ZnO/CQDs Nanocomposites for Visible Light Photodegradation of Organic Pollutants
Abstract
:1. Introduction
2. Results and Discussion
2.1. ZnO/CQDs Nanocomposites Characterization
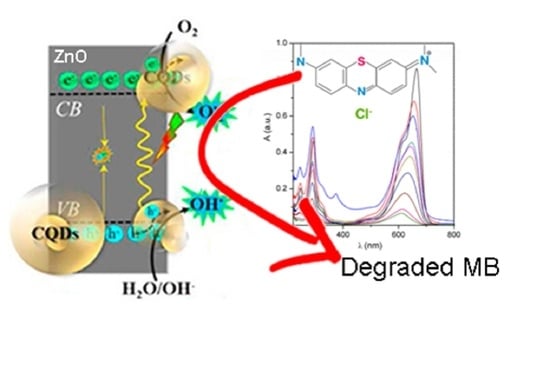
2.2. Methylene Blue (MB) Photodegradation
3. Methods and Materials
3.1. Synthesis of ZnO/CQDs Nanocomposites
3.2. Characterization Techniques
3.3. Methylene Blue Photooxidative Degradation
3.4. Reaction Products Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar]
- Yang, X.; Wang, D. Photocatalysis: From Fundamental Principles to Materials and Applications. ACS Appl. Energy Mater. 2018, 1, 6657–6693. [Google Scholar]
- Djurišić, A.B.; Chen, X.; Leung, Y.H.; Ng, A.M.C. ZnO Nanostructures: Growth, Properties and Applications. J. Mater. Chem. 2012, 22, 6526–6535. [Google Scholar]
- Kumar, S.G.; Rao, K.S.R.K. Zinc Oxide Based Photocatalysis: Tailoring Surface-Bulk Structure and Related Interfacial Charge Carrier Dynamics for Better Environmental Applications. RSC Adv. 2015, 5, 3306–3351. [Google Scholar]
- Li, D.; Li, D.K.; Wu, H.Z.; Liang, F.; Xie, W.; Zou, C.W.; Shao, L.X. Defects Related Room Temperature Ferromagnetism in Cu-Implanted ZnO Nanorod Arrays. J. Alloys Compd. 2014, 591, 80–84. [Google Scholar]
- Gupta, M.K.; Lee, J.H.; Lee, K.Y.; Kim, S.W. Two-Dimensional Vanadium-Doped ZnO Nanosheet-Based Flexible Direct Current Nanogenerator. ACS Nano 2013, 7, 8932–8939. [Google Scholar]
- Liu, X.; Hu, M.; Chu, X.; Yan, Q. Synthesis and Field Emission Properties of Highly Ordered Ti-Doped ZnO Nanoarray Structure. J. Mater. Sci. Mater. Electron. 2013, 24, 2839–2845. [Google Scholar]
- Qi, L.; Li, H.; Dong, L. Simple Synthesis of Flower-like ZnO by a Dextran Assisted Solution Route and Their Photocatalytic Degradation Property. Mater. Lett. 2013, 107, 354–356. [Google Scholar]
- Xiang, Q.; Yu, J.; Jaroniec, M. Graphene-Based Semiconductor Photocatalysts. Chem. Soc. Rev. 2012, 41, 782–796. [Google Scholar]
- Rauwel, E.; Galeckas, A.; Rauwel, P.; Sunding, M.F.; Fjellvaåg, H. Precursor-Dependent Blue-Green Photoluminescence Emission of ZnO Nanoparticles. J. Phys. Chem. C 2011, 115, 25227–25233. [Google Scholar]
- Wang, Z.L. Splendid One-Dimensional Nanostructures of Zinc Oxide: A New Nanomaterial Family for Nanotechnology. ACS Nano 2008, 2, 1987–1992. [Google Scholar]
- Polyakov, A.Y.; Nesterov, A.V.; Goldt, A.E.; Zubyuk, V.; Dolgova, T.; Yadgarov, L.; Visic, B.; Fedyanin, A.A.; Tenne, R.; Goodilin, E.A. Optical Properties of Multilayer Films of Nanocomposites Based on WS 2 Nanotubes Decorated with Gold Nanoparticles. J. Phys. Conf. Ser. 2015, 643, 012046. [Google Scholar]
- Donkova, B.; Vasileva, P.; Nihtianova, D.; Velichkova, N.; Stefanov, P.; Mehandjiev, D. Synthesis, Characterization, and Catalytic Application of Au/ZnO Nanocomposites Prepared by Coprecipitation. J. Mater. Sci. 2011, 46, 7134–7143. [Google Scholar]
- Kurtz, M.; Strunk, J.; Hinrichsen, O.; Muhler, M.; Fink, K.; Meyer, B.; Wöll, C. Active Sites on Oxide Surfaces: ZnO-Catalyzed Synthesis of Methanol from CO and H2. Angew. Chem. Int. Ed. 2005, 44, 2790–2794. [Google Scholar]
- Yuan, J.; Choo, E.S.G.; Tang, X.; Sheng, Y.; Ding, J.; Xue, J. Synthesis of ZnO–Pt Nanoflowers and Their Photocatalytic Applications. Nanotechnology 2010, 21, 185606. [Google Scholar]
- Wang, Y.; Hu, A. Carbon Quantum Dots: Synthesis, Properties and Applications. J. Mater. Chem. C 2014, 2, 6921–6939. [Google Scholar]
- Pan, M.; Xie, X.; Liu, K.; Yang, J.; Hong, L.; Wang, S. Fluorescent Carbon Quantum Dots-Synthesis, Functionalization and Sensing Application in Food Analysis. Nanomaterials 2020, 10, 930. [Google Scholar]
- Zhang, Z.; Zheng, T.; Li, X.; Xu, J.; Zeng, H. Progress of Carbon Quantum Dots in Photocatalysis Applications. Part. Part. Syst. Charact. 2016, 33, 457–472. [Google Scholar]
- Molaei, M.J. A Review on Nanostructured Carbon Quantum Dots and Their Applications in Biotechnology, Sensors, and Chemiluminescence. Talanta 2019, 196, 456–478. [Google Scholar]
- Luo, P.G.; Yang, F.; Yang, S.T.; Sonkar, S.K.; Yang, L.; Broglie, J.J.U.; Liu, Y.; Sun, Y.P. Carbon-Based Quantum Dots for Fluorescence Imaging of Cells and Tissues. RSC Adv. 2014, 4, 10791–10807. [Google Scholar]
- Singh, I.; Arora, R.; Dhiman, H.; Pahwa, R. Carbon Quantum Dots: Synthesis, Characterization and Biomedical Applications. Turkish J. Pharm. Sci. 2018, 15, 219–230. [Google Scholar]
- Sumanth Kumar, D.; Jai Kumar, B.; Mahesh, H.M. Quantum Nanostructures (QDs): An Overview. In Synthesis of Inorganic Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 59–88. [Google Scholar]
- Muthulingam, S.; Lee, I.H.; Uthirakumar, P. Highly Efficient Degradation of Dyes by Carbon Quantum Dots/N-Doped Zinc Oxide (CQD/N-ZnO) Photocatalyst and Its Compatibility on Three Different Commercial Dyes under Daylight. J. Colloid Interface Sci. 2015, 455, 101–109. [Google Scholar]
- Yu, H.; Zhao, Y.; Zhou, C.; Shang, L.; Peng, Y.; Cao, Y.; Wu, L.Z.; Tung, C.H.; Zhang, T. Carbon Quantum Dots/TiO2 Composites for Efficient Photocatalytic Hydrogen Evolution. J. Mater. Chem. A 2014, 2, 3344–3351. [Google Scholar]
- Al Ja’farawy, M.S.; Kusumandari; Purwanto, A.; Widiyandari, H. Carbon Quantum Dots Supported Zinc Oxide (ZnO/CQDs) Efficient Photocatalyst for Organic Pollutant Degradation—A Systematic Review. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100681. [Google Scholar]
- Bozetine, H.; Wang, Q.; Barras, A.; Li, M.; Hadjersi, T.; Szunerits, S.; Boukherroub, R. Green Chemistry Approach for the Synthesis of ZnO-Carbon Dots Nanocomposites with Good Photocatalytic Properties under Visible Light. J. Colloid Interface Sci. 2016, 465, 286–294. [Google Scholar]
- Yu, H.; Zhang, H.; Huang, H.; Liu, Y.; Li, H.; Ming, H.; Kang, Z. ZnO/Carbon Quantum Dots Nanocomposites: One-Step Fabrication and Superior Photocatalytic Ability for Toxic Gas Degradation under Visible Light at Room Temperature. New J. Chem. 2012, 36, 1031–1035. [Google Scholar]
- Zhang, X.; Pan, J.; Zhu, C.; Sheng, Y.; Yan, Z.; Wang, Y.; Feng, B. The Visible Light Catalytic Properties of Carbon Quantum Dots/ZnO Nanoflowers Composites. J. Mater. Sci. Mater. Electron. 2015, 26, 2861–2866. [Google Scholar]
- Zhang, X.-Y.; Liu, J.-K.; Wang, J.-D.; Yang, X.-H. Mass Production, Enhanced Visible Light Photocatalytic Efficiency, and Application of Modified ZnO Nanocrystals by Carbon Dots. Ind. Eng. Chem. Res. 2015, 54, 1766–1772. [Google Scholar]
- Han, C.; Yang, M.-Q.; Weng, B.; Xu, Y.-J. Improving the Photocatalytic Activity and Anti-Photocorrosion of Semiconductor ZnO by Coupling with Versatile Carbon. Phys. Chem. Chem. Phys. 2014, 16, 16891–16903. [Google Scholar]
- Liu, H.; Ye, T.; Mao, C. Fluorescent Carbon Nanoparticles Derived from Candle Soot. Angew. Chem. Int. Ed. 2007, 46, 6473–6475. [Google Scholar]
- Dekaliuk, M.O.; Viagin, O.; Malyukin, Y.V.; Demchenko, A.P. Fluorescent Carbon Nanomaterials: “Quantum Dots” or Nanoclusters? Phys. Chem. Chem. Phys. 2014, 16, 16075–16084. [Google Scholar]
- Li, H.; He, X.; Kang, Z.; Huang, H.; Liu, Y.; Liu, J.; Lian, S.; Tsang, C.H.A.; Yang, X.; Lee, S.T. Water-Soluble Fluorescent Carbon Quantum Dots and Photocatalyst Design. Angew. Chem. Int. Ed. 2010, 49, 4430–4434. [Google Scholar]
- Chen, X.; Zhang, W.; Wang, Q.; Fan, J. C8-Structured Carbon Quantum Dots: Synthesis, Blue and Green Double Luminescence, and Origins of Surface Defects. Carbon N. Y. 2014, 79, 165–173. [Google Scholar]
- Liu, Y.; Zhou, L.; Li, Y.; Deng, R.; Zhang, H. Highly fluorescent nitrogen-doped carbon dots with excellent thermal and photo stability applied as invisible ink for loading important information and anti-counterfeiting. Nanoscale 2017, 9, 491–496. [Google Scholar]
- Dong, Y.; Shao, J.; Chen, C.; Li, H.; Wang, R.; Chi, Y.; Lin, X.; Chen, G. Blue Luminescent Graphene Quantum Dots and Graphene Oxide Prepared by Tuning the Carbonization Degree of Citric Acid. Carbon N. Y. 2012, 50, 4738–4743. [Google Scholar]
- Yuan, F.; Yuan, T.; Sui, L.; Wang, Z.; Xi, Z.; Li, Y.; Li, X.; Fan, L.; Tan, Z.; Chen, A.; et al. Engineering Triangular Carbon Quantum Dots with Unprecedented Narrow Bandwidth Emission for Multicolored LEDs. Nat. Commun. 2018, 9, 2249–2260. [Google Scholar]
- Wu, P.; Wu, X.; Li, W.; Liu, Y.; Chen, Z.; Liu, S. Ultra-Small Amorphous Carbon Dots: Preparation, Photoluminescence Properties, and Their Application as TiO2 Photosensitizers. J. Mater. Sci. 2019, 54, 5280–5293. [Google Scholar]
- Liu, H.; Zhao, X.; Wang, F.; Wang, Y.; Guo, L.; Mei, J.; Tian, C.; Yang, X.; Zhao, D. High-Efficient Excitation-Independent Blue Luminescent Carbon Dots. Nanoscale Res. Lett. 2017, 12, 399–405. [Google Scholar]
- Song, Y.; Zhu, S.; Xiang, S.; Zhao, X.; Zhang, J.; Zhang, H.; Fu, Y.; Yang, B. Investigation into the Fluorescence Quenching Behaviors and Applications of Carbon Dots. Nanoscale 2014, 6, 4676–4683. [Google Scholar]
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The Photoluminescence Mechanism in Carbon Dots (Graphene Quantum Dots, Carbon Nanodots, and Polymer Dots): Current State and Future Perspective. Nano Res. 2015, 8, 355–381. [Google Scholar]
- Dulub, O.; Boatner, L.A.; Diebold, U. STM Study of the Geometric and Electronic Structure of ZnO(0 0 0 1)-Zn, (0 0 0 1̄)-O, (1 0 1̄ 0), and (1 1 2̄ 0) Surfaces. Surf. Sci. 2002, 519, 201–217. [Google Scholar]
- Zhang, Q. Effects of Calcination on the Photocatalytic Properties of Nanosized TiO2 Powders Prepared by TiCl4 Hydrolysis. Appl. Catal. B Environ. 2000, 26, 207–215. [Google Scholar]
- Fu, D.; Han, G.; Chang, Y.; Dong, J. The Synthesis and Properties of ZnO-Graphene Nano Hybrid for Photodegradation of Organic Pollutant in Water. Mater. Chem. Phys. 2012, 132, 673–681. [Google Scholar]
- Song, S.; Wu, K.; Wu, H.; Guo, J.; Zhang, L. Multi-shelled ZnO decorated with nitrogen and phosphorus co-doped carbon quantum dots: Synthesis and enhanced photodegradation activity of methylene blue in aqueous solutions. RSC Adv. 2019, 9, 7362–7374. [Google Scholar]
- Li, Y.; Zhang, B.P.; Zhao, J.X.; Ge, Z.H.; Zhao, X.K.; Zou, L. ZnO/Carbon Quantum Dots Heterostructure with Enhanced Photocatalytic Properties. Appl. Surf. Sci. 2013, 279, 367–373. [Google Scholar]
- Pandiyarajan, T.; Karthikeyan, B. Cr Doping Induced Structural, Phonon and Excitonic Properties of ZnO Nanoparticles. J. Nanoparticle Res. 2012, 14, 647. [Google Scholar]
- Chung, F.H. Quantitative Interpretation of X-Ray Diffraction Patterns of Mixtures. I. Matrix-Flushing Method for Quantitative Multicomponent Analysis. J. Appl. Crystallogr. 1974, 7, 519–525. [Google Scholar]
- Sali, S.; Boumaour, M.; Kechouane, M.; Kermadi, S.; Aitamar, F. Nanocrystalline ZnO Film Deposited by Ultrasonic Spray on Textured Silicon Substrate as an Anti-Reflection Coating Layer. Phys. B Condens. Matter 2012, 407, 2626–2631. [Google Scholar]
- Li, Y.; Zhao, Y.; Cheng, H.; Hu, Y.; Shi, G.; Dai, L.; Qu, L. Nitrogen-Doped Graphene Quantum Dots with Oxygen-Rich Functional Groups. J. Am. Chem. Soc. 2012, 134, 15–18. [Google Scholar]
- Xu, T.; Zhang, L.; Cheng, H.; Zhu, Y. Significantly Enhanced Photocatalytic Performance of ZnO via Graphene Hybridization and the Mechanism Study. Appl. Catal. B Environ. 2011, 101, 382–387. [Google Scholar]
- Van Zandvoort, I.; Wang, Y.; Rasrendra, C.B.; Van Eck, E.R.H.; Bruijnincx, P.C.A.; Heeres, H.J.; Weckhuysen, B.M. Formation, Molecular Structure, and Morphology of Humins in Biomass Conversion: Influence of Feedstock and Processing Conditions. ChemSusChem 2013, 6, 1745–1758. [Google Scholar]
- Heng, Z.W.; Chong, W.C.; Pang, Y.L.; Koo, C.H. An Overview of the Recent Advances of Carbon Quantum Dots/Metal Oxides in the Application of Heterogeneous Photocatalysis in Photodegradation of Pollutants towards Visible-Light and Solar Energy Exploitation. J. Environ. Chem. Eng. 2021, 9, 105199. [Google Scholar]
- Moalem-Banhangi, M.; Ghaeni, N.; Ghasemi, S. Saffron derived carbon quantum dot/N-doped ZnO/fulvic acid nanocomposite for sonocatalytic degradation of methylene blue. Synth. Met. 2021, 271, 116626. [Google Scholar]
- Velumani, A.; Sengodan, P.; Arumugam, P.; Rajendran, R.; Santhanam, S.; Palanisamy, M. Carbon quantum dots supported ZnO sphere based photocatalyst for dye degradation application. Curr. Appl. Phys. 2020, 20, 1176–1184. [Google Scholar]
- Soga, T. Nanostructured Materials for Solar Energy Conversion; Elsevier: Oxford, UK, 2006. [Google Scholar]
- Zhang, T.; Oyama, T.; Aoshima, A.; Hidaka, H.; Zhao, J.; Serpone, N. Photooxidative N-Demethylation of Methylene Blue in Aqueous TiO2 Dispersions under UV Irradiation. J. Photochem. Photobiol. A Chem. 2001, 140, 163–172. [Google Scholar]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.M. Photocatalytic Degradation Pathway of Methylene Blue in Water. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar]
- Zhou, B.; Song, J.; Zhang, Z.; Jiang, Z.; Zhang, P.; Han, B. Highly Selective Photocatalytic Oxidation of Biomass-Derived Chemicals to Carboxyl Compounds over Au/TiO2. Green Chem. 2017, 19, 1075–1081. [Google Scholar]
- Behnood, R.; Sodeifian, G. Synthesis of N Doped-CQDs/Ni Doped-ZnO Nanocomposites for Visible Light Photodegradation of Organic Pollutants. J. Environ. Chem. Eng. 2020, 8, 103821. [Google Scholar]














| Sample | β, Degree | (101) Plane, 2θ | θ, Degree | Crystallite Size, nm |
|---|---|---|---|---|
| ZnO(C) | 0.2599 | 36.2158 | 18.1079 | 33.61 |
| ZnO(C)/CQD180-12 | 0.2490 | 36.2637 | 18.1318 | 35.09 |
| ZnO(C)/CQD200-4 | 0.2469 | 36.2322 | 18.1161 | 35.39 |
| ZnO(OP)/CQD180-12 | 0.3053 | 36.1265 | 18.0632 | 28.61 |
| ZnO(OP)/CQD200-4 | 0.3139 | 36.1327 | 18.0663 | 27.83 |
| Sample | 2θ, Degree | Lattice Parameters, Å | c/a | d-Spacing, BRAGG | |
|---|---|---|---|---|---|
| c | a = b | ||||
| ZnO(C) | 36.2158 | 5.2157 | 3.2561 | 1.6018 | 2.4803 |
| ZnO(C)/CQD180-12 | 36.2637 | 5.2140 | 3.2533 | 1.6027 | 2.4771 |
| ZnO(C)/CQD200-4 | 36.2322 | 5.2140 | 3.2547 | 1.6020 | 2.4795 |
| ZnO(OP)/CQD180-12 | 36.1265 | 5.2246 | 3.2678 | 1.5988 | 2.4867 |
| ZnO(OP)/CQD200-4 | 36.1327 | 5.2246 | 3.2713 | 1.5971 | 2.4859 |
| JCPDS 36-1451 | 36.2150 | 5.2150 | 3.2560 | 1.6016 | - |
| Sample | BET (m2/g) | Vp (cm3/g) | Average Dp (nm) | dBET (nm) |
|---|---|---|---|---|
| ZnO(C) | 12 | 0.033 | 26.5 | 89.1 |
| ZnO(C)/CQD200-4 | 15 | 0.136 | 52.0 | 71.3 |
| ZnO(C)/CQD180-12 | 18 | 0.062 | 25.8 | 59.4 |
| ZnO(OP)/CQD200-4 | 7 | 0.038 | 25.7 | 152.8 |
| ZnO(OP)/CQD180-12 | 11 | 0.045 | 37.2 | 97.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toma, E.E.; Stoian, G.; Cojocaru, B.; Parvulescu, V.I.; Coman, S.M. ZnO/CQDs Nanocomposites for Visible Light Photodegradation of Organic Pollutants. Catalysts 2022, 12, 952. https://doi.org/10.3390/catal12090952
Toma EE, Stoian G, Cojocaru B, Parvulescu VI, Coman SM. ZnO/CQDs Nanocomposites for Visible Light Photodegradation of Organic Pollutants. Catalysts. 2022; 12(9):952. https://doi.org/10.3390/catal12090952
Chicago/Turabian StyleToma, Elena E., Giuseppe Stoian, Bogdan Cojocaru, Vasile I. Parvulescu, and Simona M. Coman. 2022. "ZnO/CQDs Nanocomposites for Visible Light Photodegradation of Organic Pollutants" Catalysts 12, no. 9: 952. https://doi.org/10.3390/catal12090952