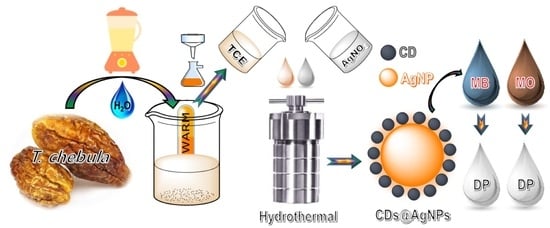
Green-Routed Carbon Dot-Adorned Silver Nanoparticles for the Catalytic Degradation of Organic Dyes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Characterization of the Synthesized CDs@AgNPs Composite
2.2. Catalytic Activity of the Synthesized CDs@AgNPs Composite towards the Degradation of Organic Dyes
3. Materials and Methods
Synthesis of the CDs@AgNP Composite
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mitchell, S.; Qin, R.; Zheng, N.; Pérez-Ramírez, J. Nanoscale engineering of catalytic materials for sustainable technologies. Nat. Nanotechnol. 2021, 16, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Chadha, U.; Selvaraj, S.K.; Ashokan, H.; Hariharan, S.P.; Mathew Paul, V.; Venkatarangan, V.; Paramasivam, V. Complex Nanomaterials in Catalysis for Chemically Significant Applications: From Synthesis and Hydrocarbon Processing to Renewable Energy Applications. Adv. Mater. Sci. Eng. 2022, 2022, 1552334. [Google Scholar] [CrossRef]
- Li, J.; Ma, C.; Zhu, S.; Yu, F.; Dai, B.; Yang, D. A Review of Recent Advances of Dielectric Barrier Discharge Plasma in Catalysis. Nanomaterials 2019, 9, 1428. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef]
- Priyadharsan, A.; Vasanthakumar, V.; Karthikeyan, S.; Raj, V.; Shanavas, S.; Anbarasan, P.M. Multi-functional properties of ternary CeO2/SnO2/rGO nanocomposites: Visible light driven photocatalyst and heavy metal removal. J. Photochem. Photobiol. A Chem. 2017, 346, 32–45. [Google Scholar] [CrossRef]
- Ye, R.; Hurlburt, T.J.; Sabyrov, K.; Alayoglu, S.; Somorjai, G.A. Molecular catalysis science: Perspective on unifying the fields of catalysis. Proc. Natl. Acad. Sci. USA 2016, 113, 5159–5166. [Google Scholar] [CrossRef]
- Thongam, D.D.; Chaturvedi, H. Advances in nanomaterials for heterogeneous photocatalysis. Nano Express 2021, 2, 012005. [Google Scholar] [CrossRef]
- Kumar, A.; Mishra, B.; Tripathi, B.P. Polydopamine assisted synthesis of ultrafine silver nanoparticles for heterogeneous catalysis and water remediation. Nano-Struct. Nano-Objects 2020, 23, 100489. [Google Scholar] [CrossRef]
- Rauf, M.A.; Ashraf, S.S. Fundamental principles and application of heterogeneous photocatalytic degradation of dyes in solution. Chem. Eng. J. 2009, 151, 10–18. [Google Scholar] [CrossRef]
- Jo, W.-K.; Kumar, S.; Isaacs, M.A.; Lee, A.F.; Karthikeyan, S. Cobalt promoted TiO2/GO for the photocatalytic degradation of oxytetracycline and Congo Red. Appl. Catal. B Environ. 2017, 201, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Hama Aziz, K.H.; Miessner, H.; Mueller, S.; Mahyar, A.; Kalass, D.; Moeller, D.; Khorshid, I.; Rashid, M.A.M. Comparative study on 2,4-dichlorophenoxyacetic acid and 2,4-dichlorophenol removal from aqueous solutions via ozonation, photocatalysis and non-thermal plasma using a planar falling film reactor. J. Hazard. Mater. 2018, 343, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Dabhane, H.; Chatur, S.; Jadhav, G.; Tambade, P.; Medhane, V. Phytogenic synthesis of gold nanoparticles and applications for removal of methylene blue dye: A review. Environ. Chem. Ecotoxicol. 2021, 3, 160–171. [Google Scholar] [CrossRef]
- Rajasekar, R.; Samuel, M.; Edison, T.N.J.I.; Raman, N. Sustainable synthesis of silver nanoparticles using Alstonia scholaris for enhanced catalytic degradation of methylene blue. J. Mol. Struct. 2021, 1246, 131208. [Google Scholar] [CrossRef]
- Kora, A.J.; Rastogi, L. Catalytic degradation of anthropogenic dye pollutants using palladium nanoparticles synthesized by gum olibanum, a glucuronoarabinogalactan biopolymer. Ind. Crops Prod. 2016, 81, 1–10. [Google Scholar] [CrossRef]
- Edison, T.N.J.I.; Atchudan, R.; Karthik, N.; Balaji, J.; Xiong, D.; Lee, Y.R. Catalytic degradation of organic dyes using green synthesized N-doped carbon supported silver nanoparticles. Fuel 2020, 280, 118682. [Google Scholar] [CrossRef]
- Bolla, P.A.; Huggias, S.; Serradell, M.A.; Ruggera, J.F.; Casella, M.L. Synthesis and Catalytic Application of Silver Nanoparticles Supported on Lactobacillus kefiri S-Layer Proteins. Nanomaterials 2020, 10, 2322. [Google Scholar] [CrossRef]
- Saha, J.; Begum, A.; Mukherjee, A.; Kumar, S. A novel green synthesis of silver nanoparticles and their catalytic action in reduction of Methylene Blue dye. Sustain. Environ. Res. 2017, 27, 245–250. [Google Scholar] [CrossRef]
- Alshorifi, F.T.; Alswat, A.A.; Mannaa, M.A.; Alotaibi, M.T.; El-Bahy, S.M.; Salama, R.S. Facile and Green Synthesis of Silver Quantum Dots Immobilized onto a Polymeric CTS–PEO Blend for the Photocatalytic Degradation of p-Nitrophenol. ACS Omega 2021, 6, 30432–30441. [Google Scholar] [CrossRef]
- El-Yazeed, W.S.A.; El-Hakam, S.A.; Salama, R.S.; Ibrahim, A.A.; Ahmed, A.I. Ag-PMA supported on MCM-41: Surface Acidity and Catalytic Activity. J. Sol-Gel Sci. Technol. 2022, 102, 387–399. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Z.; Chen, S.; Wu, J.; Guo, X.; Liu, Z. One-step hydrothermal synthesis of silver nanoparticles loaded on N-doped carbon and application for catalytic reduction of 4-nitrophenol. RSC Adv. 2015, 5, 87151–87156. [Google Scholar] [CrossRef]
- Yuan, W.; Jiang, G.; Che, J.; Qi, X.; Xu, R.; Chang, M.W.; Chen, Y.; Lim, S.Y.; Dai, J.; Chan-Park, M.B. Deposition of Silver Nanoparticles on Multiwalled Carbon Nanotubes Grafted with Hyperbranched Poly(amidoamine) and Their Antimicrobial Effects. J. Phys. Chem. C 2008, 112, 18754–18759. [Google Scholar] [CrossRef]
- Jahanbakhshi, M.; Habibi, B. A novel and facile synthesis of carbon quantum dots via salep hydrothermal treatment as the silver nanoparticles support: Application to electroanalytical determination of H2O2 in fetal bovine serum. Biosens. Bioelectron. 2016, 81, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Saravanan, R.; Karthikeyan, S.; Gupta, V.K.; Sekaran, G.; Narayanan, V.; Stephen, A. Enhanced photocatalytic activity of ZnO/CuO nanocomposite for the degradation of textile dye on visible light illumination. Mater. Sci. Eng. C 2013, 33, 91–98. [Google Scholar] [CrossRef]
- Eljiedi, A.A.A.; Kamari, A. Removal of methyl orange and methylene blue dyes from aqueous solution using lala clam (Orbicularia orbiculata) shell. AIP Conf. Proc. 2017, 1847, 040003. [Google Scholar] [CrossRef]
- Su, Y.; Shi, B.; Liao, S.; Zhao, J.; Chen, L.; Zhao, S. Silver Nanoparticles/N-Doped Carbon-Dots Nanocomposites Derived from Siraitia Grosvenorii and Its Logic Gate and Surface-Enhanced Raman Scattering Characteristics. ACS Sustain. Chem. Eng. 2016, 4, 1728–1735. [Google Scholar] [CrossRef]
- Zheng, M.; Wang, C.; Wang, Y.; Wei, W.; Ma, S.; Sun, X.; He, J. Green synthesis of carbon dots functionalized silver nanoparticles for the colorimetric detection of phoxim. Talanta 2018, 185, 309–315. [Google Scholar] [CrossRef]
- Beiraghi, A.; Najibi-Gehraz, S.A. Carbon dots-modified silver nanoparticles as a new colorimetric sensor for selective determination of cupric ions. Sens. Actuators B Chem. 2017, 253, 342–351. [Google Scholar] [CrossRef]
- Wei, X.; Cheng, F.; Yao, Y.; Yi, X.; Wei, B.; Li, H.; Wu, Y.; He, J. Facile synthesis of a carbon dots and silver nanoparticles (CDs/AgNPs) composite for antibacterial application. RSC Adv. 2021, 11, 18417–18422. [Google Scholar] [CrossRef]
- Li, H.; Huang, J.; Lu, F.; Liu, Y.; Song, Y.; Sun, Y.; Zhong, J.; Huang, H.; Wang, Y.; Li, S.; et al. Impacts of Carbon Dots on Rice Plants: Boosting the Growth and Improving the Disease Resistance. ACS Appl. Bio Mater. 2018, 1, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Atchudan, R.; Edison, T.N.J.I.; Aseer, K.R.; Perumal, S.; Karthik, N.; Lee, Y.R. Highly fluorescent nitrogen-doped carbon dots derived from Phyllanthus acidus utilized as a fluorescent probe for label-free selective detection of Fe3+ ions, live cell imaging and fluorescent ink. Biosens. Bioelectron. 2018, 99, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Salama, R.S.; El-Sayed, E.-S.M.; El-Bahy, S.M.; Awad, F.S. Silver nanoparticles supported on UiO-66 (Zr): As an efficient and recyclable heterogeneous catalyst and efficient adsorbent for removal of indigo carmine. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127089. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Chakradhar, D.; Perumal, S.; Shim, J.-J.; Lee, Y.R. Facile green synthesis of nitrogen-doped carbon dots using Chionanthus retusus fruit extract and investigation of their suitability for metal ion sensing and biological applications. Sens. Actuators B Chem. 2017, 246, 497–509. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Lee, Y.R. Nitrogen-doped carbon dots originating from unripe peach for fluorescent bioimaging and electrocatalytic oxygen reduction reaction. J. Colloid Interface Sci. 2016, 482, 8–18. [Google Scholar] [CrossRef]
- Atchudan, R.; Perumal, S.; Edison, T.N.J.I.; Albasher, G.; Sundramoorthy, A.K.; Vinodh, R.; Lee, Y.R. Lotus-biowaste derived sulfur/nitrogen-codoped porous carbon as an eco-friendly electrocatalyst for clean energy harvesting. Environ. Res. 2022, 214, 113910. [Google Scholar] [CrossRef] [PubMed]
- Atchudan, R.; Chandra Kishore, S.; Gangadaran, P.; Jebakumar Immanuel Edison, T.N.; Perumal, S.; Rajendran, R.L.; Alagan, M.; Al-Rashed, S.; Ahn, B.-C.; Lee, Y.R. Tunable fluorescent carbon dots from biowaste as fluorescence ink and imaging human normal and cancer cells. Environ. Res. 2022, 204, 112365. [Google Scholar] [CrossRef] [PubMed]
- Dager, A.; Baliyan, A.; Kurosu, S.; Maekawa, T.; Tachibana, M. Ultrafast synthesis of carbon quantum dots from fenugreek seeds using microwave plasma enhanced decomposition: Application of C-QDs to grow fluorescent protein crystals. Sci. Rep. 2020, 10, 12333. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Pang, Q.; Mai, K.; He, X.; Xu, L.; Zhou, F.; Liu, Y. Silver nanoparticle@carbon quantum dot composite as an antibacterial agent. RSC Adv. 2022, 12, 9621–9627. [Google Scholar] [CrossRef]
- Atchudan, R.; Jebakumar Immanuel Edison, T.N.; Perumal, S.; Vinodh, R.; Babu, R.S.; Sundramoorthy, A.K.; Renita, A.A.; Lee, Y.R. Facile synthesis of nitrogen-doped porous carbon materials using waste biomass for energy storage applications. Chemosphere 2022, 289, 133225. [Google Scholar] [CrossRef]
- Jyoti, K.; Singh, A. Green synthesis of nanostructured silver particles and their catalytic application in dye degradation. J. Genet. Eng. Biotechnol. 2016, 14, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Song, W.C.; Park, S.Y.; Park, G. Green Synthesis of Silver and Gold Nanoparticles via Sargassum serratifolium Extract for Catalytic Reduction of Organic Dyes. Catalysts 2021, 11, 347. [Google Scholar] [CrossRef]
- Luong, T.H.V.; Nguyen, T.H.T.; Nguyen, B.V.; Nguyen, N.K.; Nguyen, T.Q.C.; Dang, G.H. Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs. Green Process. Synth. 2022, 11, 71–83. [Google Scholar] [CrossRef]
- Somasundaram, C.K.; Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Vinodh, R.; Sundramoorthy, A.K.; Babu, R.S.; Alagan, M.; Lee, Y.R. Sustainable Synthesis of Silver Nanoparticles Using Marine Algae for Catalytic Degradation of Methylene Blue. Catalysts 2021, 11, 1377. [Google Scholar] [CrossRef]
- Edison, T.N.J.I.; Atchudan, R.; Sethuraman, M.G.; Lee, Y.R. Reductive-degradation of carcinogenic azo dyes using Anacardium occidentale testa derived silver nanoparticles. J. Photochem. Photobiol. B Biol. 2016, 162, 604–610. [Google Scholar] [CrossRef]
- Edison, T.N.J.I.; Lee, Y.R.; Sethuraman, M.G. Green synthesis of silver nanoparticles using Terminalia cuneata and its catalytic action in reduction of direct yellow-12 dye. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 161, 122–129. [Google Scholar] [CrossRef]
- Edison, T.N.J.I.; Atchudan, R.; Kamal, C.; Lee, Y.R. Caulerpa racemosa: A marine green alga for eco-friendly synthesis of silver nanoparticles and its catalytic degradation of methylene blue. Bioprocess Biosyst. Eng. 2016, 39, 1401–1408. [Google Scholar] [CrossRef]
- Suvith, V.S.; Philip, D. Catalytic degradation of methylene blue using biosynthesized gold and silver nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 118, 526–532. [Google Scholar] [CrossRef]
- Khodadadi, B.; Bordbar, M.; Nasrollahzadeh, M. Achillea millefolium L. extract mediated green synthesis of waste peach kernel shell supported silver nanoparticles: Application of the nanoparticles for catalytic reduction of a variety of dyes in water. J. Colloid Interface Sci. 2017, 493, 85–93. [Google Scholar] [CrossRef]
- Hamedi, S.; Shojaosadati, S.A.; Mohammadi, A. Evaluation of the catalytic, antibacterial and anti-biofilm activities of the Convolvulus arvensis extract functionalized silver nanoparticles. J. Photochem. Photobiol. B Biol. 2017, 167, 36–44. [Google Scholar] [CrossRef]
- Amjad, U.-E.-S.; Sherin, L.; Zafar, M.F.; Mustafa, M. Comparative Study on the Catalytic Degradation of Methyl Orange by Silver Nanoparticles Synthesized by Solution Combustion and Green Synthesis Method. Arab. J. Sci. Eng. 2019, 44, 9851–9857. [Google Scholar] [CrossRef]
- Nagar, N.; Devra, V. A kinetic study on the degradation and biodegradability of silver nanoparticles catalyzed Methyl Orange and textile effluents. Heliyon 2019, 5, e01356. [Google Scholar] [CrossRef] [PubMed]
- Bhankhar, A.; Giri, M.; Yadav, K.; Jaggi, N. Study on degradation of methyl orange-an azo dye by silver nanoparticles using UV–Visible spectroscopy. Indian J. Phys. 2014, 88, 1191–1196. [Google Scholar] [CrossRef]







| Catalyst | Reaction Time (min) | MB/MO Degradation (%) | Rate Constant (k) | Reference |
|---|---|---|---|---|
| Ag/Au composite | 20 | - (MB) | 0.668 min−1 | [48] |
| AgNPs/G. arborea | 10 | 100 (MB) | - | [17] |
| Ag NPs/peach kernel | 10/12 | 100 (MB/MO) | - | [49] |
| Ag NPs/C. arvensis | 20 | - (MB) | 0.108 min−1 | [50] |
| AS-AgNPs | 27 | 97 (MB) | 0.0007 s−1 | [13] |
| AgNPs/Marine algae | 20 | 99 (MB) | 0.106 min−1 | [44] |
| AgNPs-GS | 60 | 94 (MO) | 0.0725 min−1 | [51] |
| AgNPs/PDS | 40 | 88 (MO) | 0.00159 s−1 | [52] |
| Ag-ZX | 25 | 100 (MO) | 0.305 min−1 | [53] |
| CDs@AgNPs | 15/20 min | 95.5/99.0 | 0.204/0.128 min−1 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perumal, S.; Edison, T.N.J.I.; Atchudan, R.; Sundramoorthy, A.K.; Lee, Y.R. Green-Routed Carbon Dot-Adorned Silver Nanoparticles for the Catalytic Degradation of Organic Dyes. Catalysts 2022, 12, 937. https://doi.org/10.3390/catal12090937
Perumal S, Edison TNJI, Atchudan R, Sundramoorthy AK, Lee YR. Green-Routed Carbon Dot-Adorned Silver Nanoparticles for the Catalytic Degradation of Organic Dyes. Catalysts. 2022; 12(9):937. https://doi.org/10.3390/catal12090937
Chicago/Turabian StylePerumal, Suguna, Thomas Nesakumar Jebakumar Immanuel Edison, Raji Atchudan, Ashok K. Sundramoorthy, and Yong Rok Lee. 2022. "Green-Routed Carbon Dot-Adorned Silver Nanoparticles for the Catalytic Degradation of Organic Dyes" Catalysts 12, no. 9: 937. https://doi.org/10.3390/catal12090937