparation of IM-NiAl Catalyst
3.3. Characterization
3.4. Catalyst Evaluation
- u is the feed rate of MCH, mL/min;
- ρ is the density of MCH, g/mL;
- m is the amount of catalyst used, g;
- M is the molar mass of MCH, g/mol;
- C is the conversion rate of MCH, %;
- S is the selectivity of toluene, %.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, J.H.; Xu, Z.B.; Jiang, W.; Liu, W.; Guan, X.H. Optimal planning of distributed hydrogen-based multi-energy systems. Appl. Energy 2021, 281, 116107. [Google Scholar] [CrossRef]
- Johnston, B.; Mayo, M.C.; Khare, A. Hydrogen: The Energy Source for the 21st Century. Technovation 2005, 25, 569–585. [Google Scholar] [CrossRef]
- Pradhan, A.; Vishwakarma, M.; Dwivedi, S.K. A review: The impact of hydrogen embrittlement on the fatigue strength of high strength steel. Mater. Today Proc. 2020, 26, 3015–3019. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, X.; Xu, P.; Liu, P.; Zhao, Y.; Yang, J. Development of high pressure gaseous hydrogen storage technologies. Int. J. Hydrogen Energy 2012, 37, 1048–1057. [Google Scholar] [CrossRef]
- Hu, J.; Chandrashekhara, K. Fracture analysis of hydrogen storage composite cylinders with liner crack accounting for autofrettage effect. Int. J. Hydrogen Energy 2009, 34, 3425–3435. [Google Scholar] [CrossRef]
- Ananthachar, V.; Duffy, J.J. Efficiencies of hydrogen storage systems onboard fuel cell vehicles. Sol. Energy 2005, 78, 687–694. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Jia, Z.C.; Yuan, Z.M.; Yang, T.; Yan, Q.I.; Zhao, D.L. Development and application of hydrogen storage. J. Iron Steel Res. Int. 2015, 22, 757–770. [Google Scholar] [CrossRef]
- Biniwale, R.B.; Rayalu, S.; Devotta, S.; Ichikawa, M. Chemical hydrides: A solution to high capacity hydrogen storage and supply. Int. J. Hydrogen Energy 2008, 33, 360–365. [Google Scholar] [CrossRef]
- Bourane, A.; Elanany, M.; Pham, T.V.; Katikaneni, S.P. An over view of organic liquid phase hydrogencarriers. Int. J. Hydrogen Energy 2016, 41, 23075–23091. [Google Scholar] [CrossRef]
- Wang, N.L.; Qiu, J.E.; You, K.Y.; Yuan, X.; Luo, H.A. Microwave assisted synthesis of Sn-modified MgAlO as support for platinum catalyst in cyclohexane dehydrogenation to cyclohexene. Appl. Catal. A-Gen. 2016, 516, 9–16. [Google Scholar] [CrossRef]
- Xia, Z.; Liu, H.; Lu, H.; Zhang, Z. Study on catalytic properties and carbon deposition of Ni-Cu/SBA-15 for cyclohexane dehydrogenation. Appl. Surf. Sci. 2017, 422, 905–912. [Google Scholar] [CrossRef]
- Cromwell, D.K.; Vasudevan, P.T.; Pawelec, B.; Fierro, J. Enhanced methyl cyclhexane dehydrogenation to toluene over Ir/USY catalyst. Catal. Today 2016, 259, 119–129. [Google Scholar] [CrossRef]
- Trimpont, P.A.; Marin, G.B.; Froment, G.F. Kinetics of methylcyclohexane dehydrogenation on sulfided commercial platinum/alumina and platinum –rhenium/alumina catalysts. Ind. Eng. Chem. Fundam. 1986, 25, 544–553. [Google Scholar] [CrossRef]
- Wu, K.; Chen, F.; Wang, F.; Huang, Y.; Yang, Y. Preparation of Pt supported on mesoporous Mg–Al oxide catalysts for efficient dehydrogenation of methylcyclohexane. Int. J. Hydrogen Energy 2021, 46, 25513–25519. [Google Scholar] [CrossRef]
- Sugiura, Y.; Nagatsuka, T.; Kubo, K.; Hirano, Y.; Nakamura, A.; Miyazawa, K.; Iizuka, Y.; Furuta, S.; Iki, H.; Higo, T.; et al. Dehydrogenation of Methylcyclohexane over Pt/TiO2-Al2O3 Catalysts. Chem. Lett. 2017, 46, 1601–1604. [Google Scholar] [CrossRef]
- Li, X.Y.; Ma, D.; Bao, X.H. Dispersion of Pt Catalysts Supported on Activated Carbon and Their Catalytic Performance in Methylcyclohexane Dehydrogenation. Chin. J. Catal. 2008, 29, 259–263. [Google Scholar] [CrossRef]
- Chen, Y.R.; Tsuru, T.; Kang, D.Y. Simulation and design of catalytic membrane reactor for hydrogen production via methylcyclohexane dehydrogenation. Int. J. Hydrogen Energy 2017, 42, 26296–26307. [Google Scholar] [CrossRef]
- Yakovlev, V.A.; Gulyaeva, Y.K.; Alekseeva, M.V.; Ermakov, D.Y.; Bulavchenko, O.A.; Zaikina, O.O. High-Loaded Nickel Based Sol–Gel Catalysts for Methylcyclohexane Dehydrogenation. Catalysts 2020, 10, 1198. [Google Scholar]
- Ham, H.; Simanullang, W.F.; Kanda, Y.; Wen, Y.; Hashimoto, A.; Abe, H.; Shimizu, K.I.; Furukawa, S. Silica-Decoration Boosts Ni Catalysis for (De)hydrogenation: Step-Abundant Nanostructures Stabilized by Silica. ChemCatChem 2021, 13, 1306–1310. [Google Scholar] [CrossRef]
- Yolcular, S. Organic chemical hydride dehydrogenation over nickel catalysts supported with SiO2 for hydrogen recovery. Energy Sources Part A: Recovery. Util. Environ. Eff. 2016, 14, 2031–2034. [Google Scholar]
- Al-Shaikhali, A.H.; Jedidi, A.; Anjum, D.H.; Cavallo, L.; Takanabe, K. Kinetics on NiZn Bimetallic Catalysts for Hydrogen Evolution via Selective Dehydrogenation of Methylcyclohexane to Toluene. ACS Catal. 2017, 7, 1592–1600. [Google Scholar] [CrossRef]
- Meng, J.C.; Zhou, F.; Ma, H.X.; Yuan, X.Z.; Wang, Y.Z.; Zhang, J. A Review of Catalysts for Methylcyclohexane Dehydrogenation. Top. Catal. 2021, 64, 509–520. [Google Scholar] [CrossRef]
- Zhu, C.; Chen, S.; Pan, D.; Cui, X.; Qiao, Y.; Li, R. Ordered mesoporous alumina-supported vanadium oxides as an efficient catalyst for ethylbenzene dehydrogenation to styrene with CO2. Catal. Commun. 2018, 115, 12–16. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.E.; Chen, H.; Zhang, X.Y.; Fu, Y.C.; Shen, J.Y. Synthesis of high-surface-area Co-O-Si compl:ex oxide for skeletal isomerization of 1-hexene and hydrodesulfurization of thiophene. Chin. J. Catal. 2014, 35, 1402–1409. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.E.; Song, G.X.; Shen, J.Y.; Tian, X.C. Preparation of NiAlOx and NiSiOx Complex Oxides with High Surface Areas for the Isomerization Reactions of 1-Hexene. Catal. Lett. 2016, 146, 1934–1942. [Google Scholar] [CrossRef]
- Song, K.H.; Jeong, S.K.; Jeong, B.H.; Lee, K.Y.; Kim, H.J. Effect of the Ni/Al ratio on the performance of NiAl2O4 spinel-based catalysts for supercritical methylcyclohexane catalytic cracking. Catalysts 2021, 11, 323. [Google Scholar] [CrossRef]
- Li, L.; Hui, K.S.; Hui, K.N.; Cho, Y.R. Ultrathin petal-like NiAl layered doubleoxide/sulfide composites as an advanced electrode for high-performance asymmetric supercapacitors. J. Mater. Chem. A 2017, 5, 19687–19696. [Google Scholar] [CrossRef]
- Vandergrift, C.; Elberse, P.A.; Mulder, A.; Geus, J.W. Preparation of Silica- supported Copper Catalysts by Means of Deposition-precipitation. Appl. Catal. 1990, 59, 275–289. [Google Scholar] [CrossRef]
- Gervasini, A.; Manzoli, M.; Martra, G.; Ponti, A.; Ravasio, N.; Sordelli, L.; Zaccheria, F. Dependence of Copper Species on the Nature of the Support for Dispersed CuO Catalysts. J. Phys. Chem. B 2006, 110, 51–61. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, Y.Q.; Bao, J.G.; Wang, W.Y.; Jiang, X.M. Catalytic performance of Ni/γ-Al2O3 for hydrogen carrier methylcyclohexane dehydrogenation. Chem. Ind. Eng. Prog. 2010, 29, 484–489. [Google Scholar]
- Shukla, A.A.; Gosavi, P.V.; Pande, J.V.; Kumar, V.P.; Chary, K.; Biniwale, R.B. Efficient hydrogen supply through catalytic dehydrogenation of methylcyclohexane over Pt/metal oxide catalysts. Int. J. Hydrogen Energy 2010, 35, 4020–4026. [Google Scholar] [CrossRef]
- Jing, Y.; Wang, W.; Lei, M.; Wu, K.; Chen, G.; Huang, Y. Dehydrogenation of methylcyclohexane over Pt Sn supported on Mg Al mixed metal oxides derived from layered double hydroxides. Int. J. Hydrogen Energy 2018, 43, 9343–9352. [Google Scholar]
- Li, G.H.; Xiong, G.; Zhao, X.; Wang, W.Y.; Li, W.S.; Yang, Y.Q. Ni-Cu/γ-Al2O3-ZrO2 preparation of catalyst and its dehydrogenation performance. Petrochem. Technol. 2016, 45, 798–804. [Google Scholar]









| Catalyst | Ni Content/wt% | Specific Surface Area /(m2/g) | Pore Size/nm |
|---|---|---|---|
| Ni10Al | — | 400 | 13.44 |
| Ni15Al | — | 480 | 18.17 |
| Ni20Al | 18.78 | 359 | 13.99 |
| Ni25Al | — | 272 | 10.12 |
| IM-NiAl | 19.63 | 133 | 16.29 |
| Catalyst | T (K) | Conversion of MCH (%) | Selectivity of Toluene (%) | Reference |
|---|---|---|---|---|
| Ni20Al | 723 | 77.4 | 85.6 | This work |
| Si(x)-Ni/SiO2 | 623 | 45 | 35 | [19] |
| Ni/Al2O3 | 653 | 55.23 | 99.9 | [30] |
| Ni-Cu/γ-Al2O3-ZrO2 | 723 | 82.6 | 98.2 | [33] |
| Pt/Al2O3 | 623 | 71.2 | 97.1 | [31] |
| Pt-Sn/Mg-Al-O | 623 | 90.5 | 98.4 | [32] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Lei, Q.; Li, H.; Li, G.; Zhao, Y. Preparation of a Novel NiAlO Composite Oxide Catalyst for the Dehydrogenation of Methylcyclohexane. Catalysts 2022, 12, 958. https://doi.org/10.3390/catal12090958
Wang D, Lei Q, Li H, Li G, Zhao Y. Preparation of a Novel NiAlO Composite Oxide Catalyst for the Dehydrogenation of Methylcyclohexane. Catalysts. 2022; 12(9):958. https://doi.org/10.3390/catal12090958
Chicago/Turabian StyleWang, Dongliang, Qian Lei, Hongwei Li, Guixian Li, and Yu Zhao. 2022. "Preparation of a Novel NiAlO Composite Oxide Catalyst for the Dehydrogenation of Methylcyclohexane" Catalysts 12, no. 9: 958. https://doi.org/10.3390/catal12090958